- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocol for Isolation of Cardiomyocyte from Adult Mouse and Rat
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4412 Views: 5598
Reviewed by: Thirupugal GovindarajanRAVI THAKURDavide Botta

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2411 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 214 Views

High Content In Vitro Survival Assay of Cortical Neurons
Paolo V. Fioretti [...] Manuela Basso
Feb 5, 2026 86 Views
Abstract
The isolation of intact single adult cardiomyocytes from model animals, mouse and rat, is an essential tool for cardiac molecular and cellular research. While several methods are reported for adult mouse cardiomyocyte isolation, the viability and yield of the isolated cells have been variable. Here, we describe step-by-step protocols for high viability and yield cardiomyocyte isolation from mouse and rat, based on the use of a stable pressure Langendorff perfusion system. After the animal is euthanized or terminally anesthetized, the heart is removed from the chest and subject to Langendorff perfusion. Then, the heart is digested by perfusion with collagenase and hyaluronidase. After thorough digestion, the cardiomyocytes are dispersed and gradually recovered, the extracellular Ca2+ concentration adjusted, and cells are then ready for use. This protocol will facilitate research that requires isolated adult mouse and rat cardiomyocytes.
Keywords: HeartBackground
Cardiomyocytes isolated from adult mice and rats are a key tool for studying cardiac physiology and pathology, as well as their pharmacology and toxicology. Isolation of high-quality cardiomyocytes is the most important factor for successful experiments of this kind. The general protocol for adult rodent cardiomyocyte isolation has been well summarized by Louch et al. (2011). The rat cardiomyocyte has been isolated for almost a half-century (Powell and Twist, 1976). While the mouse cardiomyocyte is more sensitive to enzyme digestion, it has greater fragility during the isolation procedure, and its use has become practical only in subsequent decades (Zhou et al., 2000). More recently, several mouse cardiomyocytes isolation method papers have been published with modified buffers, enzymes, etc. (Li et al., 2014; Roth et al., 2014; Judd et al., 2016).
