- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Flow Cytometry-based Measurement of Reactive Oxygen Species in Cyanobacteria
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4417 Views: 3836
Reviewed by: Dennis J NürnbergAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Growth Recovery Assay and FACS-based Population Sorting Following Territorial Exclusion in Proteus mirabilis
Murray J. Tipping and Karine A. Gibbs
Mar 5, 2020 5071 Views

Flow Cytometry Analysis and Fluorescence-activated Cell Sorting of Myeloid Cells from Lung and Bronchoalveolar Lavage Samples from Mycobacterium tuberculosis-infected Mice
Alissa C. Rothchild [...] Alan H. Diercks
May 20, 2020 7208 Views

Assay for Assessing Mucin Binding to Bacteria and Bacterial Proteins
Lubov S. Grigoryeva [...] Nicholas P. Cianciotto
Mar 5, 2021 4767 Views
Abstract
Cyanobacteria are Gram-negative oxygen-producing photosynthetic bacteria that are useful in the pharmaceutical and biofuel industries. Monitoring of oxidative stress under fluctuating environmental conditions is important for determining the fitness, survival, and growth of cyanobacteria in the laboratory as well as in large scale cultivation systems. Here, we provide a protocol developed using unicellular Synechococcus elongatus PCC 7942 and filamentous Fremyella diplosiphon BK14 cyanobacteria for high-throughput oxidative stress measurement by 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) and flow cytometry (FCM). We also provide details for the optimization of cell number, dye concentration, and FCM parameters for each organism before it can be utilized to quantify reactive oxygen species (ROS). FCM-based method can be used to measure ROS in a large population of cyanobacterial cells in a high-throughput manner.
Graphical abstract:
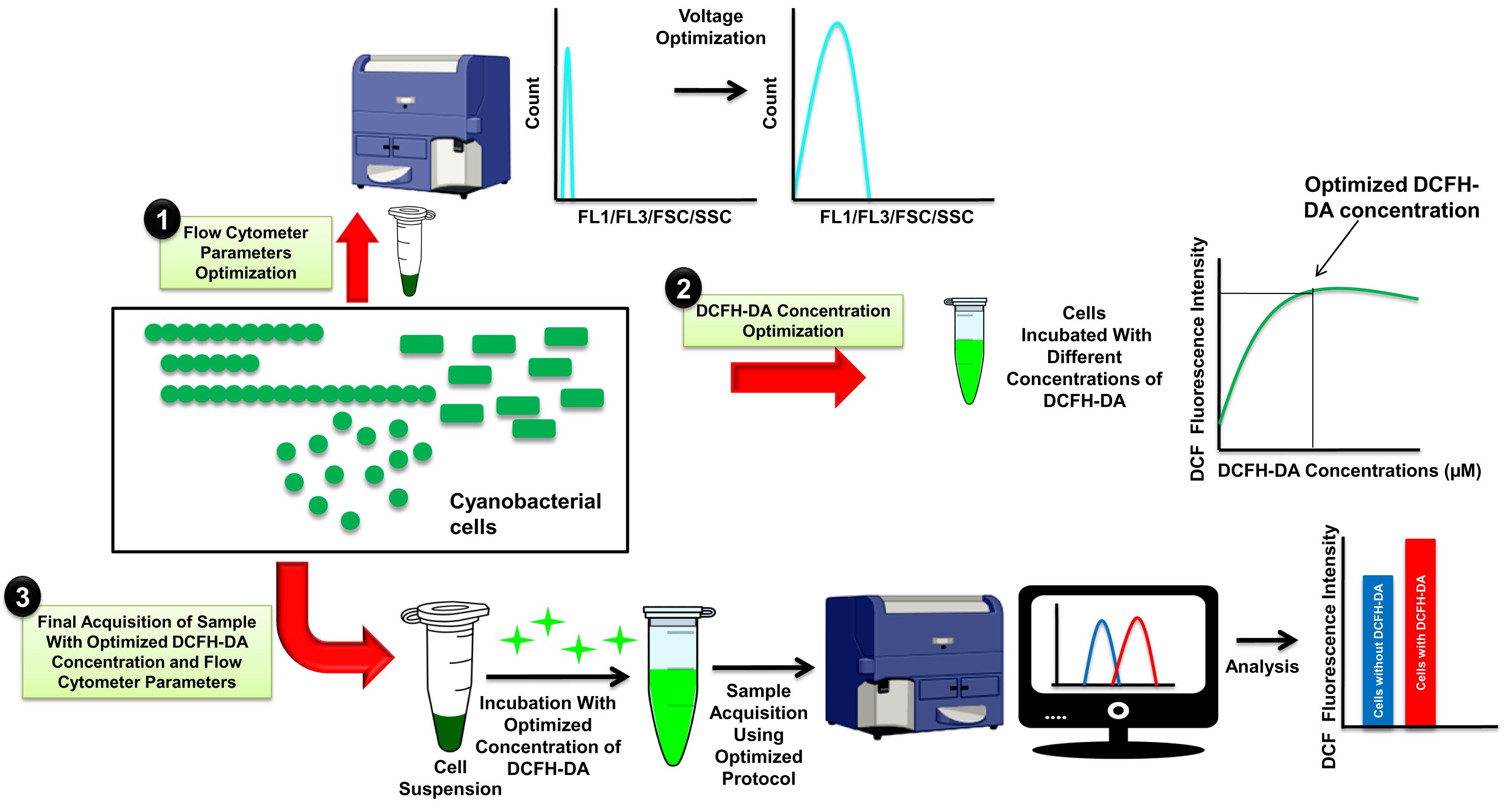
Background
