- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Anticipatory and Consummatory Responses to Touch and Food Rewards: A Protocol for Human Research
Published: Vol 12, Iss 4, Feb 20, 2022 DOI: 10.21769/BioProtoc.4325 Views: 2328
Reviewed by: Salim GasmiSrinidhi Rao Sripathy RaoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1294 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
Understanding the neural basis of reward processing is a major concern, as it holds the key to alleviating symptoms of addiction and poor mental health. However, this goal seems difficult to attain as long as research on reward processing cannot easily be compared across species and reward types, due to methodological differences and the presence of confounding factors. We recently developed an experimental paradigm that allows monitoring anticipatory and consummatory responses to matched social (touch) and nonsocial (food) rewards in adult humans. The following protocol describes in detail the materials and the paradigm, which measures reward wanting and liking with a real effort task and subjective ratings. It can also be used in combination with facial electromyography (EMG), brain imaging (e.g., fMRI), and pharmacological interventions. It is our firm belief that the field will profit greatly from more research being conducted on reward processing using this and similarly controlled paradigms, which allow for cross-species comparison.
Keywords: Reward taskBackground
Animal research has been instrumental for our understanding of reward processing. However, translation of findings from animal to human research remains difficult, including for methodological reasons. Several aspects characteristic of animal research are typically lacking in human research: decision utility and experienced utility are measured in the same task, primary rewards are provided on a trial-by-trial basis, and hedonic reactions to anticipated and consumed rewards are measured objectively, e.g., through physical effort (Berridge, 2000; Steiner et al., 2001). In contrast, in human research, wanting (i.e., decision utility—the motivation to obtain a reward) and liking (i.e., experienced utility—the hedonic response) are typically measured with self-report, and rewards are administered—if at all—at the end of the experimental session (Pool et al., 2016).
Because of these important differences in the operationalization and measurement of reward, comparing findings from human and animal research is difficult (Der-Avakian et al., 2016). There is also a lively debate around the question of whether different neural networks underlie the processing of different types of rewards (Rademacher et al., 2010; Grabenhorst and Rolls, 2011; Chevallier et al., 2012; Ruff and Fehr, 2014). Indeed, past research compared rewards that not only differed in the dimension of interest (e.g., social vs. nonsocial), but also in several uncontrolled aspects (e.g., temporal proximity, primacy, salience) (Matyjek et al., 2020).
Here, we describe a novel paradigm (Korb et al., 2020a and 2020b), which allows to measure anticipatory and consummatory responses to matched primary food and primary social (interpersonal touch) rewards, in a population of healthy human adults. The task uses several trials, and in each trial a reward is announced and received. Wanting and liking are monitored by registering the amount of physical effort exerted with a hand dynamometer, and participants’ subjective reward responses with a rating slider. Using this paradigm in combination with a pharmacological intervention and the monitoring of facial responses with electromyography (EMG) (Korb et al., 2020a), we recently found evidence suggesting that anticipatory and consummatory pleasure rely on partly different neurochemical systems, and the neurochemical bases for food and touch rewards differ to some degree. We hope that the detailed description of the paradigm provided here will inspire more scholars to adopt a translational approach and help them investigate reward processing in a way that more easily allows comparison across species and reward types. This paradigm can be combined with the monitoring of facial responses with EMG, making it comparable to the ‘taste reactivity test’ used in animals and human infants, which consists in observing facial expressions as a measure of hedonic reward appreciation (Berridge, 2000; Steiner et al., 2001). It can also be easily adapted to psychopharmacological interventions, as well as to brain imaging with clinical populations (e.g., autism spectrum condition) and healthy controls. Doing so holds the promise of a better understanding of the neural circuitry underlying the processing of and responding to different types of rewards, to ultimately overcome altered reward processing in addiction and mental health disorders.
Materials and Reagents
Food products must be stored in the manner indicated by the manufacturing company (e.g., fridge). All related materials must be food safe as approved by the authorities, handled as indicated by manufacturers, and in compliance with hygienic standards.
Nonsocial stimuli (i.e., food)
200 mL of milk, 5% fat (Halbfett Milch; Nöm AG, Austria); stored in fridge at ~4°C until use
150 mL of cocoa milk (Kakaomilch, 5%; Nöm AG, Austria); stored in fridge at ~4°C until use
Powdered sugar (Wiener Zucker Staubzucker; Agrana AG, Austria)
Water (tap water if safe for drinking and low in chlorine)
4 large plastic syringes (MonojectTM 140 mL Luer-lock; Covidien/Medtronic plc, Ireland)
Tubing (1/16 × 1/8 in. TygonS3 TM E-3603; Saint-Gobain S.A., France) (Note 1)
4 female Luer to hose barb 1/16 in. tubing adapter (Cole-Parmer Instrument Co., U.S.A.)