To date, all the published cardiomyocyte isolation methods are based on Langendorff retrograde perfusion through the aorta with enzyme solutions. There are two Langendorff perfusion methods that are well used: 1) in stable flow perfusion, a fine peristaltic pump is used to pump the solutions to the Langendorff perfused heart; 2) in stable pressure perfusion, gravity is used to drive the buffers into the perfused heart. Because of differences in the use of the perfusion system, digestion enzymes, perfusion buffer, etc., there is no one universal method to produce a large yield of high quality, viable cardiomyocytes from adult rodent hearts. In this protocol, based on our experience with both stable flow perfusion (Zhang et al., 2013) and stable pressure perfusion (Zhang et al., 2017, 2018, 2020), we recommend using the stable pressure Langendorff perfusion system, and we present herein optimized adult mouse and rat cardiomyocyte isolation protocols that are easy to follow and result in high yield and cell viability.
Materials and Reagents
23 G needle (BD, catalog number: 305194), trimmed the tip and covered by a thin silastic tube (Warner, catalog number: PE-50)
18 G needle (BD, catalog number: 05196), trimmed the tip and covered by a thin silastic tube (Warner, catalog number: PE-160)
50 mL beaker (Fisher, catalog number: FB-100-50)
60 mm diameter tissue culture dish (Falcon, catalog number: 353002)
1 mL Pipette tips (Thermo Scientific, catalog number: 94056710)
NaCl (Fisher, catalog number: S271-3)
KCl (Fisher, catalog number: P217-500)
HEPES (Fisher, catalog number: BP310-100)
NaH2PO4·H2O (Fisher, catalog number: BP330-500)
KH2PO4 (J.T. Baker, catalog number: 3246-01)
MgSO4·7H2O (Sigma, catalog number: M2773-500G)
Glucose (Fisher, catalog number: D16-1)
2,3-Butanedione monoxime, BDM (Alfa Aesar, catalog number: A14339)
Taurine (ACROS ORGANICS, catalog number: 166541000)
L-Glutathione, Reduced (UBPBio, catalog number: P1030-100)
CaCl2 (Fisher, catalog number: BP510-100)
Bovine serum albumin, BSA (Sigma, catalog number: A7906-100)
Collagenase Type 2 (Warthington, catalog number: LS004177)
Hyaluronidase from bovine testes (Sigma, catalog number: H3506-5G)
Pentobarbital sodium (Oak Pharmaceuticals, catalog number: NDC 76478-501-50)
Heparin (Fresenius Kabi, catalog number: 63323-540-11)
Fetal bovine serum (HyClone, SH30396.03)
Oxygen (Praxair, with Prostar platinum oxygen modulator)
Mouse cardiomyocyte isolation buffers (see Recipes)
Rat cardiomyocyte isolation buffers (see Recipes)
Equipment
Forceps (World Precision Instruments, catalog number: 15915)
Langendorff perfusion system with ~90 cm water pressure with an isolation rig (Double Baker Warming Coil, Harvard Apparatus, catalog number: PY8 50-8382) (Figure 1)
Circulating Water Bath (Julabo, CORIO CD)
Water Bath (Precision, catalog number: 51221048)