Cyanobacteria are a monophyletic group of Gram-negative bacteria that are found in a variety of habitats and produce oxygen similar to higher plants during photosynthesis (Dvořák et al., 2017). Cyanobacteria have evolved different mechanisms to adapt to a wide range of environmental conditions. These ecologically important organisms are well-known for their significant contribution to global carbon dioxide and nitrogen fixation, and as a result, they contribute significantly to the productivity of ecosystems (Kanno et al., 2017). Cyanobacteria have shown their potential in bioenergy and valuable chemical production due to their minimal growth requirements, high photosynthetic efficiency, amenability to genetic modification, and installation of novel metabolic pathways in their primary metabolic chassis (Rajneesh et al., 2017a). However, oxidation and reduction processes related to photosynthesis and respiration are affected by changing environmental conditions such as light quality and quantity, pH, salinity, temperature, and nutrient limitation. Altered oxidation and reduction processes in cyanobacterial thylakoid membranes result in generation of reactive oxygen species (ROS), which consequently cause oxidative stress (Niyogi, 1999). Increased levels of ROS in cyanobacteria are known to damage lipids, DNA, RNA, pigments, and proteins. ROS also results in photoinhibition due to damage to the oxygen-evolving complex and the D2 protein of photosystem II (Niyogi, 1999; Latifi et al., 2009). As a result, the development, growth, and survival of cyanobacterial cells are impaired by high levels of ROS that are generated under fluctuating environmental conditions (Niyogi, 1999; Latifi et al., 2009). Therefore, rapid and accurate methods to measure ROS are required for monitoring the health of cyanobacterial cells in cultivation systems that are used in commercial setups as well as in basic biological studies.