4 hose barb union 1/16 in. tubing connector (Cole-Parmer Instrument Co., U.S.A.)
Milk system cleaner (WoldoClean/Woldoshop GmbH, Germany)
Felt-tip pen, flexible ruler or meter tape (at least 10 cm), scissors and everyday use tape
Equipment
Setting: A laboratory (quiet room) with a table, two chairs, and a black curtain hanging so that it divides the table in two sides. The curtain blocks participants from seeing the experimenter, who is sitting on the other side
Computer system: A laptop running on Windows (HP EliteBook workstation 8570; Hewlett-Packard Inc., U.S.A.), connected to a supplementary monitor (resolution at 1280 × 1024 pixels; model: Flatron L2000CQ; LG Corp., South Korea) and a supplementary keyboard (Lenovo Group Ltd., China, model: SK-8825)
Earphones (3.5 mm jack connector, compatible with pc audio line-out)
Hand dynamometer (Vernier Software & Technology, model: HD-BTA)
Four independent syringe pumps (PHD ultra 4400 PC; Harvard Apparatus, model: 70-3510)
Adjustable mechanical arm to support tubing
Scale (Steinberg Systems, model: SBS-LW-7500)
Software
Matlab (Mathworks Inc., U.S.A.) with the Cogent 2000 and Cogent Graphics toolboxes (RRID:SCR_015672) to control the task and the pump delivery system. Alternatively, similar toolboxes to Cogent can be used (e.g., psychtoolbox-3).
Matlab and R (R Core Team, 2020) to pre-process the data and run statistics
Procedure
General notes
Prescreen participants for any contraindication to the stimuli (e.g., diabetes, food allergies, forearm skin conditions).
Prescreen participants to only include those who indicate liking the proposed food and touch stimuli (i.e., milk, cocoa milk, receiving forearm caresses).
Ask participants not to eat in the four hours preceding the experiment (Note 2).
To carry out the present paradigm, approval from the local ethics commission is necessary. The protocol does not pose major physical or psychological risks for participants; however, each participant must be informed about the main procedures beforehand and must be free to interrupt the procedures at any moment, without providing further explanations.
Task Description
General description
The task delivers social (i.e., touch) and nonsocial (i.e., food) rewarding stimuli in segregated blocks.
Touch stimuli consist of gentle caresses, delivered at specific speeds by a trained experimenter to the participant’s forearm. Food stimuli consist of sweet milk with different concentrations of cocoa, delivered through a tubing system to the participant’s mouth. The task entails three different levels of reward magnitude (high, low, very low), tailored on the subjective ranking performed by the participant before the main task (see point E.2). The reward magnitudes differ in the pace at which caresses are delivered, and in the concentration of cocoa in the administered milk.
In each trial, a high or low possible reward is announced. Participants obtain the reward if their effort pressing the hand dynamometer is sufficient, otherwise, the very low reward is delivered.
The paradigm allows to record aspects of reward processing in humans with different measures (self-report, physical effort, facial responses) and across four crucial phases: pre-effort anticipation (announcement of possible reward), post-effort anticipation (waiting for upcoming reward), delivery (reward consumption), and relax (reward appreciation).
Variables
The task allows the manipulation of:
Type of reward (2): social (i.e., touch) and nonsocial stimuli (i.e., food).
Magnitude of reward (2/3): high, low, very low (not for pre-effort anticipation).
The task allows the measurement of (Note 3):
Wanting of the possible reward, as explicit subjective rating.
Wanting of the possible reward, as effort/force applied to the dynamometer.
Liking of the obtained reward, as explicit subjective rating.
Design
Two blocks for each type of reward; interleaved (i.e., ABAB/BABA).
Sixteen trials for each block; 8 high and 8 low possible rewards. Note that despite the same number of high and low possible rewards being announced, the number of high, low, and very low rewards obtained may differ per participant, as this is determined by the force that participants exert trail-by-trial, to obtain the possible rewards (and by chance, as force is converted into probability).
Stimuli
Each stimulus is associated with one representative icon (Figure 1).
Social (touch)
A trained experimenter (Note 4) administers caresses to the participant’s forearm. Using the extended index and middle finger, deliver a continuous back and forth movement at paced speed as prompted by auditory cues. Apply very soft pressure (as for tickling) over a 9 cm-long area on the posterior (hairy) side of participant’s forearm (we chose to caress the left instead of the right forearm for practical reasons). Consider the use of a dry powder or (gluten free) flour to smoothen the skin-to-skin contact and avoid unpleasant rubbing (depends also on room and skin temperature). The following pace of caresses was chosen based on the literature [humans perceive as more pleasant caresses that are in the speed range that elicits C-tactile fiber activation, i.e., 1–10 cm/s (Löken et al., 2009; Taneja et al., 2021)] and pilot testing.