Pump for circulating the enzyme buffer (Fisher, Minipump Variable Flow, catalog number: EF20714B)
Centrifuge for 15 ml tube (Thermo, CENTRA CL2)
Procedure
The mouse and rat cardiomyocyte isolation protocols share the same experimental device settings (Figure 1, and Video 1):
Langendorff perfusion system with ~90 cm water pressure (Figure 1A, note height of the water column). Using the Double Baker Warming Coil to switch between the perfusion buffer and enzyme digestion buffer (Figure 1B).
Clean the isolation rig (Figure 1, indicated with red box) and tubes with 70% ethanol one time by filling the left 50 mL tube to the full level with 70% ethanol, after which the ethanol will go to all the left tubes and the left part of the rig. Repeat for the right tube. Drain the 70% ethanol. Fill the tubes with autoclaved H2O and drain two times.
Turn on the circulating water bath (Figure 1A, lower left) and the regular 37°C water bath (see Video 1).
The peristaltic pump is used only to circulate perfusion enzyme buffer released from the heart back into the 50 mL perfusion tube, as the former fills and the latter is exhausted.
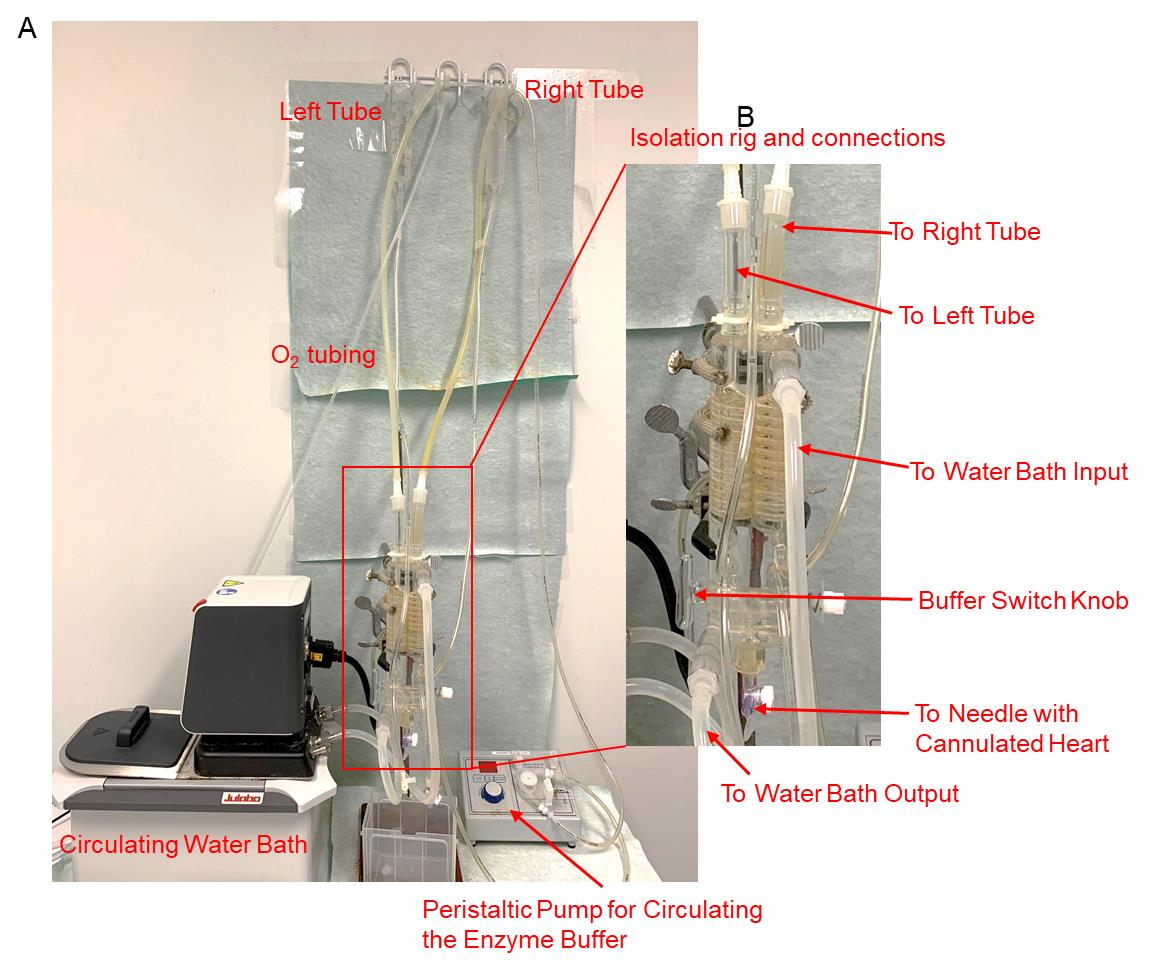
Figure 1. Adult cardiomyocyte isolation system. (A) The heart perfusion setup. (B) A zoomed view of the isolation rig and its connections. The needle with the cannulated heart will be connected to the bottom part of the rig (see the arrow indicated by “To Needle with Cannulated Heart”).Video 1. Perfusion system introductionPart I: Adult mouse cardiomyocyte isolationPart I: Adult mouse cardiomyocyte isolation
Prepare the isolation and digestion buffers (see Recipes for Mouse cardiomyocyte isolation buffers). Fill the system with 80 mL of mouse cardiomyocyte isolation buffer into the left tube (Figure 1A, the total volume of 50 mL syringe tube plus the tubes system is approximately 110 mL) and 50 mL of enzyme digestion buffer into the right tube (Figure 1A) and bubble with oxygen delivered by tubing that reaches the bottom of both the left and the right 50 mL tubes (Figure 1A). Connect a 23 G needle covered by a thin plastic tube (to prevent a loose tie, Figure 2A) to a syringe filled with perfusion buffer.
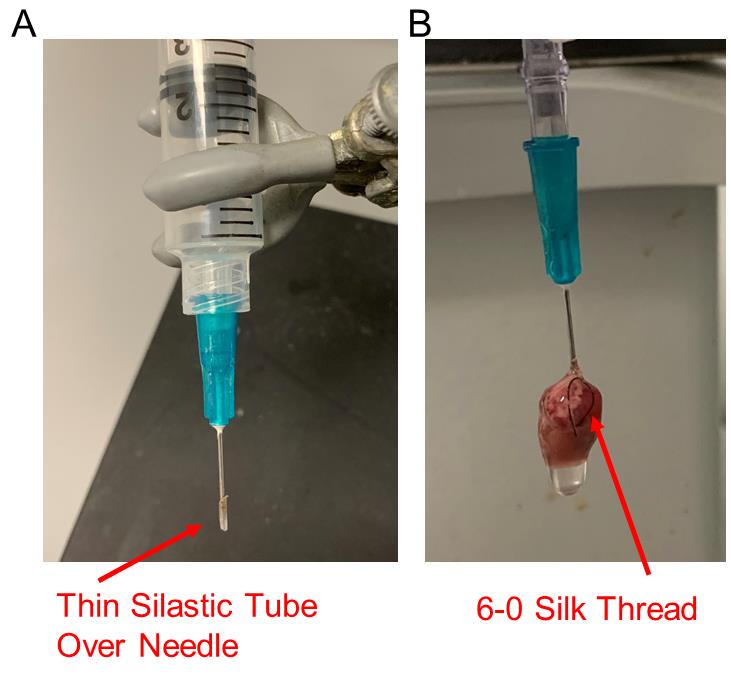
Figure 2. Mouse heart needle cannulation and perfusion. (A) A 23 G needle with a silastic tube cover to facilitate the seal to the aorta and prevent a loose tie. (B) A typical perfused mouse heart. The heart is cannulated to the 23 G needle and ligated using 6-0 silk thread.Euthanize the mouse by cervical dislocation. Spray the mouse chest with 70% ethanol.