Earlier, we developed a method to measure ROS levels in cyanobacteria using 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA), a live cell-permeable fluorescent probe that can be visualized by fluorescence microscopy (Rastogi et al., 2010; Rajneesh et al., 2017b). DCFH-DA is a well-known fluorescent probe for detecting ROS in live cells. It hydrolyzes inside the cell to DCFH by the activity of esterases. DCFH cannot cross the cell membrane, and it is mostly non-fluorescent, but it emits green fluorescence when oxidized to dichlorofluorescein (DCF) by intracellular ROS (Kalyanaraman et al., 2012). The intensity of green fluorescence can be measured at 530 nm after excitation at 485 nm. Previous methods have been widely used to measure ROS levels in various organisms in addition to cyanobacteria (Rastogi et al., 2010; Rajneesh et al., 2017b; Li et al., 2017; Basso et al., 2018). However, despite its wide application, fluorescence microscopy-based methods are limited to small sample sizes (the number of cells analyzed is an individual choice, but analysis of at least 50 cells per replicate is recommended), and therefore, the entire population is not well represented (Mondal et al., 2020). Also, imaging a large number of cells in the darkroom is not easy as it is a time-consuming process, and longer exposure of samples to excitation light could result in a high background of green fluorescence due to photooxidation of the dye. Regular imaging for longer periods can also cause dry, itchy, and weary eyes, as well as headaches. Cell morphology is another disadvantage of fluorescence microscopy-based ROS monitoring, as visualizing and estimating ROS in small spherical shape cells is not possible (Mondal et al., 2020). However, FCM-based ROS-monitoring methods overcome the abovementioned limitations of fluorescence microscopy-based methods and provide a high-throughput platform to monitor the ROS in a large number of cells of various morphology. Furthermore, it allows simultaneously recording data on cell size and shape, granularity, chlorophyll, and phycobiliproteins while monitoring the ROS levels. The forward scatter (FSC) parameter of FCM provides information about cell size and shape, while the internal complexity, i.e., granularity, of a cell can be determined by measuring side scatter. Granules and the nucleus are two cellular components that influence side scatter; however, the nucleus is absent in cyanobacteria. Similar to fluorescence microscopy-based ROS-detection methods described earlier (Rastogi et al., 2010; Rajneesh et al., 2017b), this protocol can be optimized for different organisms for successful monitoring of ROS.
Materials and Reagents
1.5 mL amber microcentrifuge tubes (Abdos, catalog number: P10204A)
3 mL round bottom polystyrene tubes (Becton Dickinson, catalog number: 156758)
20 mL 1 M HEPES pH 8.0 (Himedia, catalog number: RM380-500G)
Glass slides (Borosil, catalog number: BT409100P02)
Cover slips (Borosil, catalog number: 9115S01)
1 mL pipette tips (Abdos, catalog number: P10109)
200 µL pipette tips (Abdos, catalog number: P10140)
10 µL pipette tips (Abdos, catalog number: P10116)
1 cm quartz cuvette (Shimadzu, catalog number:226-85010-92)
0.2 µm size Millipore membrane filter (Merck, catalog number: GSWP04700)
BD FACS Clean Solution (0.1% Sodium hypochlorite) (Becton Dickinson, catalog number: 340345)
Hydrogen Peroxide (Merck, catalog number: 1.93408.0521)
Milli-Q water
Exponentially growing cultures of Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803 and Fremyella diplosiphon BK14 grown in BG11 medium supplemented with 20 mM HEPES (Allen, 1968).
Note: Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803, and Fremyella diplosiphon BK14 (Kehoe and Grossman, 1996) were grown in BG11 liquid medium supplemented with 20 mM HEPES. Cells were inoculated from a solid BG11+20 mM HEPES agar plate. Synechococcus elongatus PCC 7942 and Synechocystis sp. PCC 6803 were grown under PAR (photosynthetically active radiation) (~80 µmol m-2 s-1), while Fremyella diplosiphon BK14 was grown in red light (Red LED Fluorescent light, Havells, India) (~20 µmol m-2 s-1) with ~150 rpm shaking at 25°C. The growth curves of all organisms were monitored, and cultures were maintained in the exponential phase by regular sub-culturing.
DCFH-DA (Sigma-Aldrich, catalog number: D6883)
Ethanol (Merck, catalog number: 1.00983.0511)
Disodium hydrogen phosphate (Na2HPO4) (Merck, catalog number: 1.93622.0521)
Sodium dihydrogen phosphate (NaH2PO4) (Merck, catalog number: 1.93624.0521)
Sodium chloride (NaCl) (SRL, catalog number: 41721)
2 mM (w/v) DCFH-DA Stock solution (1 mL) (see Recipes)
0.1 M PBS pH 7.4 (100 mL) (see Recipes)
Equipment
BD FACSCalibur flow cytometer (Becton Dickinson, catalog number: 342975)
UV-VIS Spectrophotometer 1800 (Shimazdu, catalog number: 80626)
Nikon 90i eclipse fluorescence microscope (Nikon, Japan)
Cooling Centrifuge (Remi, India/NEYA16R)
Software
BD CellQuest Pro (Becton Dickinson, USA)
NIS elements AR software 4.0 (Nikon, Japan)
Procedure
Optimization of FCM parameters
Take 50 mL exponentially growing cultures of unicellular (S. elongatus PCC 7942 and Synechocystis sp. PCC 6803) and filamentous (Fremyella diplosiphon BK14) cyanobacteria grown under the abovementioned conditions (item 14 of Materials and Reagents).