“slow” (pace 6 cm/s)
“fast” (pace 21 cm/s)
“very fast” (pace 27 cm/s)
Nonsocial (food) (Note 5)
Syringes attached to electrically-operated and computer-controlled pumps deliver liquid food (milk) through the tubing directly into the participant’s mouth. The amount of food delivered is constant at 2 mL in each trial. Note that, to control for caloric inconsistencies between cocoa milk and plain milk, we added sugar to the plain milk (see point C.2.c for procedures).
“chocolate” (cocoa milk 100%)
“mix” (cocoa milk 25% + sugared milk 75%)
“milk” (sugared milk 100%)
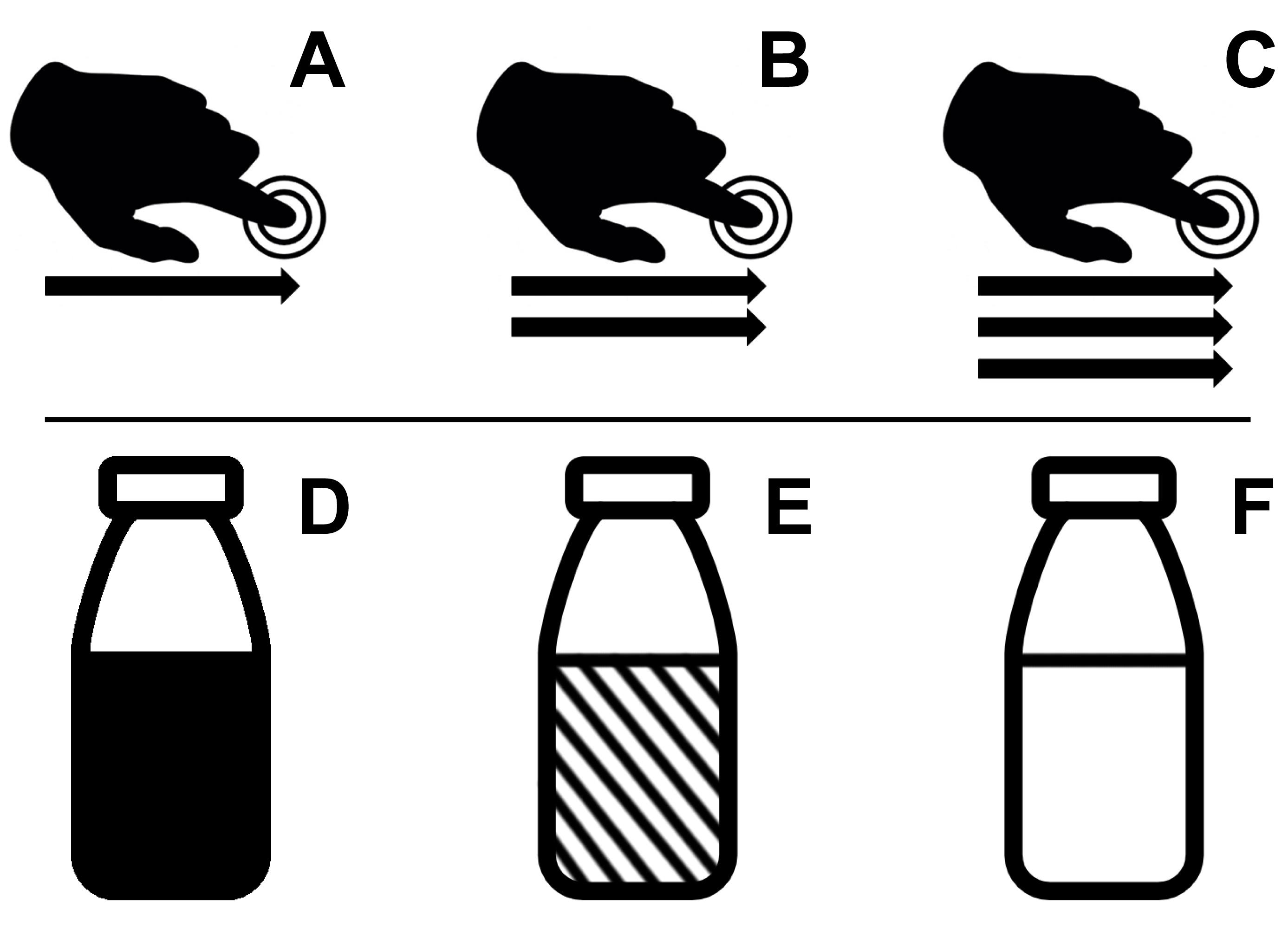
Figure 1. Stimuli and representative icons. A-C. Social touch stimuli; A) “slow”, B) “fast”, C) “very fast”. D-F. Nonsocial food stimuli; D) “chocolate”, E) “mix”, F) “milk”.Trial sequence (Note 6) (Figure 2, Videos 1 and 2)
Text, pictures and graphics are displayed on a dark background to limit excessive luminosity in a dim environment. Touch and food trials last on average 38 and 50 s, respectively.