Open the chest cavity and quickly take out the heart with approximately 2 mm of aorta. Put the heart in the mouse cardiomyocyte isolation buffer on ice. Gently press the heart to remove blood in the chambers.
Cannulate the aorta to the 23 G tube-covered needle and tie the aorta to the cannula with 6-0 silk thread. The tip of the cannula should be just above the aortic valve. Gently flush the heart with 2–3 mL perfusion buffer to ensure that there is no leak and to flush out most of the blood (see Video 2).
Note: The total time to cannulate the heart should be less than 1 min.
Video 2. Mouse heart canulationTransfer the cannulated heart to the Langendorff perfusion system, attaching the 23 G needle (and heart) to the fitting at the lower end of the rig (Figures 1B and 2B). Perfuse the heart with mouse cardiomyocyte isolation buffer from the left tube for approximately 5 min, during which time approximately 15 mL of isolation buffer should flow through. During this time, gently press the heart to further remove any blood left in the chamber until the efflux is clear.
Note: Any blood left in the heart can neutralize the digestion buffer enzymes and lower the digestion efficiency.
Switch to the digestion buffer (right tube, 250 U/mL collagenase II + 0.2 mg/mL hyaluronidase) by turning the Buffer Switch Knob (Figure 1B) 180°, catch the efflux digestion buffer in a 60 mm tissue culture dish; turn on the circulating pump (Figure 1A) to gradually add this digestion buffer back into the right tube containing fresh digestion buffer (Figure 1A). At that point, also add 40 µL of CaCl2 (100 mM stock) to the circulating digestion buffer, to result in ~80 µM CaCl2. Thus, the heart will be digested by buffer without Ca2+ at the very beginning, but as the digestion buffer with Ca2+ gradually reaches the heart, the heart accommodates to the high efficiency 80 µM CaCl2 digestion buffer.
Note: 80 µM Ca2+ accelerates the digestion but does not damage the cells.
Perfuse with digestion buffer for 20–30 min until the heart becomes soft.
Note: The heart should swell and look soft, pinkish, but not pale white, as the latter means the perfusion buffer does not reach the heart tissue. A pale white heart could be an issue with the aorta cannulation or blood clotting in the coronary arteries. The constant-pressure flow rate will increase during perfusion as the heart swells.
Excessive digestion will reduce the cardiomyocyte viability. The endpoint of digestion should therefore be judged by the softness of the heart. When very soft and flexible, detach the heart from the cannulated syringe needle, remove the atria, and tear apart the ventricle tissue into 3–4 pieces in 5 mL digestion buffer (from the circulated perfused digestion buffer) using forceps in the collection dish.
Leave the tissue in 5 mL of digestion buffer for 5 min. Pipette the tissue up and down 5–6 times in a 1 mL pipette tip.
Note: Cut the end of the 1 mL tip to approximately 3 mm diameter to make sure that the tissue fragments easily go through the tip).
The cells will disperse from the tissue into the digestion buffer: the cells will be in the buffer, and tissue clumps will be in the bottom of the dish. Collect the suspended isolated cells with a 1 mL pipette tip to a 15 mL tube, and then add 2 mL of stopping buffer to the cells in the tube.
Add 5 mL of enzyme digestion buffer to the leftover tissue to further digest 5 min more and disperse the cells as in step 9. Collect the dispersed cells and add 2 mL of stopping buffer. Repeat steps 9 and 10 until the tissue becomes white and only connective tissue is left.
Combined all the isolated cells. Centrifuge at 100 × g for 20 s or let the cells settle down for 10 min.
