- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Dual-target Bridging ELISA for Bispecific Antibodies
Published: Vol 12, Iss 19, Oct 5, 2022 DOI: 10.21769/BioProtoc.4522 Views: 4125
Reviewed by: Chiara AmbrogioMigla MiskinyteAnonymous reviewer(s)
Abstract
Bispecific antibodies (BsAbs) are typically monoclonal antibody (mAb)–derived molecular entities engineered to bind to two distinct targets, including two antigens or two epitopes on the same antigen. When compared to parental monoclonal antibodies or combinational therapies, the generated BsAbs have the ability to bridge the two targets and thus may offer additional clinical benefits. Characterizing BsAbs’ ability to bind to both targets simultaneously is critical for their biotherapeutic development. A range of bi-functional quantitative bridging assays to enable target-specific capture and detection of binding properties include enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR), and cell-based flow cytometry. Developing suitable and robust cell-based bioassays is more challenging than non-cell-based binding assays because cell-based assays with complex matrices can be inherently variable and often lack precision. Compared to SPR, ELISA has a rapid setup and readily available method, being widely and extensively applied in almost every laboratory. Here, we describe a dual-target bridging ELISA assay that characterizes the ability of a HER2(human epidermal growth factor receptor 2)/PD-L1(programmed cell death ligand 1) BsAb in binding to both HER2 and PD-L1 simultaneously, a prerequisite for its envisioned mode of action.
Graphical abstract:

Background
Engineered bispecific antibodies (BsAbs) that recognize two separate antigens or epitopes are an emerging class of next-generation biological therapeutics. Such antibodies, capable of engaging multiple targets, shed new light on clinical treatments with the prospect of additive or synergistic mechanisms of action (MoA) and/or superior potency compared to monoclonal antibody (mAb) or combination therapies (Kontermann, 2012; Register et al., 2021). Many BsAbs are currently marketed as therapeutics in several disease areas, and more than 100 BsAbs have progressed into clinical pipelines (Kaplon et al., 2022; Register et al., 2021).
The binding assays are necessary components of in vitro BsAb characterization (Saldanha et al., 2018). They must be readily conducted during initial product development phases for the candidate screening and stability assessments and can therefore potentially be used as MoA-reflective potency assays (Lee et al., 2017). The bioassay strategy is outlined to characterize the independent or simultaneous binding affinities of a BsAb to their dual-antigen targets and demonstrate the full biological binding activity, which is the prerequisite of the envisioned MoA (Register et al., 2021).
Bridging ELISA is a type of sandwich ELISA that has been widely used in bi-functional quantitative assays that cover both binding events simultaneously. This assay is based on a bridging format and utilizes an immobilized capture recombinant antigen 1 in a solid phase (usually polystyrene microplates), followed by the addition of a biotinylated version of the antigen 2, to form the antigen 1–BsAb–antigen 2 bridging complex. The horseradish peroxidase (HRP)–labeled streptavidin is used as the detector. Major advantages of this method are the rapid setup, easy preparation of reagents, and effortless handling when compared to other bridging bioassays [e.g., surface plasmon resonance (SPR) or cell-based flow cytometry]. By applying this bridging approach, several BsAbs, including PD-L1/TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domain), HER2/PD-1(programmed death protein 1), 4-1BB(CD137) (tumor necrosis factor receptor superfamily 9)/HER2, and OX40(CD134) (tumor necrosis factor receptor superfamily 4)/4-1BB (Hinner et al., 2019; Ljungars et al., 2020; Chu et al., 2022; Mu et al., 2022), have been determined to being capable of binding to both targets simultaneously. The bridging ELISA assay should be viewed as a potential new standard and well-established procedure for measuring dual-target binding. Here, we focus on HER2(human epidermal growth factor receptor 2)/PD-L1(programmed cell death ligand 1) BsAb and describe an effective dual-target binding ELISA protocol that allows the measurement of a bispecific drug binding to both targets in a single assay format.
