- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Investigation of Microvesicle Uptake by Mouse Lung-marginated Monocytes in vitro
Published: Vol 12, Iss 3, Feb 5, 2022 DOI: 10.21769/BioProtoc.4307 Views: 4151
Reviewed by: Gal HaimovichRajesh ThippeshappaKrisztina Vukman

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2237 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1336 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1464 Views
Abstract
Extracellular microvesicles (MVs) are released into the circulation in large numbers during acute systemic inflammation, yet little is known of their intravascular cell/tissue-specific interactions under these conditions. We recently described a dramatic increase in the uptake of intravenously injected MVs by monocytes marginated within the pulmonary vasculature, in a mouse model of low-dose lipopolysaccharide-induced systemic inflammation. To investigate the mechanisms of enhanced MV uptake by monocytes, we developed an in vitro model using in vivo derived monocytes. Although mouse blood is a convenient source, monocyte numbers are too low for in vitro experimentation. In contrast, differentiated bone marrow monocytes are abundant, but they are rapidly mobilized during systemic inflammation, and thus no longer available. Instead, we developed a protocol using marginated monocytes from the pulmonary vasculature as an anatomically relevant and abundant source. Mice are sacrificed by terminal anesthesia, the lungs inflated and perfused via the pulmonary artery. Perfusate cell populations are evaluated by flow cytometry, combined with in vitro generated fluorescently labelled MVs, and incubated in suspension for up to one hour. Washed cells are analyzed by flow cytometry to quantify MV uptake and confocal microscopy to localize MVs within cells (O'Dea et al., 2020). Using this perfusion-based method, substantial numbers of marginated pulmonary vascular monocytes are recovered, allowing multiple in vitro tests to be performed from a single mouse donor. As MV uptake profiles were comparable to those observed in vivo, this method is suitable for physiologically relevant high throughput mechanistic studies on mouse monocytes under in vitro conditions.
Graphic abstract:
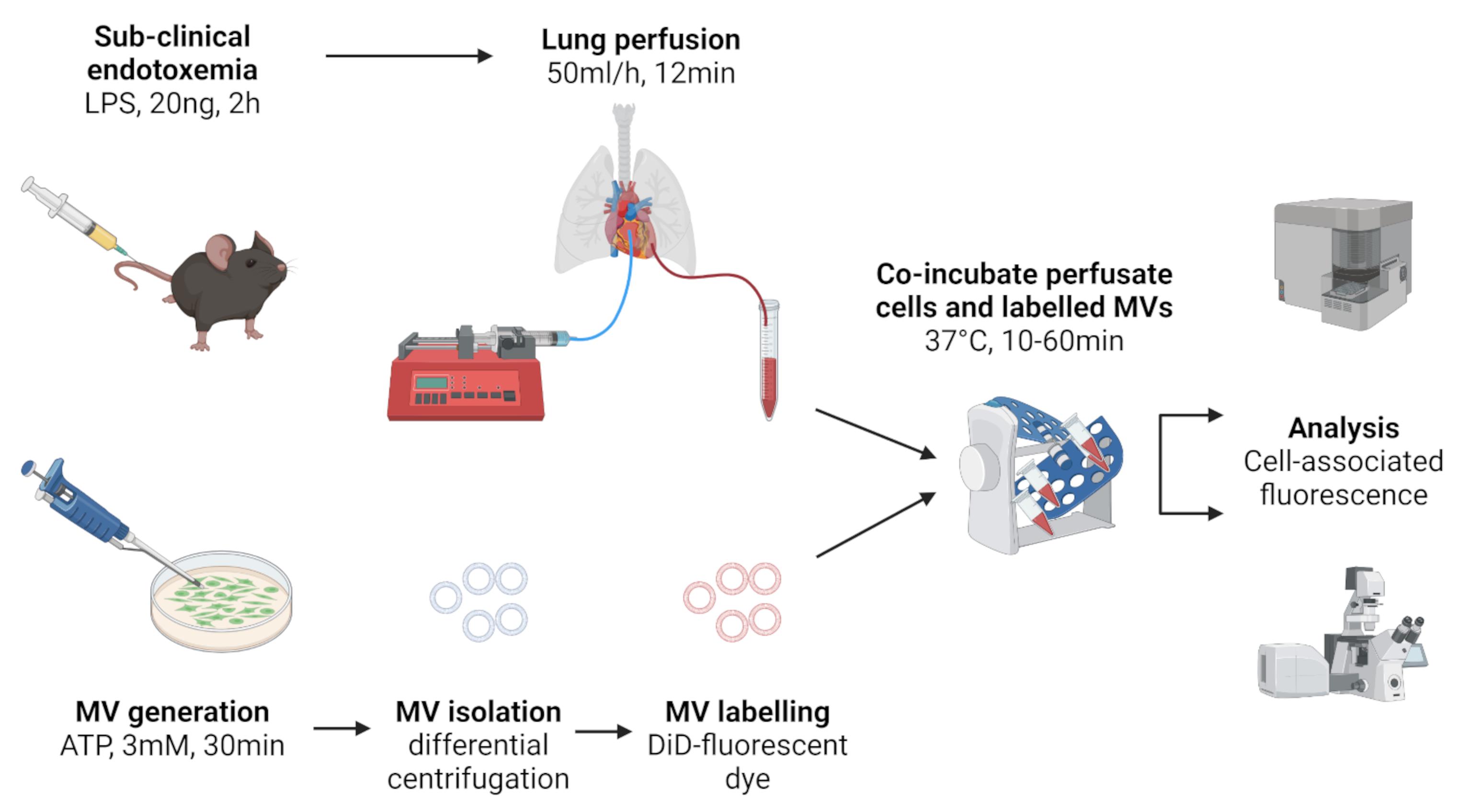
Figure 1. Schematic of lung perfusate cell harvest and co-incubation with in vitro generated MVs. Created with BioRender.com.