Remove the supernatant, add 10 mL of fresh stop buffer and resuspend the cells.
Add 10 µL of CaCl2 (100 mM) four times (40 µL total) with a 5 min interval between each addition; each time, mix the solution by gently pipetting up and down three times. This accomplishes gradual Ca2+ restoration to avoid high Ca2+ concentration stress.
Centrifuge the cells at 100 × g for 20 s or let the cells settle down for 10 min.
Remove the supernatant and resuspend the cells in the appropriate buffer or medium for your experimental use. See the primary isolated mouse cardiomyocytes’ appearance, Figure 4A.
Part II: Adult rat cardiomyocyte isolation
The procedure for rat cardiomyocyte isolation is very similar to that of mouse cardiomyocyte isolation. The major differences are in the perfusion buffer recipe, enzyme amount, and cutting of the ventricle tissue into small chunks.
Prepare the isolation and digestion buffers (see Recipes for rat cardiomyocyte isolation buffers). Fill the system with 80 mL of 1 mM Ca2+ perfusion buffer (left tube, Figure 1A) and 50 mL of Ca2+ free perfusion buffer (right tube, Figure 1A) and bubble with oxygen by O2 tubes reaching the bottom of both left and right 50 mL tubes (Figure 1A). Connect an 18 G needle covered by a thin silastic tube (to prevent a loose tie around the aorta, Figure 3) to a syringe filled with 5 mL of 1 mM Ca2+ perfusion buffer.
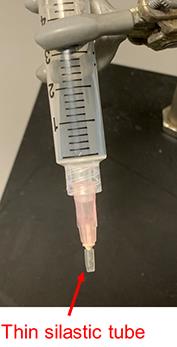
Figure 3. Rat heart cannulation needle. An 18 G needle covered with silastic tubing to prevent a loose tie.Anesthetize the rat by i.p. injection with pentobarbital sodium (150 mg/Kg) and heparin (10,000 U in 0.5 mL) to prevent blood clotting. Pinch the foot pad. When the rat has no response, then move to the next step.
Spray the rat chest with 70% ethanol. Open the chest wall, cut the ribs and quickly remove the heart with 3–5 mm aorta attached. Put the heart in 1 mM Ca2+ perfusion buffer on ice. Gently press the heart to remove blood in the chambers.
Cannulate the aorta with the 18 G needle and tie the aorta to the cannula with 4-0 silk thread. The tip of the cannula should be just above the aortic valve. Flush 3–4 mL buffer into aorta to check that there is no leak and to remove most of the blood (see Video 3). Transfer the heart to the isolation rig.
Video 3. Rat Heart CanulationPerfuse with 1 mM Ca2+ isolation buffer for 5 min to remove any blood left in the heart chamber, until the efflux is clear.
Switch to Ca2+-free perfusion buffer for 5 min. The heart will gradually stop beating.
Add 20 mL of concentrated digestion buffer (~6,500 U collagenase II and 12 mg hyaluronidase) to the Ca2+-free perfusion buffer that remains in the tube (~60 mL). The total volume is 80 mL.
Catch the efflux digestion buffer with a 50 mL beaker and submerge the heart in the digestion buffer. Recirculate the digestion buffer, as in the mouse protocol.
After 5 min, add 150 µL of 100 mM CaCl2 three times at 1 min intervals into the digestion buffer to gradually increase the Ca2+ concentration. The final Ca2+ concentration will be approximately 500 µM to accelerate the digestion.
The heart should swell and look soft, pinkish, but not pale white, as the latter means that the perfusion buffer has not reached the heart tissue. A pale white heart could be an issue with the aorta cannulation or blood clotting in the coronary arteries. The flow rate will increase during perfusion as the heart swells. Total digestion time is 40–60 min.
When the heart becomes soft, detach the heart from the cannulated syringe needle, remove the atria and aorta, and cut the ventricle tissue into small chunks (~2 mm cubes).
Add 15 mL of enzyme digestion buffer (from the circulated digestion buffer) and gently swirl in a 37°C water bath for 5 min.