Central fixation cross; duration 3 s on average (varying randomly between 2 and 4 s).
Announcement of the possible reward; duration 2 s.
Icon announces the highest possible reward (i.e., high or low) that the participant can obtain in the current trial.
Rating of wanting; duration 4 s.
Continuous visual analog scale ranging from “not at all” (left anchor) to “very much” (right anchor). Using the keyboard, participants are instructed to move the cursor along the continuum to express the degree of wanting of the announced possible reward. In each trial, the scale appears with the cursor placed randomly in a central position (25% to the left and right of the midpoint).
Physical effort; duration 4 s.
An empty bar fills up with red color from bottom (0%) to top (100%) according to the force applied to the hand dynamometer, calibrated on individual maximum voluntary contraction (MVC).
Participants are instructed to squeeze the dynamometer according to their desire (wanting) to obtain the announced reward. The likelihood of getting such a reward is directly proportional to the peak of the force exerted (i.e., 100% and 0% of MVC exerted correspond to 100% and 0% probability of receiving the announced reward, respectively).
Announcement of obtained reward; duration 2 s.
Icon announces the obtained reward, either the previously announced (high or low) or the very low reward.
Preparation for reward delivery; duration 6 s.
Participants are instructed to wait for stimulus delivery. In touch blocks, the trained experimenter receives an auditory cue through earphones priming the pace of the caress to be delivered.
Reward delivery; duration 7 s.
Icon indicates the stimulus being delivered, either high, low, or very low reward.
In touch blocks, the experimenter receives the auditory cue through earphones driving the pace of the caress being delivered; in food blocks, instructions signal 2.5 s of delivery and 4.5 s of swallowing: the pump pushes the plunger for the first 2.5 s delivering the 2 mL of liquid, then pulls the plunger a little, enough to avoid liquid from leaking.
Relaxation; duration 6 s.
Participants are instructed to relax and enjoy the stimulus they just experienced.
Rating of liking; duration 4 s.
Identical to the rating of wanting; participants express the degree of liking of the stimulus just experienced.
Only for food blocks: preparation for rinsing; duration 5 s.
Participants are instructed to expect water delivery.
Only for food blocks: rinsing; duration 7 s.
Participants receives 2 mL of water to rinse their mouth. Instructions signal 2.5 s of delivery and 4.5 s of swallowing, as for the reward delivery phase.
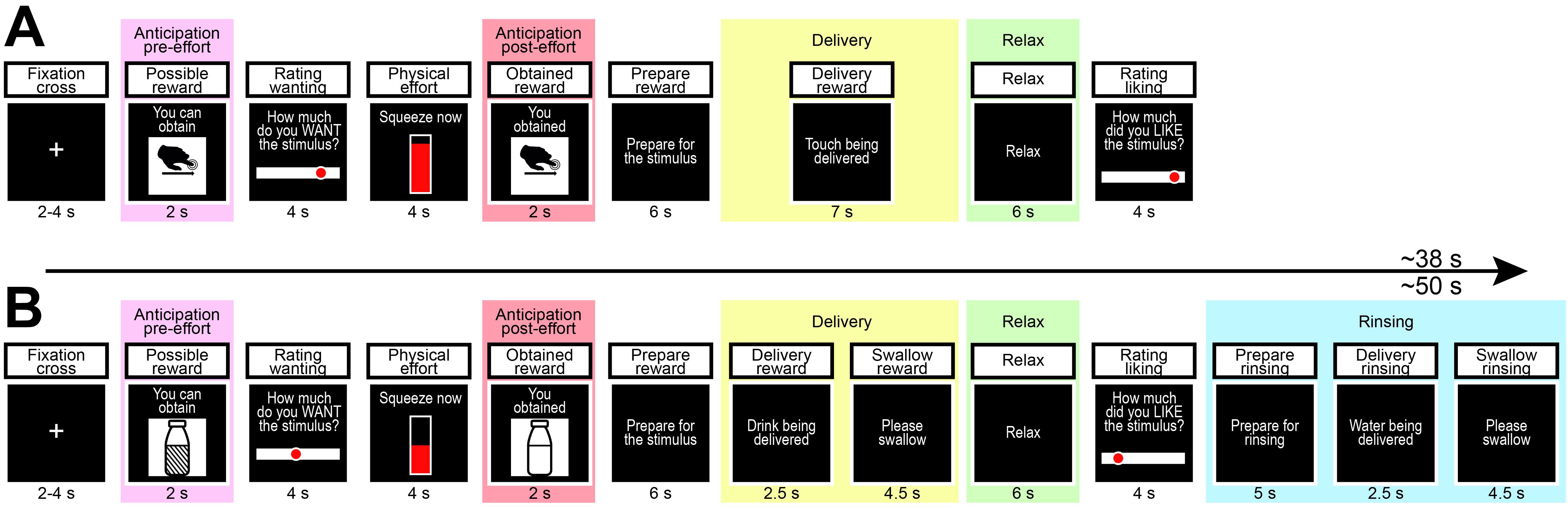
Figure 2. Trial Sequence. Description, screens, duration onscreen, and main phases (colored panels) of the task. A) Touch reward task (average duration 38 s); in this representative trial, the highest possible reward that the participant can obtain is the “slow” touch (e.g., high reward); the participant rates the wanting and exerts a strong physical effort; the participant is informed that they have secured the highest possible reward; the participant receives the reward, enjoys the reward, and rates its liking. B) Food reward task (average duration 50 s); in this representative trial, the highest possible reward that participant can obtain is the “mix” (e.g., low reward); the participant provides a medium rating of wanting, and exerts a moderate effort; the participant is informed that they will receive the “milk” (e.g., very low reward) instead; the very low reward is delivered, and after a relax phase the participant rates its liking; water is then delivered to rinse the mouth and prepare for the next trial.