Materials and Reagents
96-well microplate (Greiner Bio-One, catalog number: 650061)
HER2/PD-L1 BsAb protein (Chen et al., 2021)
Recombinant extracellular domain of human programmed cell death ligand 1 (C-6His)(PD-L1-ECD) Novoprotein, catalog number: CM06)
Recombinant human epidermal growth factor receptor 2 (C-6His) (HER2) (Novoprotein, catalog number: CP69)
1% casein in PBS (Thermo Fisher Scientific, catalog number: 37582)
TMB substrate kit (Thermo Fisher Scientific, catalog number: 34021)
High sensitivity streptavidin-HRP (Thermo Fisher Scientific, catalog number: 21130)
EZ-Link Sulfo-NHS-LC-LC-Biotin kit (Thermo Fisher Scientific, catalog number: 21338)
Sulfuric acid (Sinopharm Chemical Reagent Co., Ltd, catalog number: 100216008)
PBS (Hyclone, catalog number: 16777-249)
Tween-20 (Sigma-Aldrich, catalog number: P1379)
0.05% PBST (v/v) (see Recipes)
2 M sulfuric acid solution (H2SO4) (see Recipes)
Trastuzumab (Selleck, catalog number: A2007)
Equipment
SpectraMax M5e microplate reader (Molecular Devices, catalog number: 89212-400)
Software
GraphPad Prism 9.0 (GraphPad Software, www.graphpad.com)
Procedure
Antigens preparation
Prepare the biotin-antigen—biotinylated-HER2 protein—used for detection. First, conjugate the HER2 protein using the EZ-Link Sulfo-NHS-LC-LC-Biotin kit, according to manufacturer’s instructions.
Prepare human PD-L1-ECD protein (capture antigen) at a final concentration of 2 μg/mL in PBS buffer.
Coating plate with PD-L1-ECD antigen
Coat each well of a 96-well microplate with 50 µL of 2 µg/mL PD-L1-ECD protein. Cover the plate with a lid and incubate at 4 °C overnight without agitation.
Blocking and addition of HER2/PD-L1 BsAb
The next day, remove the coating solution and wash the plate three times with 200 µL of PBS per well.
Block the coated microplate by adding 200 µL of 1% casein in PBS buffer to each well using a multichannel pipette. Incubate for 1 h at 37 °C.
Discard the blocking solution and wash the microplate three times with 200 µL of 0.05% PBST per well.
Prepare three-fold serial dilutions of HER2/PD-L1 BsAb and control antibody (e.g., trastuzumab) in 1% casein in PBS buffer (e.g., 200 nM, 66.7 nM, 22.2 nM, 7.4 nM, 2.5 nM, 0.823 nM, 0.274 nM, 0.091 nM, 0.030 nM, 0.010 nM, 0.003 nM, and 0.001 nM).
Add 50 μL of each diluted antibody to each well in triplicate and incubate for 1 h at 37 °C.
Incubation with HER2/PD-L1 BsAb and addition of biotinylated-HER2 antigen
After incubation with the HER2/PD-L1 BsAb and control antibody, wash the microplates three times with 200 µL of 0.05% PBST to each well.
Pipette 50 µL of pre-prepared biotinylated-HER2 protein at 2 µg/mL in 1% casein in PBS buffer to each well and incubate for 1 h at 37 °C.
Discard the biotinylated-HER2 protein solution and wash the microplates three times with 200 µL of 0.05% PBST to each well.
Add 50 μL of 1:1,000 diluted streptavidin-HRP in 1% casein in PBS buffer to each well for detection, and incubate for 1 h at 37 °C.
Discard the detection solution and wash the microplates three times with 200 µL of 0.05% PBST to each well.
Addition of substrate and development
Prepare TMB substrate solution A and B at 1:1 ratio according to manufacturer’s instructions. Add 50 μL of freshly prepared substrate to each well and incubate for 10 min at room temperature.
After sufficient color development, add 50 μL of stop solution with 2 M sulfuric acid (H2SO4) to each well and measure the absorbance (optical density, OD) at 450 nm using a SpectraMax M5e microplate reader.
Data analysis
Subtract the background signal from the measured OD450nm values and normalize them. Transform all values of the HER2/PD-L1 BsAb concentration or control antibody to logarithmic scale (base 10, log10).
Plot the background corrected and normalized OD450nm values (Y-axis, corresponding to the fraction of occupied HER2/PD-L1 BsAb binding to both HER2 and PD-L1 sites simultaneously) against the logarithm of the HER2/PD-L1 BsAb or control antibody concentrations (X-axis, log10 scale).
Fit the concentration-response data using a 4 parameter logistic model by non-linear regression, with GraphPad Prism 9.0 software (Figure 1).