Background
Extracellular vesicles (EVs) are a family of cell-derived membrane-encapsulated particles produced constitutively, but also in response to inflammatory stimuli, or activation of programmed cell death pathways (Yanez-Mo et al., 2015). Carrying functional molecular cargoes, EVs have considerable potential as short- and long-range intercellular communication vehicles. Microvesicles (MVs) are an EV subclass derived directly from the cell membrane that display lineage-specific surface markers enabling identification of the parental cell source. During diseases associated with systemic inflammation, such as sepsis and severe burns injury, circulating levels of platelet- and myeloid cell-derived MVs are increased acutely and associated with clinical severity (Nieuwland et al., 2000; Joop et al., 2001; O'Dea et al., 2016). As these MV subtypes carry pro-inflammatory molecular cargoes, they may play a key role in long-range propagation of organ injury during systemic inflammation. Despite this potential, little is known of MV trafficking within the circulation and their cell/tissue-specific interactions during systemic inflammation.
We previously developed a lipopolysaccharide (LPS)-induced model of sub-clinical endotoxemia to evaluate cell/tissue specific interactions of circulating MVs under systemic inflammatory conditions in vivo. In vitro generated MVs labelled with the fluorescent lipophilic dye, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine (DiD) were injected intravenously and their cellular uptake quantified in lung, liver, and spleen cell suspensions by flow cytometry (O'Dea et al., 2020). Using in vivo analysis of cell-associated MVs rather than biodistribution methods, which measure total tissue/organ associated MV label (Willekens et al., 2005; Wiklander et al., 2015), enabled us to determine the relative uptake of MVs by different cell populations within and between organs. We found that during systemic inflammation there is a dramatic increase in MV uptake by pulmonary intravascular ‘marginated’ monocytes, resulting in an overall ≥20-fold increase in uptake by the lungs.
To enable more detailed analyses of the enhanced MV-monocyte interactions during systemic inflammation, we developed a novel in vitro model of MV uptake by in vivo derived pulmonary vascular perfusate cells. In vitro analysis of intravascular monocyte functions, including uptake of MVs, is severely constrained in mice due to their low abundance and small volumes of blood; this problem is further exacerbated by withdrawal of monocytes from the circulation by their margination to vascular beds of lungs, liver, and other tissues during systemic inflammation (O'Dea et al., 2005 and 2009; Hamon et al., 2017). Although differentiated monocytes can be obtained from the bone marrow reserve, such preparations are mixed with immature precursors and, moreover, much of the mature monocyte pool is rapidly released into the circulation during inflammation (O'Dea et al., 2009; Shi et al., 2011). Conversely, the pulmonary circulation is a major vascular site for monocyte accumulation, marginated in significant numbers within its vast capillary network under both resting and inflammatory conditions (Doherty and Worthen, 1994; Kuebler and Goetz, 2002). As such, the lungs represent a previously ‘untapped’ source for in vitro evaluation of monocytes and neutrophils, which also accumulate in substantial numbers within the pulmonary circulation during inflammation (van Eeden et al., 1997; Yipp et al., 2017).