Remove growth medium by centrifugation at 6,000 × g and 25°C for 10 min and dilute the samples with 0.1 M PBS buffer to different O.D.750, i.e., 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8. Make 1 mL final volume of each cell concentration in a 1.5 mL microcentrifuge tube.
Clean nozzle of BD FACSCalibur with 0.1% sodium hypochlorite solution and Milli-Q water.
Note: Turn on the BD FACSCalibur flow cytometer 10–15 min before conducting the experiment. Calibration of BD FACSCalibur with QC beads at regular intervals is recommended.
Draw two dot plots in BD CellQuest Pro software, i.e., Forward Scatter (FSC) vs. Side Scatter (SSC) and FSC vs. Fluorescence channel 3 (FL3). Similarly, draw two histogram plots of Fluorescence channel 1 (FL1) and FL3. Set log scale for all parameters. FL1 and FL3 are two Fluorescence channels that detect DCF fluorescence (green fluorescence, emission range 515–545 nm) and autofluorescence of Chlorophyll (Chl) ɑ (red fluorescence, emission range >670 nm), respectively. The FSC vs. SSC plot provides information about cell size, shape, and granularity; the FSC vs. FL-3 plot is used to select the cyanobacterial population-based on Chl ɑ autofluorescence and cell size. The FL-1 and FL-3 histogram plot provides the fluorescence intensity of DCF and Chl ɑ, respectively.
Transfer samples from step 2 to a 3 mL round bottom polystyrene tube and acquire all samples with a low flow rate (12 μL/min), adjusting the PMT voltages of FSC, SSC, FL1, and FL3 for different organisms, as shown in Figure 1A. The adjusted optimal PMT voltage is used for analyzing all the samples of the same organism, which provides fluorescence intensity from the cell in the absence of DCFH-DA. This background fluorescence of cells is excluded while measuring the mean fluorescence intensity of DCF in DCFH-DA treated cells to estimate ROS levels. Set FSC as the primary parameter and FL3 as the secondary parameter.
Acquire 50,000–100,000 events. Keep sample acquisition rate below 1,000 events/second to avoid exposure of more than one cell to laser beam at the same time.
Note the minimum O.D.750 (from step 2) value that gives sample acquisition rate between 500–1,000 events/second to determine the appropriate cell density of each cyanobacterium required for getting proper signals from FCM.

Figure 1. Optimization of different flow cytometry (FCM) parameters for measurement of reactive oxygen species (ROS) levels using 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA). FCM histogram diagram for optimizing PMT voltage of different fluorescent channels of a flow cytometer (A). Graph of optimization of DCFH-DA concentration for test organism (B). Dot plot diagram of gating the population of interest based on FL3 (Chlorophyll ɑ) fluorescence and FSC-H (relative size) (C). Dot plot diagram of FSC-H vs. SSC-H showing relative size and cellular complexity, respectively, of the test organism (D).Optimization of DCFH-DA dye concentration
Take 10 mL sample of optimized O.D.750 (refer to steps 2–7 of section A) for each organism in a 15 mL Falcon tube. Add 100 mM H2O2 to each sample and incubate for 1 h under growth conditions described in item 14 of the Materials and Reagents section.
After incubation, pellet cells by centrifugation at 6000 × g and 25°C for 10 min, and wash the cells twice with 10 mL of 0.1 M PBS (pH 7.4).
After washing, suspend the cells in 10 mL of 0.1 M PBS and divide into 1 mL of aliquots.
Add different concentrations (0, 10, 15, 20, 25, 30, 35, and 40 µM) of DCFH-DA to 1 mL of cell aliquots using DCFH-DA stock solutions (item 20 of Materials and Reagents section).
Incubate cells for 1 h in the dark with ~150 rpm rocking at 25°C.
Analyze cells using FCM immediately after 1 h of incubation.
Wash FCM and set BD CellQuest Pro software with optimized PMT voltage (refer to section A).
Acquire samples with low flow rate (12 μL/min).