After the first swirl, do not pipette the chunks, but gently pipette the first swirl solution, which contains mostly dead cells, and discard it. Add 15 mL of enzyme digestion buffer, swirl the 2nd time for 5 min, and now pipette the chunks up and down to disperse the cardiomyocytes. The cells will be suspended in the buffer, and the tissue will be at the bottom of the dish. Collect the dispensed cells with the 1 mL pipette and add to a 15 mL tube to which you then add 2 mL of stopping buffer. Add 15 mL of enzyme digestion buffer to the rest of the chunks for the 3rd digestion. Repeat 3–4 times till the chunks become white and only connective tissue is left. Combine all the isolated cells. Centrifuge at 100 × g for 20 s. Resuspend the cells in 20 mL of stopping buffer and place 10 mL in each of two 15 mL tubes. Add 25 µL of 100 mM CaCl2 in each tube three times at 5 min intervals to gradually restore the Ca2+. After each addition, gently turn the 15 mL tube upside down to mix the Ca2+.
Centrifuge at 100 × g for 20 s. Resuspend the cells in the appropriate buffer or medium for the experimental use. See the isolated rat cardiomyocyte appearance after 24 h culture in Figure 4B.
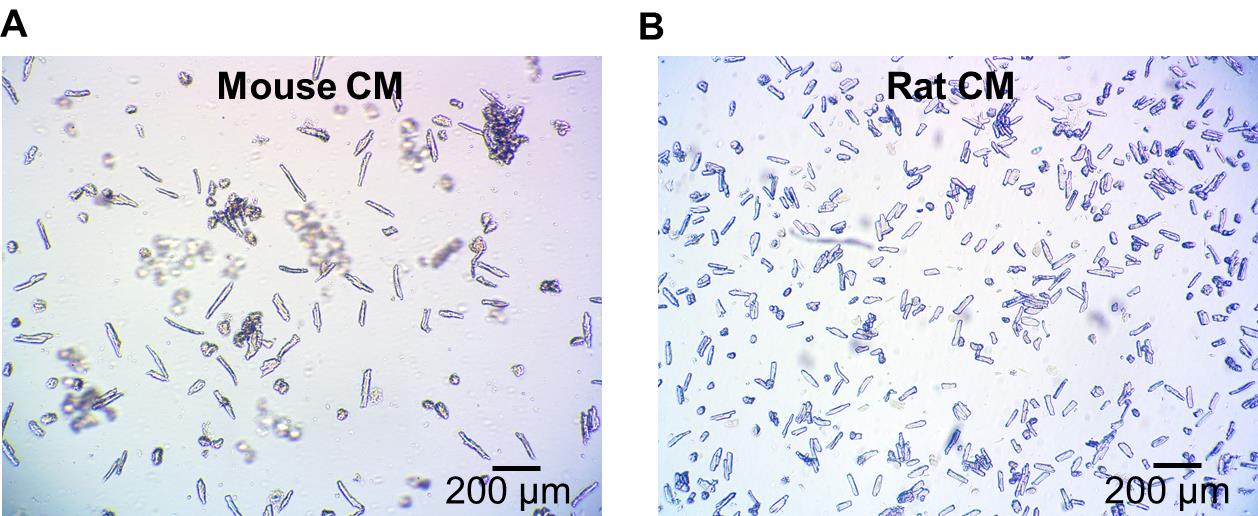
Figure 4. Isolated mouse and rat cardiomyocytes. (A) Acute isolated mouse cardiomyocytes. (B) Rat cardiomyocytes after 24 h culture.
Notes
Collagenase Type 2 activity varies between different batches. Purchase a small bottle of several batches from the vendor and test which batch works best.
Recipes
Mouse cardiomyocyte isolation buffers
Mouse cardiomyocyte perfusion buffer (pH 7.4, adjusted with NaOH)
The solution contains:
113 mM NaCl; 4.7 mM KCl; 1.2 mM MgSO4·7H2O; 0.6 mM NaH2PO4; 0.6 mM KH2PO4; 10 mM HEPES; 10 mM Glucose; 10 mM Butanedione monoxime; 5 mM Taurine
Note: Filter all the buffers and solutions through 0.22 μm filter.