Here, we describe in detail our protocol (summarized in Figure 1) for routine recovery of pulmonary vascular cells and evaluation of MV uptake by monocyte subsets and neutrophils, the findings of which were reported recently by us (Figure 6, Figure 7, and Supplementary Figure S2 in O'Dea et al., 2020). In brief, using this method we determined that MV uptake by monocytes was energy-dependent, involved endocytic processes, and could be inhibited in the presence of phosphatidylserine-enriched liposomes, β3 integrin receptor blocking peptide, and scavenger receptor A blockers.
Materials and Reagents
Culture dishes, 60 mm × 15 mm (Corning, tissue-culture treated, catalog number: CLS430166)
PVC OD 0.61 mm × ID 0.28 mm (SteriHealth, catalog number: I-10317)
Polyethylene tubing OD 1.27 mm × ID 0.86 mm (Portex, catalog number: 800/100/260)
15 mL collection tube (Falcon, polypropylene, catalog number: 352096)
1.5 mL microfuge tube (Starlab, catalog number: S1615-5510)
5 mL flow cytometry tube (Falcon, round bottom polypropylene, catalog number: 35200)
96 well black plate (Thermo Scientific, flat bottom non-treated, catalog number: 237108)
27 G needle (VWR, catalog number: 613-3834)
25 G needle (VWR, catalog number: 613-4952)
19 G needle (VWR, catalog number: 613-3938)
1 mL hypodermic syringe (Terumo, catalog number: SS-01T1)
4-0 curved-needle suture (Medisave, catalog number: W586H)
5-0 suture (Covidien, Sofsilk, catalog number: S-182)
2-0 suture (Covidien, Sofsilk, catalog number: S-185)
Straight hemostatic forceps (Fine Science Tools, catalog number: 1301414)
C57/BL6 male mice (Charles River Laboratories, Margate, UK)
J774A.1 macrophage cell line (European Collection of Authenticated Cell Cultures (ECACC), ECACC-91051511)
Lipopolysaccharide (LPS) (InvivoGen, Ultrapure Escherichia coli O111:B4, catalog number: tlrl-3pelps)
Adenosine 5'-triphosphate (ATP) (Bio-Techne, disodium salt, catalog number: 3245)
Heparin sodium (Wockhardt, 1,000 I.U./mL, catalog number: FP1086)
Human Serum Albumin (HSA) (BioIVT, 25% Solution, catalog number: GEM-800-120-S)
Fetal Bovine Serum (FBS) (Sigma, heat inactivated/sterile filtered, catalog number: F9665)
RPMI-1640 medium (Sigma, catalog number: R5886)
Penicillin-streptomycin (Sigma, catalog number: P4333-100mL)
Antibodies for flow cytometry (see Table 1)
Table 1. Fluorophore-conjugated antibodies for flow cytometric analysis of perfusate cells.
Antigen Fluorophore Dilution Clone Company/Catalog no. F4/80 FITC 1:400 BM8 Biolegend/123108 CD11b PE 1:400 M1/70 Biolegend/101208 Ly-6C PE-Cy7 1:400 HK1.4 Biolegend/128018 Ly-6G APC-Cy7 1:400 1A8 Biolegend/127624 Ly-6G/Ly-6C (Gr-1) APC 1:400 RB6-8C5 Biolegend/108412 AccuCheck Counting Beads (ThermoFisher Scientific, catalog number: PCB100)
Vybrant® DiD cell-labeling solution (ThermoFisher Scientific, catalog number: V-22887)
Diluent C (Sigma, catalog number: CGLDIL-6X10mL)
Phosphate buffered saline (PBS) with Ca2+/Mg2+ (Sigma, catalog number: D8662-500mL)
Phosphate buffered saline (PBS) 10× (Fisher scientific, 20 l, catalog number: BP399-20)
Hank’s Buffered Salt Solution (HBSS) without Ca2+/Mg2+ (Sigma, catalog number H6648)
Hank’s Buffered Salt Solution (HBSS) with Ca2+/Mg2+ (Sigma, catalog number: 55037C)
TritonTM X-100 (Sigma, catalog number: X100-500ML)
Ethylenediaminetetraacetic acid (EDTA) (ThermoFisher Scientific, catalog number: 15575020)
Sodium azide (Sigma, catalog number: S2002)
Perfusion buffer (see Recipes)
Assay buffer (see Recipes)
Flow cytometry buffer (see Recipes)
Equipment
Syringe pump (Harvard Apparatus, model: 11/55-1199)
Refrigerated microfuge (Eppendorf, model: 5417R; angle rotor: FA45-30-11)
Refrigerated benchtop centrifuge (Heraeus, model: Multifuge 3S-R)
Fluorescence plate reader (BioTek, model: Flx-800)
Flow cytometer (Beckman-Coulter, model: CyAn ADP)
Blood Test Tube Rotator (Stuart Scientific, model: SB1)
Incubator (Memmert, model: IN55)
Software
KC4 data analysis software (BioTek, https://www.biotek.com/)
FlowJoTM Software Version 10.5.3 (Ashland, OR, https://www.flowjo.com/)
Procedure
Harvest of pulmonary vascular cells
Inject C57/BL6 mice with a low-dose of LPS (20 ng in 0.2 mL of 100 ng/mL solution in PBS) via the tail vein using a 27 G needle and a 1 mL syringe.
