- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
MiniSOG2-mediated Specific Photoablation of Motor Neurons in Ascidian Embryos
(*contributed equally to this work) Published: Vol 12, Iss 20, Oct 20, 2022 DOI: 10.21769/BioProtoc.4537 Views: 1777
Reviewed by: Sunanda MarellaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Explant Culture of the Embryonic Mouse Spinal Cord and Gene Transfer by ex vivo Electroporation
Mariko Kinoshita-Kawada [...] Jane Y. Wu
Sep 20, 2019 6967 Views

Electroporation of Whole-Mount Postnatal Rodent Retinas for Advanced Functional Assays
Chien-Ting Huang [...] Chih-Tien Wang
Jan 20, 2026 137 Views
Abstract
When understanding the neuronal function of a specific neural circuit, single-cell level photoablation of a targeted cell is one of the useful experimental approaches. This protocol describes a method to photoablate specific motor neurons via the mini singlet oxygen generator (miniSOG2), a light–oxygen–voltage (LOV)-based optogenetic tool used for ablating targeted cells in arbitrary areas. MiniSOG2 could induce the cell death pathway by generating reactive oxygen species (ROS) upon blue light illumination. Photoablation of a specific cell using the miniSOG2 was performed to show that, in Ciona intestinalis type A (Ciona robusta), a single pair of motor neurons, MN2/A10.64, is necessary to drive their tail muscle contraction. The membrane targeted miniSOG2 combined with neuron-specific promoter (pSP-Neurog::miniSOG2-CAAX) was electroplated into the Ciona egg and transiently expressed at specific neurons of the embryo. MN2 labeled with pSP-Neurog:mCherry-CAAX was irradiated using a 440-nm laser from the lateral side for 10 min to ablate its neural function. The behavior of the embryo before and after the irradiation was recorded with a high-speed camera.
Graphical abstract:
Background
To understand neuronal function during development of specific neural circuits, several inhibitors of neurotransmitters or the knock-down/knock-out (KD/KO) of the neuron-specific gene have been used in previous ascidian research. However, inhibitors suppress the function of not only the targeted neuron but also the same types of other neurons, and the gene KD/KO method cannot artificially regulate the timing of inhibition. Therefore, specific ablation of the targeted neuron in the appropriate timeframe and area has been considered to be difficult. On the other hand, the mini singlet oxygen generator, or miniSOG2 (Makhijani et al., 2017), can be genetically introduced to the specific neurons and then ablate them at a given time using laser irradiation. Combined with the various tissue- and cell-specific promoters in Ciona (https://www.aniseed.cnrs.fr/aniseed/gene/?choice=find_cisreg) provides general information for ascidian-specific promoters), it was possible to ablate different neuron subtypes even at a single cell level. This method will be a strong tool for understanding not only the function of neurons but also the function of non-neuronal cells during ascidian development.
Materials and Reagents
Glass-based Petri dish (glass diameter ϕ12 mm or ϕ27 mm, IWAKI)
MiniSOG2 construct driven by your chosen promoter, e.g., pSP-Neurog::miniSOG2-CAAX. Plasmid coding miniSOG2 (pcDNA3.1_miniSOG2 T2A H2B-EGFP) (Addgene, catalog number: 87410). The DNA sequence of miniSOG2-CAAX is shown in Table 1.
Arbitrarily chosen fluorescent protein driven by the same promoter used for identifying miniSOG2 positive cells, such as pSP-Neurog::mCherry-CAAX. DNA sequence of mCherry-CAAX is shown in Table 1.