Determine the minimum concentration of dye required for in vivo detection of ROS for each organism by analyzing the titration curve, as shown in Figure 1B.
Detection of in vivo ROS using FCM
After determining the optimum cell and dye concentration for each organism (refer to sections A and B), take cyanobacterial cells from different experimental conditions. Here, we used different cyanobacteria that were grown in conditions mentioned in item 14 of the Materials and Reagents section.
Pellet cells by centrifugation at 6,000 × g and 25°C for 10 min, and wash the cells twice with 1 mL of 0.1 M PBS (pH 7.4). After washing, resuspend cells in 1 mL of 0.1 M PBS buffer.
Incubate cells with the determined concentration of dye for 1 h in the dark with rocking.
After incubation, separate samples in two microcentrifuge tubes and immediately acquire one of them with the help of a BD FACSCalibur flow cytometer using optimized settings. Use another aliquot of cells for ROS detection by fluorescence microscopy, as described by Rastogi et al. (2010) (Figure 2).
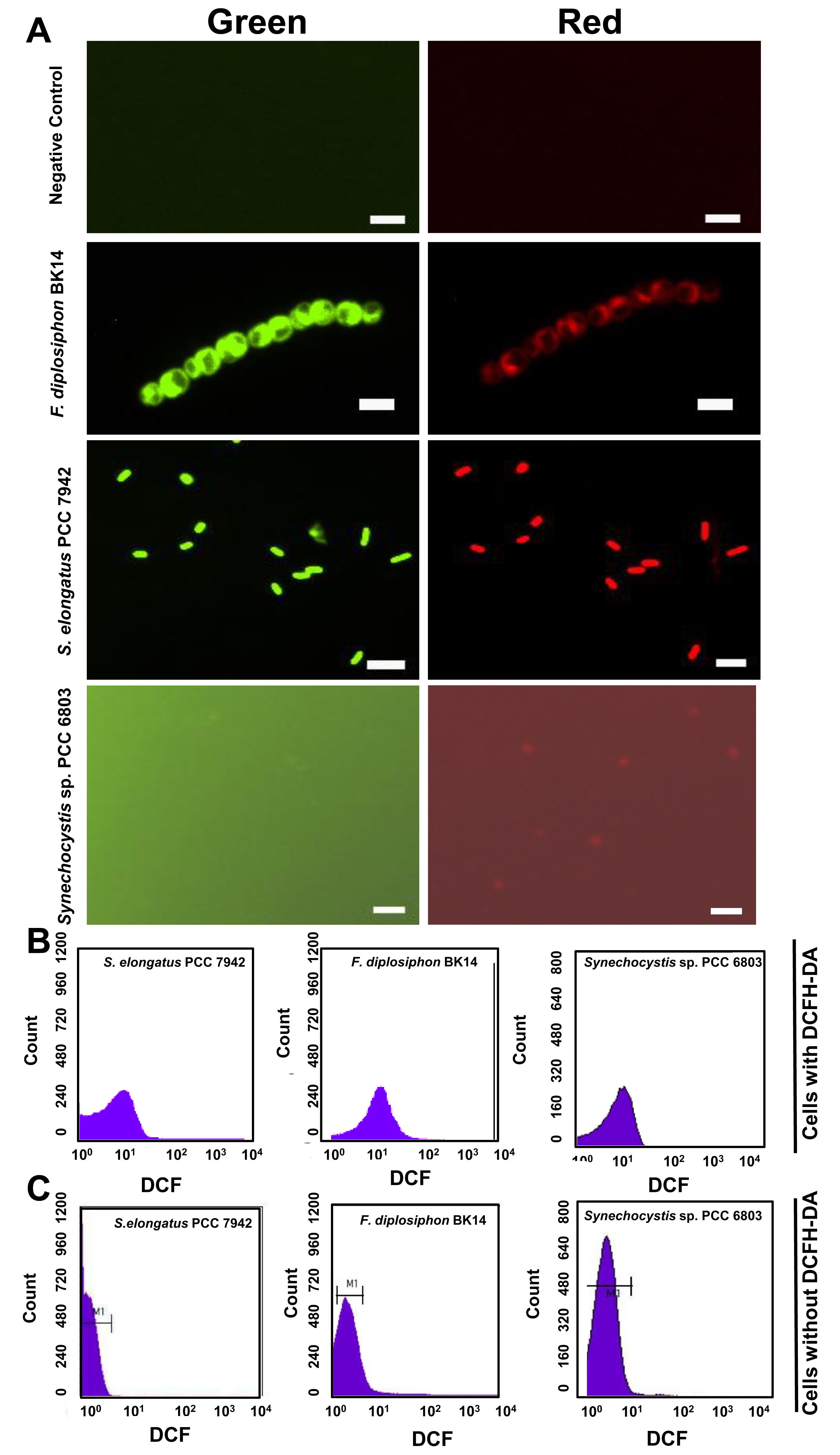
Figure 2. Measurement of reactive oxygen species (ROS) in Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803, and Fremyella diplosiphon BK14 using 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) and fluorescence microscopy or Flow cytometry (FCM). Dichlorofluorescein (DCF) fluorescence showing ROS levels (Green) and autofluorescence of photosynthetic pigments (Red) in F. diplosiphon BK14, S. elongatus PCC 7942, and Synechocystis sp. PCC 6803 (A). Fluorescence from DCFH-DA only sample without cells represents the negative control. Images were acquired using a ×40 objective. Scale bars, 5 μm. FCM histograms showing fluorescence of dichlorofluorescein (DCF), i.e., ROS level, in S. elongatus PCC 7942, F. diplosiphon BK14, and Synechocystis sp. PCC 6803 (B). Histograms showing background fluorescence of FL1 channel from S. elongatus PCC 7942, F. diplosiphon BK14, and Synechocystis sp. PCC 6803 without DCFH-DA (C).Acquire at least 50000 events at a low flow rate (12 μL/min).
Save the data in FCM format.
Data analysis
The flowchart (Figure 3) shows steps involved in the detection of ROS (steps 1–3; refer to sections A, B, and C), and data analysis.
Using BD CellQuest Pro software, gate cyanobacterial population with the help of FSC vs. FL3 (x-axis FSC and y-axis FL3) (Figure 1C).