After 2 h, sacrifice the mouse by intraperitoneal pentobarbitone anesthetic overdose. Check the pedal reflex and confirm death by severing the femoral artery.
Carry out midline thoracotomy to expose the chest cavity, clamp down the rib cage to both sides, and pull the diaphragm down with a hemostat clamp.
Inject 20 units of heparin (0.1 mL of 200 units/mL solution) into the right ventricle via a 27 G needle and a 1 mL syringe.
Perform tracheostomy and instill 20 mL/kg of air with a syringe to inflate the lungs.
Pass a 4-0 curved-needle suture through the apex of the heart to secure it in place with clear visualization of both ventricles for subsequent cannulation (Figure 2, upper panel).
Loop a 5-0 suture under the twist of the pulmonary artery and aorta and throw a loose knot.
Loop a 2-0 suture under the apex of heart and throw a loose knot.
Poke a hole in the right ventricle with a 25 G needle and insert PVC tubing (OD 0.61 mm × ID 0.28 mm) into the pulmonary artery (soften tubing end by pulling the end gently and ensure tubing is filled with perfusate) (Figure 2, lower panel).
Tighten the 5-0 suture around the cannulated pulmonary artery, to secure the inflow cannulation.
Poke hole in the left ventricle with 19 G needle and insert polyethylene tubing (OD 1.27 mm × ID 0.86 mm) into the left ventricle, taking care not to rupture the heart.
Tighten the 2-0 suture around the apex of the heart to secure the outflow cannulation.
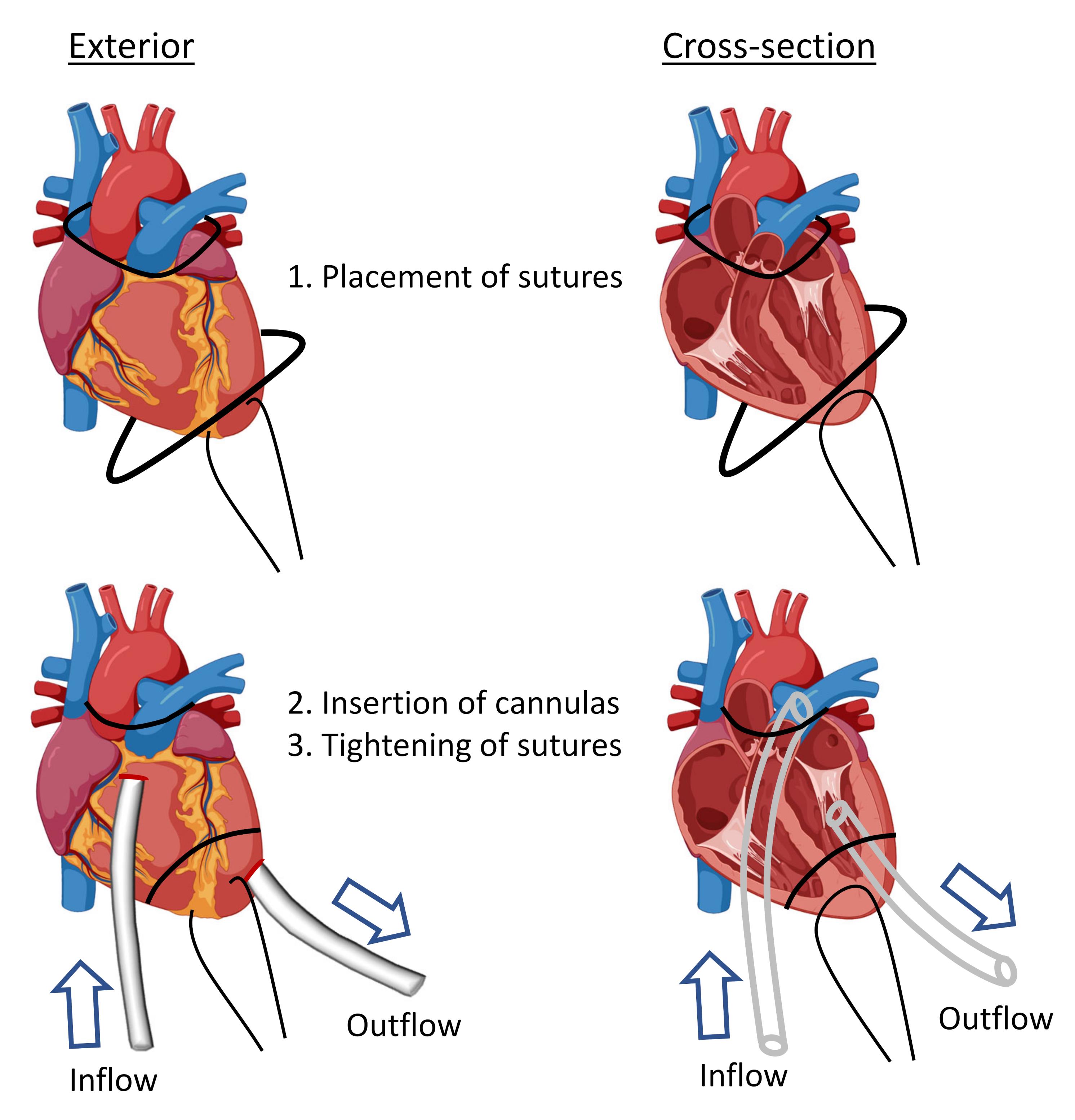
Figure 2. Diagram showing procedure for cannulation of pulmonary artery (perfusate inflow) and left ventricle (perfusate outflow). Created with BioRender.com.Start perfusion with perfusion buffer (see Recipes) in a syringe pump at 10 mL/h for 1 min, to observe the outflow of blood into a 15 mL collection tube on ice.
Increase perfusion to 50 mL/h for 12 min, to collect a 10 mL volume of pulmonary vascular cells.
Centrifuge cells at 300 × g for 10 min in a refrigerated benchtop centrifuge with swinging-bucket rotor.
To remove any residual plasma, wash perfusate cells twice with 5 mL of assay buffer (see Recipes).