Mouse heart digestion buffer
Mouse cardiomyocyte perfusion buffer + Collagenase (300 U/mL) + Hyaluronidase (0.5 mg/mL)
Mouse heart stop digestion buffer
Mouse cardiomyocyte perfusion buffer + BSA (2.5%) + CaCl2 (0.1 mM) + Fetal Bovine Serum (5%)
100 mM CaCl2 stock solution
Rat cardiomyocyte isolation buffers
Rat cardiomyocyte perfusion buffer (pH 7.4, adjusted with NaOH) with or without 1 mM Ca2+
The solution contains:
118 mM NaCl; 4.8 mM KCl; 10 mM HEPES; 1.2 mM KH2PO4; 1.2 mM MgSO4·7H2O; 11 mM Glucose; 1.0 mM CaCl2·2H2O
Note: For the Ca2+ free perfusion buffer, no Ca2+ added.
Rat heart digestion buffer
In 20 mL Ca2+ free perfusion buffer, add 6,500 U collagenase II and 12 mg hyaluronidase.
Rat heart stop digestion buffer
2% BSA in 0.1 mM Ca2+ perfusion buffer.
100 mM CaCl2 stock solution
Acknowledgments
This work was supported by NIA (P01AG001751) to P.S.R. and AHA (19CDA34660311), UW CTMR pilot award under P30AR074990 and Bronson Foundation intramural grant to H.Z.
These protocols are developed based on the experience gained in the laboratories of Dr. Heping Cheng and Dr. Wang Wang, to whom H.Z. is very grateful.
This protocol is linked to the Bio-protocol partner journal eLife with the original research paper DOI: 10.7554/eLife.60827, PMID: 33319746.
Competing interests
The authors declare no conflict of interest.
References
- Judd, J., Lovas, J. and Huang, G. N. (2016). Isolation, Culture and Transduction of Adult Mouse Cardiomyocytes. J Vis Exp(114): 54012.
- Li, D., Wu, J., Bai, Y., Zhao, X. and Liu, L. (2014). Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp(87): 51357.
- Louch, W. E., Sheehan, K. A. and Wolska, B. M. (2011). Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol 51(3): 288-298.
- Powell, T. and Twist, V. W. (1976). A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun 72(1): 327-333.
- Roth, G. M., Bader, D. M. and Pfaltzgraff, E. R. (2014). Isolation and physiological analysis of mouse cardiomyocytes. J Vis Exp(91): e51109.
- Zhang, H., Alder, N. N., Wang, W., Szeto, H., Marcinek, D. J. and Rabinovitch, P. S. (2020). Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. Elife 9: e60827.
- Zhang, H., Gong, G., Wang, P., Zhang, Z., Kolwicz, S. C., Rabinovitch, P. S., Tian, R. and Wang, W. (2018). Heart specific knockout of Ndufs4 ameliorates ischemia reperfusion injury. J Mol Cell Cardiol 123: 38-45.
- Zhang, H., Shang, W., Zhang, X., Gu, J., Wang, X., Zheng, M., Wang, Y., Zhou, Z., Cao, J. M., Ji, G., Zhang, R. and Cheng, H. (2013). Beta-adrenergic-stimulated L-type channel Ca2+ entry mediates hypoxic Ca2+ overload in intact heart. J Mol Cell Cardiol 65: 51-58.
- Zhang, H., Wang, P., Bisetto, S., Yoon, Y., Chen, Q., Sheu, S. S. and Wang, W. (2017). A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration. Cardiovasc Res 113(2): 160-170.
- Zhou, Y. Y., Wang, S. Q., Zhu, W. Z., Chruscinski, A., Kobilka, B. K., Ziman, B., Wang, S., Lakatta, E. G., Cheng, H. and Xiao, R. P. (2000). Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279(1): H429-436.
Article Information
Copyright
Zhang and Rabinovitch. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhang, H. and Rabinovitch, P. S. (2022). Protocol for Isolation of Cardiomyocyte from Adult Mouse and Rat. Bio-protocol 12(10): e4412. DOI: 10.21769/BioProtoc.4412.
- Zhang, H., Alder, N. N., Wang, W., Szeto, H., Marcinek, D. J. and Rabinovitch, P. S. (2020). Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. Elife 9: e60827.
Category
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell viability > Cell survival
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