Materials preparation
As preparation requires several steps, we advise organizing the setting and materials before the arrival of the participant.
Room
Set up a long black curtain to isolate the participant from the rest of the room. Place a table transversal to the curtain. Such disposition prevents the participant from having visual feedback of their forearm while it is being caressed in the touch blocks, and it allows the participant to focus without distractions on the tactile sensation of the caress.
On the participant’s side of the curtain (Figure 3A), place a comfortable chair (preferably without castors and moving parts). On the desk in front of the participant, place the supplementary monitor, the hand dynamometer (participants will operate it with their left hand) and the supplementary keyboard (participants will operate it with their right hand). A smaller numeric keypad or a response button box can be used instead. Adjust the mechanical arm that will hold the tubes for food delivery at the height of the participant’s mouth.
On the experimenter’s side of the curtain (Figure 3B), place a small, thin pillow (covered with a fresh paper or tissue, to be replaced for each participant) on the table next to the curtain. Place the laptop close to the pillow running parallel to the curtain. With this setting, during touch blocks the experimenter can control the laptop while comfortably caressing the participant’s forearm that is resting on the pillow.
Place the pumps on a flat surface, preferably out of the participant’s sight. We arranged them on a small side table with castors.
Food stimuli
Thoroughly wash hands before handling food stimuli and setting up the pump system.
Remove the milk and the cocoa milk from the fridge approximately 30 min before the experiment. Do so consistently across sessions and subjects to minimize major differences in the temperature of the delivered stimuli.
Using the scale, add sugar to the milk in order to equalize its caloric content with that of the cocoa milk, then shake. In our case, the declared caloric content per 100 mL is 46 kcal for the milk and 65 kcal for the cocoa milk. To equalize these values, we mix the milk with 4.8 g of sugar, which has 400 kcal per 100 g.
Fill the four syringes
“Milk” syringe contains 80–100 mL of sugared milk.
“Chocolate” syringe contains 80–100 mL of cocoa milk.
“Mix” syringe contains 80–100 mL of a mix including 25% of cocoa milk and 75% of sugared milk.
“Water” syringe contains 100–120 mL of fresh still water.
Remove air bubbles from the syringes.
Fix each syringe to the relative pump.
Connect the tubes to the syringe tips through the hose-to-Luer connectors.
Cut four pieces of tubes of ~10 cm. Connect them to the tubes through the hose barb union connectors. Tape them together and cut out excessive parts (make sure that tape is not entering the participant’s mouth). These tubes will come in contact with the mouth of the participant, so use new and clean tubes, handle in compliance with the hygienic standards.
Tape the tubes to the mechanical arm (Figure 3C).
Computer
Connect the supplementary monitor, the supplementary keyboard, the hand dynamometer, the pumps, and the earphones to the laptop. The synergy of these instruments is controlled via Matlab during the task.
Pumps
Activate the pumps to push the syringes until the tubes are completely filled with the beverages.
Preparation of the participant
Mark the area to be caressed with a felt-tip pen.
Keep hand in pronation, mark the head of the ulna.
Mark 9 cm on a straight line from the head of ulna towards the belly of the extensor muscles of the hand (towards the elbow), on the hairy side of the forearm.
Let the participant sit centrally before the monitor, with their face at ~80 cm.
Place their left forearm beyond the curtain, lying comfortably on the pillow.
The left hand holds the dynamometer to perform power grip, the right hand controls the keyboard.
Assist participants in placing the end of the tubes in their mouth by adjusting the mechanical arm to the optimal position (Figure 3A and 3C).