Resuspend cells in assay buffer (~1 mL) and place on ice.
To analyze cell recoveries, pipette a small volume (e.g., 25 µL) into a 5 mL flow cytometry tube, and add an equal volume of fluorophore-conjugated antibody mix: anti-CD11b to identify granulocytes and monocytes; anti-F4/80 to identify monocytes; anti-Gr-1 to identify neutrophils and Ly6C-positive monocytes (Table 1). Incubate in a refrigerator for 30 min.
Wash cells with flow cytometry buffer (see Recipes).
Resuspend in flow cytometry buffer, add 10 µL of Accucheck counting beads, and analyze by flow cytometry.
Generation and DiD fluorescence labelling of MVs in vitro
Plate 4 × 106 J774A.1 cells in 60 × 15 mm culture dishes in a 5 mL volume of RPMI-1640/10% FBS. Incubate at 37°C, 5% CO2/air overnight.
Remove media and gently rinse adherent cells three times with 5 mL of PBS (with Ca2+/Mg2+).
Gently pipette 3 mL of ATP (3 mM) in PBS (with Ca2+/Mg2+) onto cells and incubate for 30 min at 37°C, 5% CO2/air. Extracellular ATP is used to induce rapid of release MVs from macrophages via activation of the P2X7 receptor signaling pathway.
Harvest supernatant into microfuge tubes, place on wet ice to cool, and centrifuge in an angle rotor refrigerated microfuge at 300 × g for 10 min at 4°C to remove cells.
Carefully collect supernatant using a 0.2 mL pipette and transfer to fresh tubes. Centrifuge in an angle rotor refrigerated microfuge at 20,800 × g for 30 min at 4°C to pellet MVs.
Carefully remove supernatant and discard. Wash again with 1 mL of assay buffer, and re-suspend MV pellet using 0.2 mL of assay buffer.
Add 40 µL of a 30 µM DiD/Diluent C stock solution to the MV suspension (final DiD concentration: 5 µM) and incubate for 7 min in the dark at room temperature. Diluent C is used as a diluent for lipophilic fluorescent dye labeling of cell membranes.
Wash MVs twice with 1 mL of assay buffer by centrifugation at 20,800 × g for 30 min at 4°C.
Carefully remove supernatant and re-suspend MV pellet in 0.2 mL of assay buffer.