Egg and sperm dissected from Ciona matured adults
Natural sea water
Table 1. Sequences for miniSOG2-CAAX and mCherry-CAAX
Equipment
Confocal laser scanning microscope (CLSM), inverted type (OLYMPUS, Olympus fv1000)
Optical power meter (HIOKI, optical power meter 3664)
Optical sensor (HIOKI, optical sensor 9742)
High-speed camera (WRAYMER, WRAYCAM-VEX230M)
Stereoscopic microscope (OLYMPUS, Olympus SZX12)
Software
Olympus fv1000 viewer (OLYMPUS)
Procedure
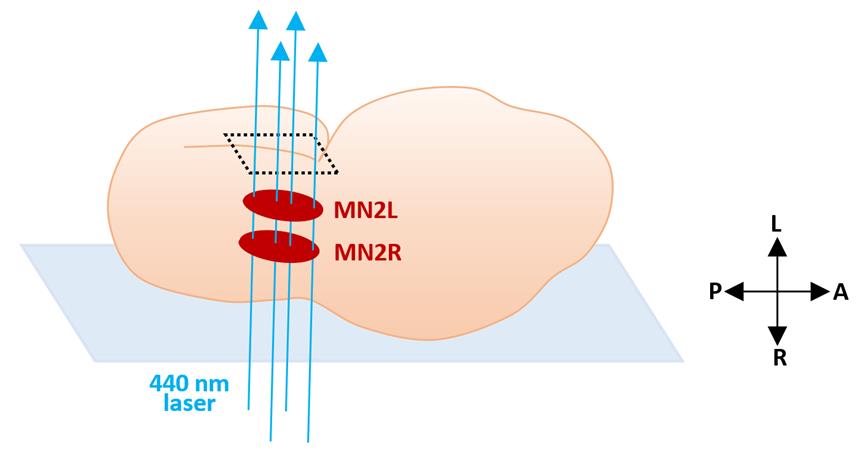
Electroporate the miniSOG2 construct together with a fluorescence marker into Ciona fertilized egg (Christiaen et al., 2009). In the case of photoablating MN2R and MN2L, pSP-Neurog::miniSOG2-CAAX can be used to express miniSOG2 at MN2/A10.64, and pSP-Neurog::mCherry-CAAX to label the position of MN2 and determine the region of interest (ROI) for laser irradiation (Figure 1).
Tip: In the case of Ciona, electroporation should be conducted 30 min after fertilization. Both constructs will be observable after the embryo develops to mid-tailbud II (St. 22) (Akahoshi et al., 2021).
Place embryo on a glass-based Petri dish with natural sea water.
To evaluate the influence on photoablation of the targeted single neuron, image embryos with a high-speed camera (200 to 500 fps) attached to a stereoscopic microscope, before performing laser irradiation.
Place your embryo under a CLSM (Olympus fv1000).
Set the ROI for laser irradiation of your targeted neuron. Select the target neuron from the neurons labeled with fluorescence protein (e.g., mCherry. Excite the red fluorescence of mCherry with a 559-nm laser). To avoid photoablating other neurons, carefully check whether the ROI is restricted to your targeted neuron only, before conducting laser irradiation. Either Olympus 20× or 40× oil immersion lenses can be used as objectives.