Place a large cup on the table below the end of the tubes to collect leaking drops.
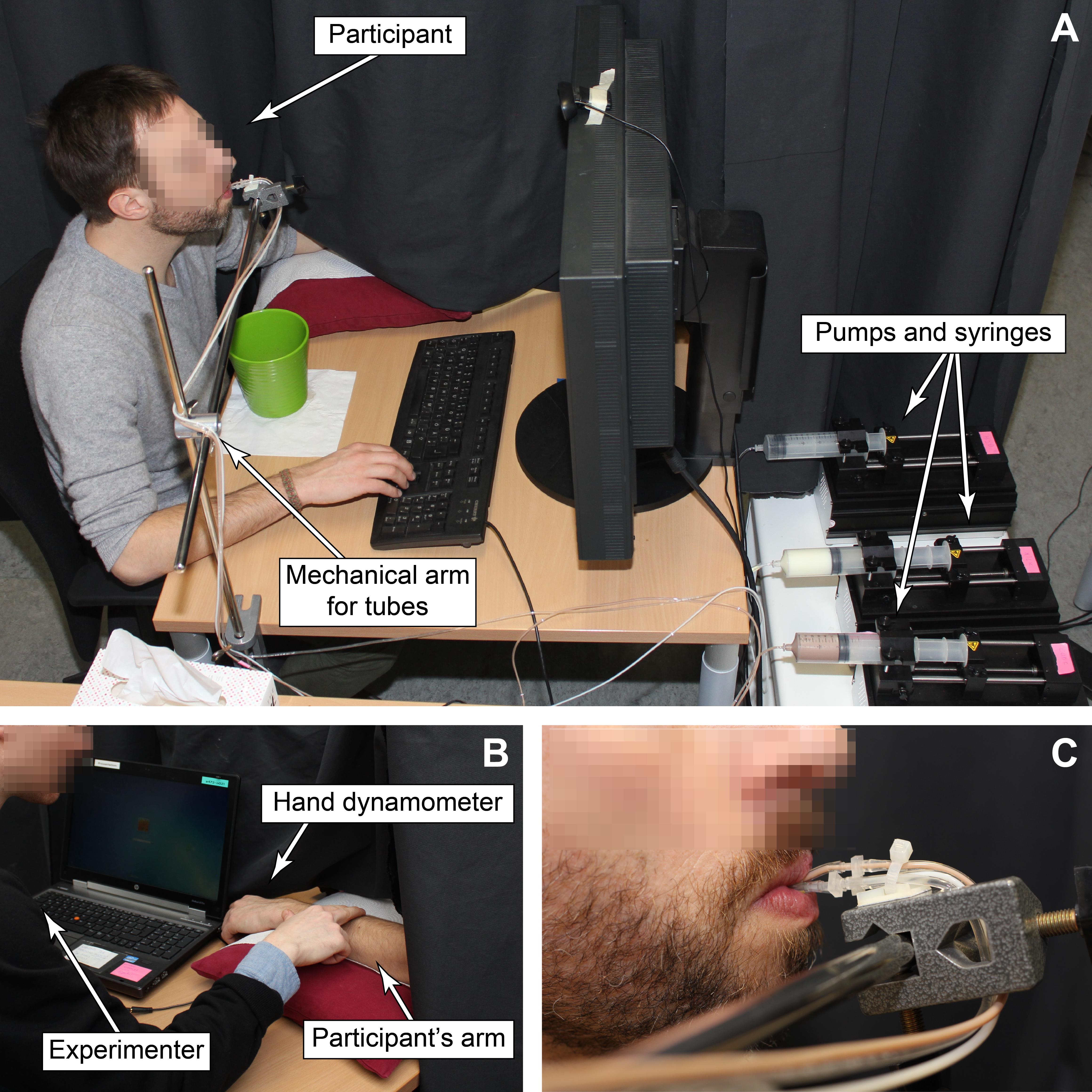
Figure 3. Experimental setup. A) Participant’s side: The participant sits in front of the secondary screen; the right hand is on the secondary keyboard; their left arm lies on the pillow beyond the black curtain; computer-controlled pumps push the syringes (these can also be placed elsewhere); the food stimuli flow through the tubes to the participant’s mouth; a mechanical arm holds the tubes. B) Experimenter’s side: The experimenter sits in front of the computer and participant’s left arm; through the earphones, the experimenter receives prompts for delivering the touch stimuli to the participant’s arm. C) One end of the tubes is tied to the mechanical arm; short tube parts are connected to the tubes and lie in the participant’s mouth.
Initial procedures
Before explaining the task, instruct participants to squeeze the hand dynamometer as strong as possible, performing a power grip for 3 s across three successive trials. Consider the peak value as participant’s MVC. The MVC is used to individually calibrate the dynamometer during the main task. Therefore, it is very important that the hand dynamometer is held in the same position during the MVC measurement and the later experiment.