Transfer 5 µL of MV suspension to a 96 well black plate and dilute with 90 µL of PBS. Lyse MVs by adding 5 µL of Triton X-100 (10%). Determine DiD fluorescence in a Biotek FLx800 plate reader at 635 nm/680 nm excitation/emission wavelengths.
Adjust MV concentration according to DiD fluorescence units (FU)/mL (see Data analysis).
MV uptake assay
Divide pulmonary vascular cells into microfuge tubes (1.5 mL) standardized to Ly-6Chigh monocyte density (1 × 105/mL) in a 0.5 mL volume.
Add DiD-labelled MVs to cells (see Data analysis).
Cover tubes with aluminum foil, place on a rotating wheel, and incubate at 37°C for up to 1 h.
Place tubes on wet ice to cool and then centrifuge in an angle rotor refrigerated microfuge at 300 × g for 10 min to pellet cells.
Discard the supernatant and wash cells twice at 300 × g for 10 min with cold PBS.
Incubate cells with fluorophore-conjugated anti-F4/80, anti-CD11b, anti-Ly6C and anti-Ly6G (Table 1) in a refrigerator for 30 min.
Wash cells with flow cytometry buffer.
Resuspend in flow cytometry buffer and analyze by flow cytometry.
Cell-associated DiD fluorescent is detected using the 635 nm excitation laser and FL8-665/20 nm emission channel of a Beckman Coulter CyAn ADP flow cytometer.
Data analysis
Analysis of perfusate cells by flow cytometry
After collection of perfusate and resuspension of cells in assay buffer, a small volume was removed for quantification of monocyte subsets and neutrophils. Using the antibody panel: anti-F4/80-FITC, anti-CD11b-PE, and anti-Gr-1-APC (Table 1), we identified neutrophils (CD11b+, Gr-1+, F4/80–), Ly-6Chigh monocytes (CD11b+, Gr-1+, F4/80+), and Ly-6Clow monocytes (CD11b+, Gr-1–, F4/80+) (Figure 3). Increased proportions of neutrophils and Ly-6Chigh monocytes were evident in lung perfusates of low-dose LPS treated mice compared to untreated mice, consistent with our previous findings of increased margination to the lungs following the same low-dose LPS treatment (O'Dea et al., 2009). Calculation of total cell numbers in perfusates indicated that recovery of Ly-6Chigh monocytes increased several-fold in LPS treated mice (n = 5, P < 0.01) (Figure 4). With a mean recovery of 4.8±1.3 × 105 Ly-6Chigh monocytes, it was possible to perform multiple in vitro tests using only a single LPS-treated mouse donor.