Generate histogram diagram of FL1 as shown in Figure 2.
Record the mean fluorescence intensity (MFI) of the FL1 histogram from the gated population of cyanobacterial cells, which depicts the ROS level.
MFI of FL1 channel histogram can also be represented as a bar diagram to depict the ROS level.
The values of FL1 from different organisms can be analyzed using one-way ANOVA with Tukey post hoc function or other suitable statistical analysis.

Figure 3. Steps involved in the detection of reactive oxygen species (ROS) and data analysis.
Notes
To optimize the concentration of DCFH-DA, use a concentration of H2O2 that gives maximum ROS in the organism while killing less than 10% of the cells (Mondal et al., 2020). A live-dead assay can be performed by using different concentrations of H2O2 and the live cell-permeable nucleic acid binding fluorescent dye SYBR Green I. Cells were treated with different concentrations of H2O2, and live cells were detected using Sybr Green I and FCM to determine the concentration of H2O2 that causes less than 10% killing (Mondal et al., 2020).
Flow cytometers have limitations regarding cell/filament size. The nozzle can analyze approximately 1 µm to 50 µm cell/filament length. Though the size range may vary, it is recommended to be careful when using filamentous cyanobacteria or organisms which make clumps or aggregates. Long filaments may clog the nozzle. It is advised to prepare short filament by using a sonicator before acquisition of sample through FCM, if required. However, impact of sonication on ROS levels and cell survival should be analyzed in advance. Samples can be sonicated in an ultrasonic bath sonicator for different time intervals, and fragmentation can be monitored by light microscopy. Filaments of Fremyella diplosiphon BK14 are shorter (<50 µm), and therefore fragmentation was not required for this cyanobacterium.
DCFH-DA is a light sensitive fluorescence probe. It is advised to perform the entire experiment in minimal light and avoid direct exposure of samples and dye to light.
Alternatively, the cell concentration can be determined by direct cell counting or turbidity measurement of the cell sample as scattering of light. A minimum of 1×105 cells are usually required for the acquisition in FCM.
Recipes
2 mM (w/v) DCFH-DA Stock solution (1 mL)
1 mL of 100% ethanol (Molecular Grade).