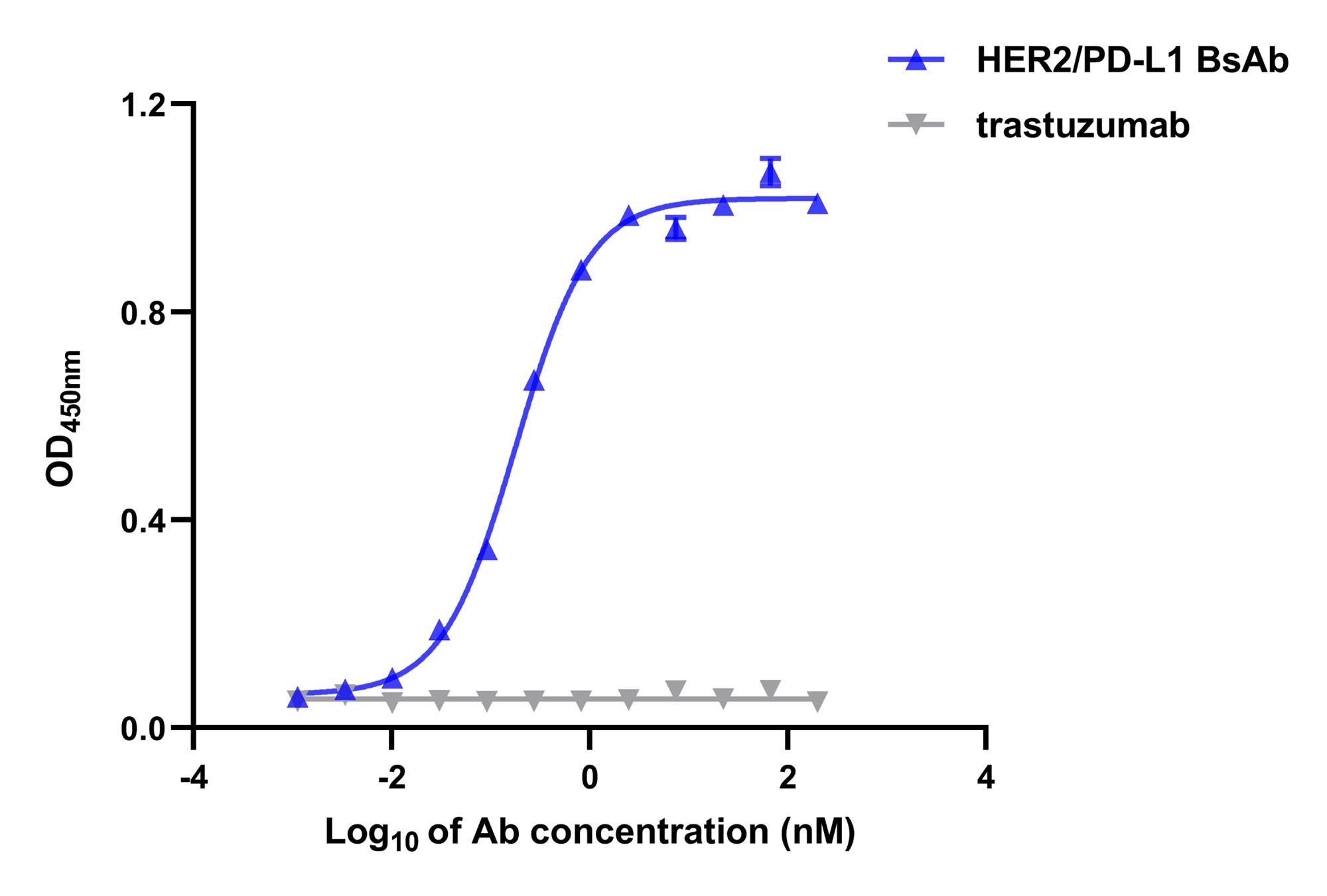
Figure 1. Binding curves of the dual-target bridging ELISA. HER2/PD-L1 BsAb could simultaneously bind to human PD-L1-ECD and HER2, as determined by the bridging ELISA, in which PD-L1-ECD proteins were coated onto the plates, and biotinylated-HER2 proteins were used as detection agent. Trastuzumab was used as control antibody. Data are shown as mean ± SEM (n = 3).