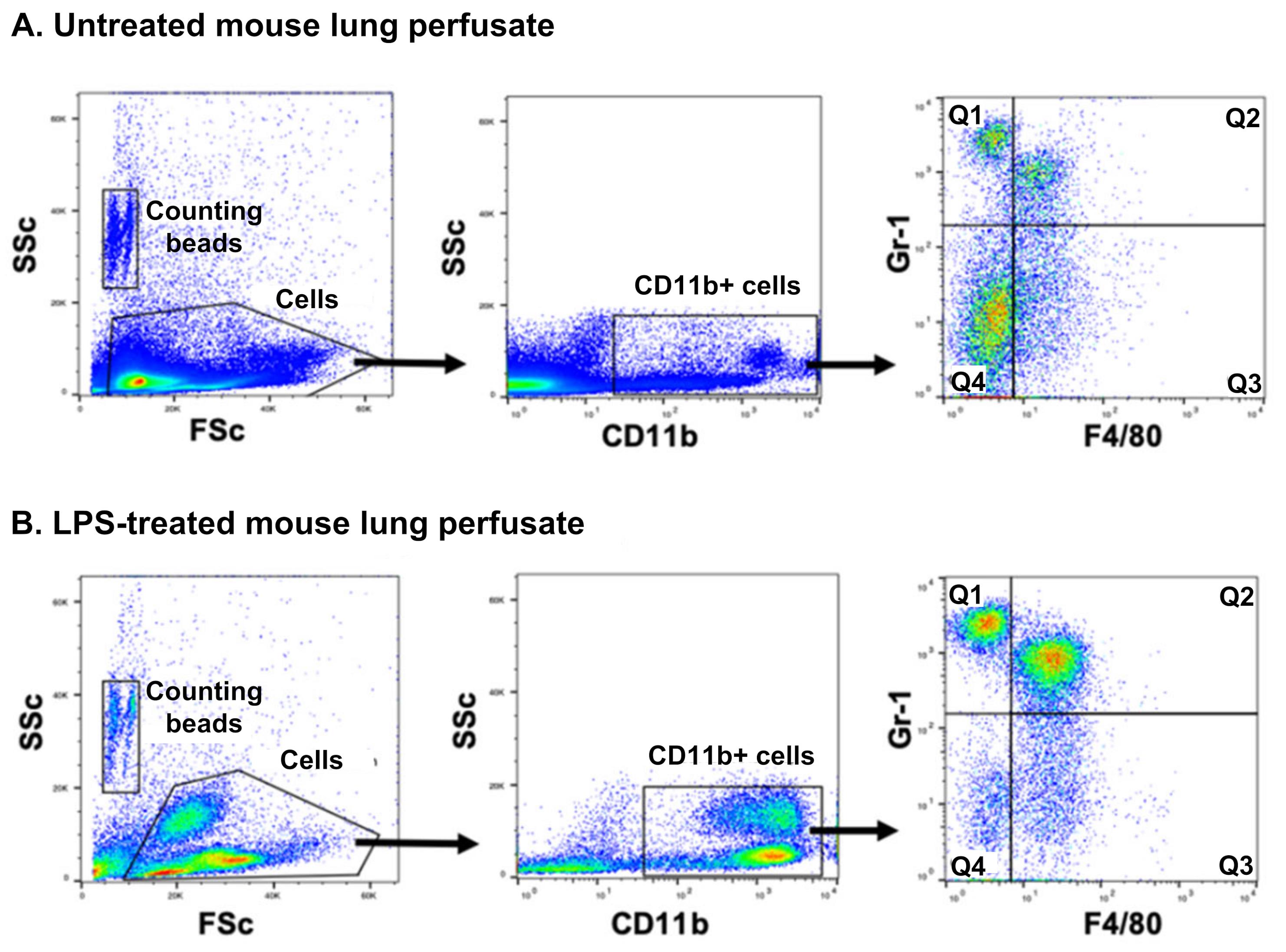
Figure 3. Flow cytometric analysis of myeloid leukocytes from pulmonary perfusates of untreated and LPS-treated mice. Pulmonary perfusates obtained from untreated and low-dose LPS (20 ng for 2 h)-treated mice were stained for CD11b, F4/80, and Gr-1 (anti-Ly6C and -Ly6G). Flow cytometry was used to distinguish each cell population based on their surface marker expression: monocytes were identified as CD11b+ and F4/80+, and their subsets as Gr-1+ (Q2), or Gr-1– (Q3), which differentiates them based on their Ly6C antigen expression. Neutrophils were identified as a CD11b+, F4/80–, and Gr-1+ population (Q1). Counting beads were used to calculate cell numbers in samples. Note that eosinophils (CD11b+) express F4/80, but are separated from F4/80+ monocytes by using a CD11b+/low side-scatter (SSc) parent gate (not shown).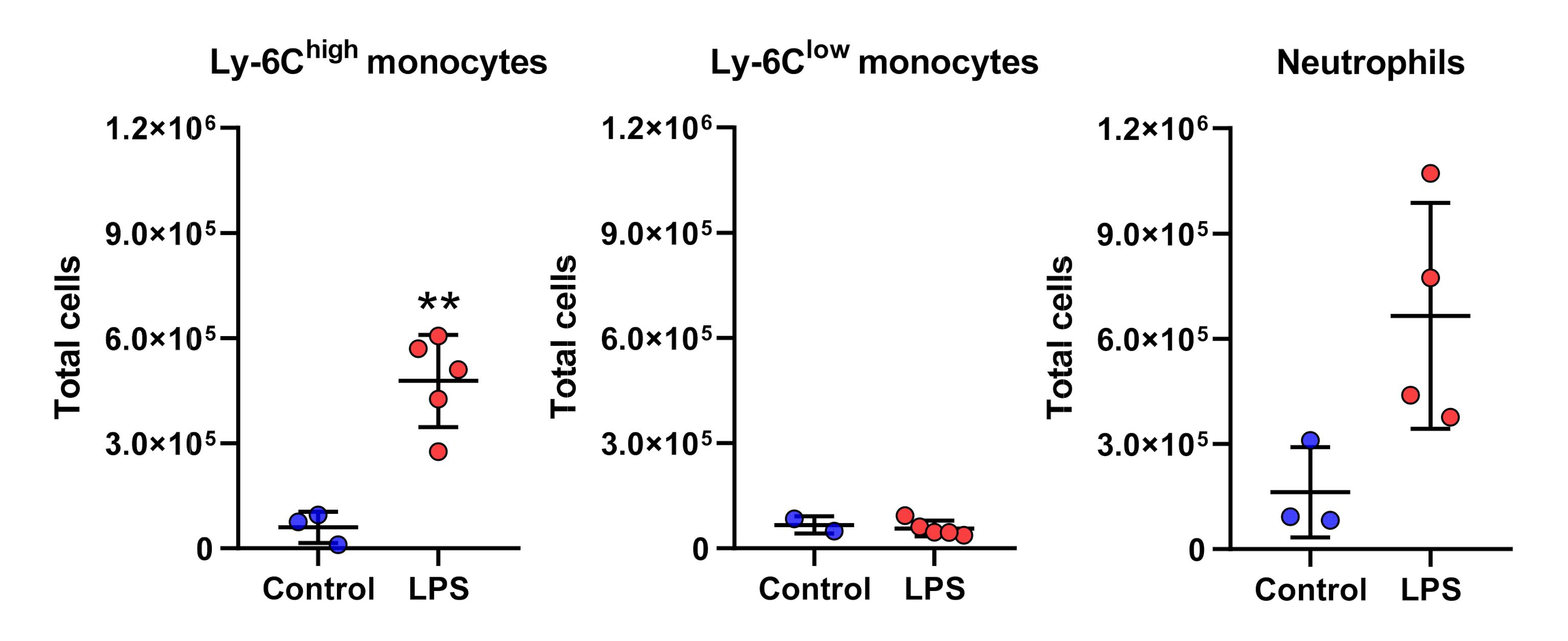
Figure 4. Total number of myeloid leukocytes recovered from pulmonary perfusates of untreated and LPS-pretreated mice. Ly-6Chigh monocytes, Ly-6Clow monocytes and neutrophils in the pulmonary perfusates from untreated and low-dose LPS (20 ng for 2 h)-treated mice were quantified by flow cytometry. Compared to untreated control mice, higher numbers of Ly-6Chigh monocytes were present in pulmonary perfusates of low-dose LPS-treated mice. Mean ± SD, n = 2-5, ** P < 0.01, two-tailed unpaired t-tests.MV generation and labeling
MVs were produced from the mouse J774.1 macrophage cell line, by ATP stimulation of the P2X7 receptor signaling pathway. This method produces early release of MVs that have surface marker expression profiles similar to those of the J774.1 parent cells, suggesting their direct derivation from the plasma membrane (O'Dea et al., 2020, see Figure 1B). Incorporation of the DiD label in the washed MV