Dissolve 0.974 mg of DCFH-DA in 1 mL of 100% ethanol.
Mix by vortexing and store in the dark at -20°C (Can be stored for up to 3 months).
0.1 M PBS pH 7.4 (100 mL)
Mix 40 mL of 0.2 M Disodium hydrogen phosphate (Na2HPO4) stock with 10 mL of sodium dihydrogen phosphate (NaH2PO4) stock.
Add 0.9 g NaCl and stir until dissolved.
Bring the volume to 100 mL with distilled H2O and adjust the pH to 7.4.
Filter through 0.2 µm size Millipore membrane filter before use.
Store at 4°C.
Acknowledgments
This work was supported by the funding from Institute of Eminence incentive grant, Banaras Hindu University (R/Dev/D/IOE/Incentive/2021-2022/32399) and SERB, New Delhi, India, in the form of Early Career Research Award (ECR/2016/000578) to Shailendra P. Singh. Soumila Mondal is thankful to UGC and BHU for providing UGC Research Fellowship. The author acknowledges DBT Interdisciplinary School of Life Sciences (ISLS) BHU for flow cytometry and fluorescence microscopy facility. This protocol was adapted from the procedure published by Mondal et al. (2020). Careful reading and suggestions from Pankaj K. Maurya and Anjali Gupta is also acknowledged.
Competing interests
The authors declare no competing financial and non-financial interests.
References
- Allen, M. M. (1968). Simple conditions for growth of unicellular blue-green algae on plates (1, 2). J Phycol 4(1): 1-4.
- Basso, V., Garcia, A., Tran, D. Q., Schaal, J. B., Tran, P., Ngole, D., Aqeel, Y., Tongaonkar, P., Ouellette, A. J. and Selsted, M. E. (2018). Fungicidal potency and mechanisms of theta-defensins against multidrug-resistant Candida species. Antimicrob Agents Chemother 62(6): e00111-18.
- Dvořák, P., Casamatta, D. A., Hašler, P., Jahodářová, E., Norwich, A. R. and Poulíčková, A. (2017). Diversity of the cyanobacteria. In: Modern topics in the phototrophic prokaryotes (pp. 3-46). Hallenbeck, P. C. (Ed.). Springer.
- Kalyanaraman, B., Darley-Usmar, V., Davies, K. J., Dennery, P. A., Forman, H. J., Grisham, M. B. and Ischiropoulos, H. (2012). Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic Biol Med 52(1): 1-6.
- Kanno, M., Carroll, A. L. and Atsumi, S. (2017). Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat Commun 8: 14724.
- Kehoe, D. M. and Grossman, A. R. (1996). Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273(5280): 1409-1412.
- Latifi, A., Ruiz, M. and Zhang, C. C. (2009). Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33(2): 258-278.
- Li, S. Y., Cheng, H., Qiu, W. X., Zhang, L., Wan, S. S., Zeng, J. Y. and Zhang, X. Z. (2017). Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials 142: 149-161.
- Mondal, S., Kumar, V. and Singh, S. P. (2020). Oxidative stress measurement in different morphological forms of wild-type and mutant cyanobacterial strains: Overcoming the limitation of fluorescence microscope-based method. Ecotoxicol Environ Saf 200: 110730.
- Niyogi, K. K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333-359.
- Rajneesh, Pathak, J., Chatterjee, A., Singh, S. P. and Sinha, R. P. (2017b). Detection of reactive oxygen species (ROS) in cyanobacteria using the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA). Bio-protocol 7(17): e2545.
- Rajneesh, Singh, S. P., Pathak, J. and Sinha, R. P. (2017a). Cyanobacterial factories for the production of green energy and value-added products: An integrated approach for economic viability. Renew Sust Energ Rev 69: 578-595.
- Rastogi, R. P., Singh, S. P., Häder, D. -P. and Sinha, R. P. (2010). Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2',7'-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397(3): 603-607.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Mondal, S. and Singh, S. P. (2022). Flow Cytometry-based Measurement of Reactive Oxygen Species in Cyanobacteria. Bio-protocol 12(10): e4417. DOI: 10.21769/BioProtoc.4417.
Category
Microbiology > Microbial physiology > Stress response
Plant Science > Phycology > Physiology
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