Measure the 440-nm laser power density with an optical power meter and an optical sensor. Optimize dichroic filters or other optical equipment in your microscope so that the laser power density is at least more than 15 µW/cm2.
Perform 440-nm laser irradiation of the ROI from the embryo’s lateral side (Figures 1, 2) for 10 min.
Tip: Every minute, turn the 440-nm laser off and ensure that embryo movement does not shift the ROI out of range from its original position. If the embryo moved during irradiation, reset the ROI to the targeted neuron again, and restart laser irradiation.
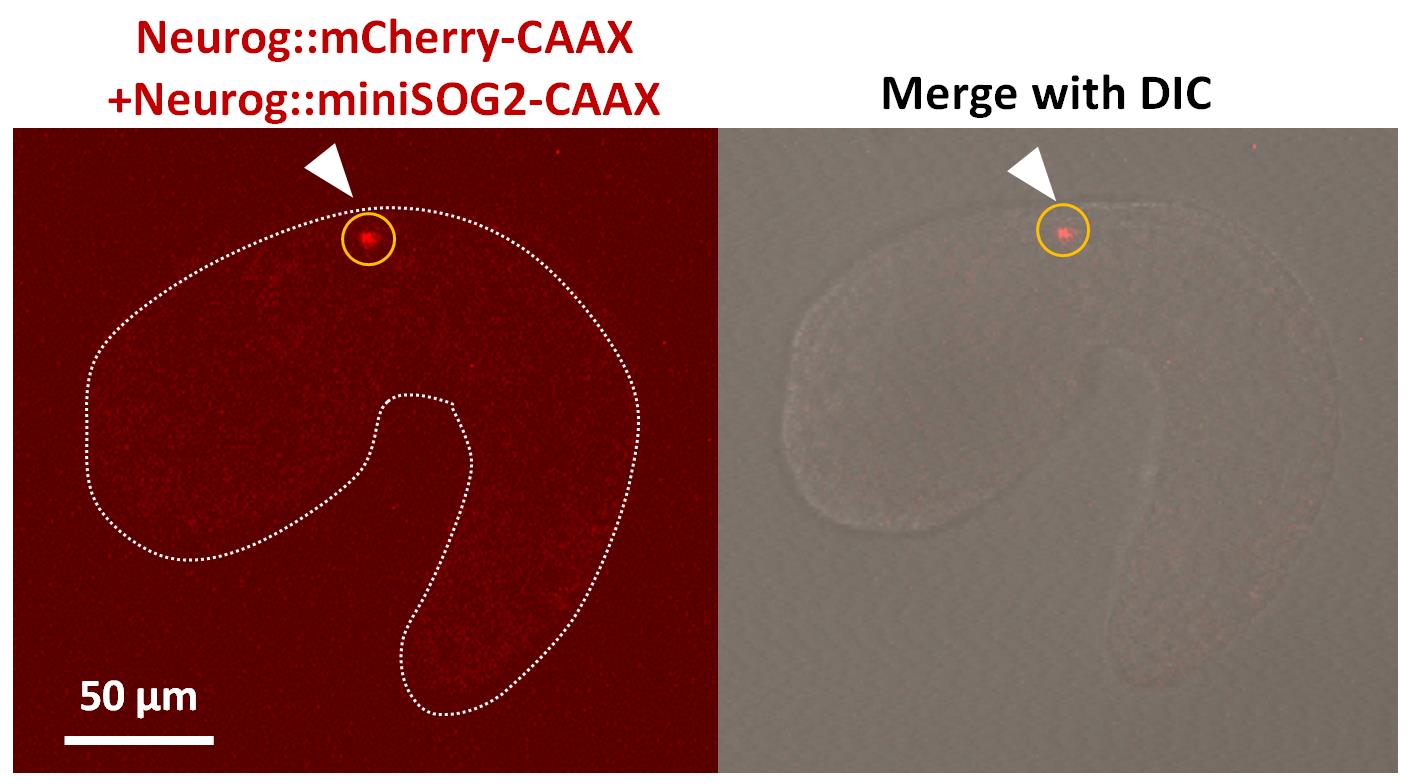
Figure 1. miniSOG2-transduced Ciona embryo. Lateral view of the fluorescence image (left) of a Ciona embryo (St. 22) electroporated with pSP-Neurog::mCherry-CAAX and pSP-Neurog::miniSOG2-CAAX. The image merged with differential interference contrast (DIC) is shown in the right panel. The signal of mCherry-CAAX seen in MN2 is indicated with a white arrowhead. ROI for laser irradiation is indicated with a yellow circle.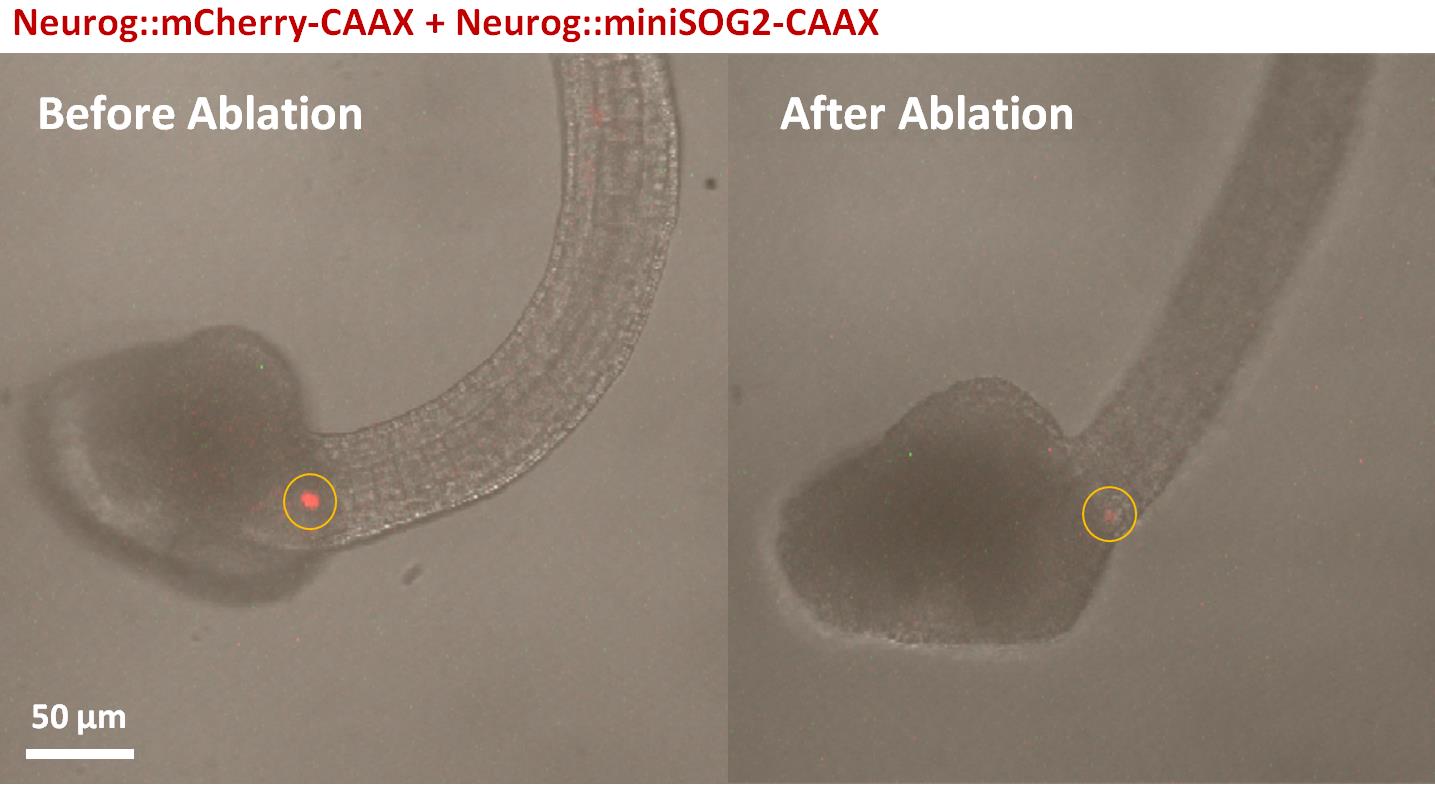
Figure 2. MiniSOG2-transduced Ciona embryo before and after laser irradiation. Lateral view of the fluorescence image merged with differential interference contrast (DIC) of Ciona embryo (St. 24) electroporated with pSP-Neurog::mCherry-CAAX and pSP-Neurog::miniSOG2-CAAX. The mCherry-CAAX signal seen in MN2 is indicated with a white arrowhead. ROI for laser irradiation is indicated with yellow circles. Left; before laser irradiation. Right; after laser irradiation.As a negative control, photoablate other labeled neurons, or the same neuron only transduced with mCherry, not miniSOG2.
Thirty minutes after irradiation, image the irradiated embryos with a high-speed camera again. Check whether any changes occured in their behavior due to photoablation of the targeted neuron, and compare these changes with the embryos irradiated as control (Video 1).