Stimuli ranking (Note 7)
Every participant experiences all touch and food stimuli, presented in two separated blocks and pseudo-randomized order.
Per each reward type, a complete set of three stimuli is administered, followed by a task asking them to rank the stimuli according to their liking.
The individual ranking is entered in the system for the main task and defines the high, low, and very low rewarding stimuli.
Instruct the participants about task procedures and dynamics.
Task familiarization: participants complete four practice trials (2 touch and 2 food).
Task administration (Videos 1 and 2)
Task duration is of ~50 min. One touch block lasts ~10 min, one food block lasts ~13 min, one touch trial lasts ~38 s, and one food trial lasts ~50 s.
Counterbalance the order of touch and food blocks across subjects.
Alternate touch and food blocks.
Allow short breaks between blocks, for participants to rest and the experimenter to prepare for a subsequent block.
Final procedures
At the end of the task, we advise collecting a second measure of participant’s MVC. This may reveal muscle fatigue and/or habituation throughout the task.
Remove and discard the tube parts that were in contact with the participant’s mouth. Clean the inside of the tubes with detergent, and rinse with warm water.
Video 1. Social task.Video 2. Nonsocial task.Videos depicts three representative trials of the social (Video 1) and the nonsocial (Video 2) tasks. Note that some details are slightly different than those described in the manuscript, as the task has been adapted for different environments and populations (Note 6).
Data analysis
We exclude from the analyses subjects who could not complete at least 16 trials (50%) of either touch or food blocks. Analyses of rating of wanting, rating of liking, and effort exerted are conducted with separate statistical models. Ratings reflect visual analog scale values ranging from -100 to 100; effort values are expressed as the ratio (e.g., percentage) between the force exerted and the individual MVC. We remove outlier trials, defined as trials in which values deviate from the subject’s mean by more than two standard deviations (indicatively, 4 to 7 trials per participant, on average).
For statistics, we recommend fitting linear mixed models, rather than using ANOVA, as they are less sensitive to differences in the number of trials per condition. Details on the analyses can be found in the Analyses section of the original research articles (Korb et al., 2020a and 2020b); associated data and analyses scripts are available on the OSF repository platform (bit.ly/3At2pZW; osf.io/vu8dz).
Notes
The length of the tubing required can vary according to various factors. For example, it depends on the distance at which the pumps are placed from the participant, and whether three or four pumps are used. Also, tubing should be replaced regularly (e.g., once a month, depending on the number of sessions completed), and some extra tubing (~10 cm) is needed for each participant, to replace the end parts in contact with their mouth. Therefore, assuming a pump-participant distance of 3 m using four pumps, 12 m of tubing are needed. Thus, a study involving 50 participants would require approximately 40 m of tubing, considering mouth-end changes and tube substitution thrice.
To control for hunger and satiety factors, we ask participants to have a light meal and to avoid the consumptions of stimulating drinks (e.g., coffee, black tea or energy drinks) on the day of the experiment. Moreover, we ask the participants not to consume solid foods or beverages high in nutrients (e.g., high protein beverages) in the four hours immediately preceding the experimental appointment, while everything else is allowed (e.g., water, fruit tea etc.).
The behavioral task is suitable to be combined with other techniques, such as neuroimaging or facial electromyography (Korb et al., 2020a and 2020b).
To minimize the possibility that caresses in the touch condition could be experienced as a sexual reward (sexual rewards are typically considered social rewards, yet social rewards are not necessarily sexual), we matched the experimenter’s gender with the participant’s sexual orientation. Therefore, in Korb et al. (2020a and 2020b) only self-reported heterosexual participants were enrolled and experimenters delivering caresses were of the same gender as the participant. In other studies of the group (under preparation), we have recruited participants regardless of their sexual orientation, whereupon the experimenter’s gender was opposite to the declared participants’ sexual preference. Further, to limit unpredictable personal preferences, there were always two experimenters in the room, so that the participants could not know which one was delivering the touch stimuli.
In more recent versions of the task, we delivered 1.5 mL instead of 2 mL of food stimuli (and water) on a trial-by-trial basis. Such a volume is sufficient to be tasted properly and limits the phenomena of satiety.
The trial sequence and the timing reported here are not identical to those in Korb et al. (2020a and 2020b) (see supplementary figures), yet they share the overall structure. In different versions of the paradigm (such as in Videos 1 and 2), we decided to eliminate certain screens (e.g., remove preparation to effort) and adjust the timings of others (e.g., limit to 4 s the ratings of wanting and liking). Therefore, here we report the modified trial sequence which shortens the overall task duration and makes the timings more similar across the touch and food conditions. Note that the adoption of the task for fMRI environments may require further adjustments to be considered such as adding jittered intervals within the trial, to enable the independent estimation of the hemodynamic response to non-orthogonal within-trial conditions.