Notes
In this ELISA method, use a multichannel pipette in each step to add solutions to the wells of a 96-well microplate. In solution removal and washing steps, throw out the solutions directly into the sink. None of the plate incubation steps require agitation.
Recipes
0.05% PBST (v/v)
Tween-20, 0.05%, 500 μL
PBS, 999.5 mL
Total, 1,000 mL
2 M sulfuric acid solution (H2SO4)
Sulfuric acid (98%), 2 M, 3 mL
ddH2O, 897 mL
Total, 1,000 mL
Acknowledgments
This work was supported by the China National Major Scientific and Technological Special Project for “Significant New Drugs Innovation and Development” (2019ZX09732002-006) and the National Natural Science Foundation of China (81872785 and 81673347). The protocol described here was adapted from previously published work (Chen et al., 2021).
Competing interests
M. P. and Y.-L. C received stipends from Shanghai Mabstone Biotechnology, Ltd. Y. W., L. T., W. W., and C. W. are employed by Dartsbio Pharmaceuticals, Ltd.
References
- Chen, Y. L., Cui, Y., Liu, X., Liu, G., Dong, X., Tang, L., Hung, Y., Wang, C. and Feng, M. Q. (2021). A bispecific antibody targeting HER2 and PD-L1 inhibits tumor growth with superior efficacy. J Biol Chem 297(6): 101420.
- Chu, W., Xu, H., Wang, Y., Xie, Y., Chen, Y. L., Tan, X., Huang, C., Wang, G., Wang, Q., Luo, W., et al. (2022). HER2/PD1 bispecific antibody in IgG4 subclass with superior anti-tumour activities. Clin Transl Med 12(4): e791.
- Hinner, M. J., Aiba, R. S. B., Jaquin, T. J., Berger, S., Durr, M. C., Schlosser, C., Allersdorfer, A., Wiedenmann, A., Matschiner, G., Schuler, J., et al. (2019). Tumor-Localized Costimulatory T-Cell Engagement by the 4-1BB/HER2 Bispecific Antibody-Anticalin Fusion PRS-343. Clin Cancer Res 25(19): 5878-5889.
- Kaplon, H., Chenoweth, A., Crescioli, S. and Reichert, J. M. (2022). Antibodies to watch in 2022. MAbs 14(1): 2014296.
- Kontermann, R. E. (2012). Dual targeting strategies with bispecific antibodies. MAbs 4(2): 182-197.
- Lee, H. Y., Schaefer, G., Lesaca, I., Lee, C. V., Wong, P. Y. and Jiang, G. (2017). “Two-in-One” approach for bioassay selection for dual specificity antibodies. J Immunol Methods 448: 74-79.
- Ljungars, A., Schiott, T., Mattson, U., Steppa, J., Hambe, B., Semmrich, M., Ohlin, M., Tornberg, U. C. and Mattsson, M. (2020). A bispecific IgG format containing four independent antigen binding sites. Sci Rep 10(1): 1546.
- Mu, S., Liang, Z., Wang, Y., Chu, W., Chen, Y. L., Wang, Q., Wang, G. and Wang, C. (2022). PD-L1/TIGIT bispecific antibody showed survival advantage in animal model. Clin Transl Med 12(5): e754.
- Register, A. C., Tarighat, S. S. and Lee, H. Y. (2021). Bioassay Development for Bispecific Antibodies-Challenges and Opportunities. Int J Mol Sci 22(10): 5350.
- Saldanha, M., Dandekar, P. and Jain, R. (2018). A Regulatory Perspective on Testing of Biological Activity of Complex Biologics. Trends Biotechnol 36(3): 231-234.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Pei, M., Wang, Y., Tang, L., Wu, W., Wang, C. and Chen, Y. L. (2022). Dual-target Bridging ELISA for Bispecific Antibodies. Bio-protocol 12(19): e4522. DOI: 10.21769/BioProtoc.4522.
- Chen, Y. L., Cui, Y., Liu, X., Liu, G., Dong, X., Tang, L., Hung, Y., Wang, C. and Feng, M. Q. (2021). A bispecific antibody targeting HER2 and PD-L1 inhibits tumor growth with superior efficacy. J Biol Chem 297(6): 101420.
Category
Biological Engineering > Biomedical engineering
Drug Discovery > Drug Screening
Biochemistry > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