Video 1. Behavior of control or miniSOG2-transducedCiona embryos before and after laser irradiation.Tail movements of both a control (pSP-Neurog::mCherry-CAAX) embryo and a miniSOG2-transduced (pSP-Neurog::mCherry-CAAX & pSP-Neurog::miniSOG2-CAAX) embryo, before and after 10 min of laser irradiation. Videos were captured with a high-speed camera. Tail movement of the control embryo occurred every 30 sec, and there was no significant change after laser irradiation. However, the miniSOG2-transduced embryo became immobile after laser irradiation.
Acknowledgments
This protocol was adapted from Makhijani et al. (2017) and Akahoshi et al. (2021).
Competing interests
The authors declare that they have no competing interests.
References
- Akahoshi, T., Utsumi, M. K., Oonuma, K., Murakami, M., Horie, T., Kusakabe, T. G., Oka, K. and Hotta, K. (2021). A single motor neuron determines the rhythm of early motor behavior in Ciona. Sci Adv 7(50): eabl6053.
- Christiaen, L., Wagner, E., Shi, W. and Levine, M. (2009). Electroporation of transgenic DNAs in the sea squirt Ciona. Cold Spring Harb Protoc 2009(12): pdb prot5345.
- Makhijani, K., To, T. L., Ruiz-Gonzalez, R., Lafaye, C., Royant, A. and Shu, X. (2017). Precision Optogenetic Tool for Selective Single- and Multiple-Cell Ablation in a Live Animal Model System. Cell Chem Biol 24(1): 110-119.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Utsumi, M. K., Akahoshi, T., Oka, K. and Hotta, K. (2022). MiniSOG2-mediated Specific Photoablation of Motor Neurons in Ascidian Embryos. Bio-protocol 12(20): e4537. DOI: 10.21769/BioProtoc.4537.
- Akahoshi, T., Utsumi, M. K., Oonuma, K., Murakami, M., Horie, T., Kusakabe, T. G., Oka, K. and Hotta, K. (2021). A single motor neuron determines the rhythm of early motor behavior in Ciona. Sci Adv 7(50): eabl6053.
Category
Neuroscience > Development > Electroporation
Developmental Biology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