The cocoa/milk dosage and the touch speeds were chosen based on pilot experiments, which also showed a fairly consistent ranking of the stimuli across the subjects interviewed. Typically, for touch stimuli, slower speed equates to higher reward magnitude (i.e., first “slow”, second “medium”, and third “fast”) and, for food stimuli, higher cocoa concentration is linked to higher reward magnitude (i.e., first “chocolate”, second “mix”, and third “milk”). However, in Korb et al. (2020a, 2020b) individual variability was taken into account and reward magnitudes were individually adjusted, according to participant’s stimulus preferences as expressed before the main task. Frequently, the stability of the initial preferences was weak along the duration of the experiment and, especially those participants expressing atypical ranking changed their opinion, adhering to the expected preferences as the task advanced. In other versions of the task, the stimulus magnitudes were selected by experimenters according to typical rankings and were not adjusted individually. Nevertheless, under certain experimental circumstances, when one can expect high interindividual or intergroup variability (e.g., testing of clinical groups), the implementation of the individual ranking may be relevant. In further experiments adopting this paradigm, we acknowledged that two or three exposures for each of the stimuli may better account for some intraindividual variability, and thus lead to a more reliable ranking. Nevertheless, keep in mind that too many ranking trials right before the experiment may lead to unwanted habituation or satiety effects.
Acknowledgments
The present protocol describes the task and the procedures adopted in Korb et al. (2020a and 2020b) which have been supported by the Vienna Science and Technology Fund (WWTF) with a grant (CS15-003) awarded to Giorgia Silani and Christoph Eisenegger. We thank Sebastian Götzendorfer, Claudia Massaccesi and Phillip Stepnicka for their prominent role in the development of the task.
Competing interests
The authors declare no competing interests in connection with this protocol.
Ethics
The Ethical Committee of the Medical University of Vienna approved all the procedures and the studies (EK N. 1393/2017). Every participant involved in the experiments expressed informed consent for taking part in the studies.
References
- Berridge, K. C. (2000). Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24(2): 173-198.
- Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S. and Schultz, R. T. (2012). The social motivation theory of autism. Trends Cogn Sci 16(4): 231-239.
- Der-Avakian, A., Barnes, S. A., Markou, A. and Pizzagalli, D. A. (2016). Translational Assessment of Reward and Motivational Deficits in Psychiatric Disorders. Curr Top Behav Neurosci 28: 231-262.
- Grabenhorst, F. and Rolls, E. T. (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci 15(2): 56-67.
- Korb, S., Götzendorfer, S. J., Massaccesi, C., Sezen, P., Graf, I., Willeit, M., et al. (2020a). Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards. Elife 9: e55797.
- Korb, S., Massaccesi, C., Gartus, A., Lundström, J. N., Rumiati, R., Eisenegger, C., et al. (2020b). Facial responses of adult humans during the anticipation and consumption of touch and food rewards. Cognition 194.
- Löken, L. S., Wessberg, J., Morrison, I., McGlone, F., and Olausson, H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12: 547-548.
- Matyjek, M., Meliss, S., Dziobek, I., and Murayama, K. (2020). A Multidimensional View on Social and Non-Social Rewards. Front Psychiatry 11: 1-8.
- Pool, E., Sennwald, V., Delplanque, S., Brosch, T., and Sander, D. (2016). Measuring wanting and liking from animals to humans: A systematic review. Neurosci Biobehav Rev 63: 124-142.
- R Core Team (2020). R: A Language and Environment for Statistical Computing.
- Rademacher, L., Krach, S., Kohls, G., Irmak, A., Gründer, G., and Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49(4): 3276-3285.
- Ruff, C. C., and Fehr, E. (2014). The neurobiology of rewards and values in social decision making. Nat Rev Neurosci 15(8): 549-562.
- Steiner, J. E., Glaser, D., Hawilo, M. E., and Berridge, K. C. (2001). Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 25(1): 53-74.
- Taneja, P., Olausson, H., Trulsson, M., Svensson, P., and Baad-Hansen, L. (2021). Defining pleasant touch stimuli: a systematic review and meta-analysis. Psychol Res 85(1): 20-35.
Article Information
Copyright
Chiappini et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chiappini, E., Silani, G. and Korb, S. (2022). Anticipatory and Consummatory Responses to Touch and Food Rewards: A Protocol for Human Research. Bio-protocol 12(4): e4325. DOI: 10.21769/BioProtoc.4325.
- Korb, S., Götzendorfer, S. J., Massaccesi, C., Sezen, P., Graf, I., Willeit, M., et al. (2020a). Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards. Elife 9: e55797.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