preparations was evaluated by anti-CD11b+ immunoaffinity separation of MVs (O'Dea et al., 2020, Figure 1C), with the majority of DiD fluorescence found to be associated with the CD11b+ MV fraction (>90%) and only minor amounts in the unbound fraction (<3%). Serial dilutions of DiD-labeled MV preparations indicated a linear relationship between total DiD fluorescence, measured using the fluorescence plate reader method, and DiD-positive MV counts, determined by flow-cytometry (O'Dea et al., 2020, Figure 1D). Using these protocols, 1,000 DiD fluorescence units were equivalent to ~5 × 105 MVs. Note that, because of MV size heterogeneity (~0.1-1.0 µm diameter) we prefer to standardize MV concentrations by DiD fluorescence rather than by MV counts.
MV uptake by perfusate cells
DiD-labelled MVs were added to perfusate cells standardized to a total of 5 × 104 Ly-6Chigh monocytes in 0.5 mL of assay media in microfuge tubes. Data for MV uptake dose responses and kinetics were reported previously (O'Dea et al., 2020, Supplementary Figure S2: C and D). For the final protocol, incubations were performed for 1 h with DiD-labeled MVs, used at a minimum concentration of 25,000 FU/mL (~1.25 × 107 MVs/mL), or a maximum of 100,000 FU/mL (~5 × 107 MVs/mL). MV uptake was compared in lung perfusates of untreated and LPS treated donor mice, using anti-Ly6C and anti-Ly6G antibodies, instead of the anti-Gr-1 antibody (Table 1), to identify monocyte subsets and neutrophils (Figure 5 & O'Dea et al., 2020, Figure 6A). As part of the uptake method evaluation, we performed side-by-side comparisons of DiD-labeled MV preparations versus insoluble DiD, prepared by incubation with assay buffer/diluent C and centrifugation without MVs. Despite incubating perfusate cells with equivalent amounts of DiD fluorescence (25,000 FU/mL), uptake of insoluble DiD was minimal (Figure 5C) compared to Ly-6Chigh monocyte uptake of DiD-labeled MVs (Figure 5B, R2), indicating that the minor contamination of MV preparations with unbound DiD dye (<3%, see above) is unlikely to have a significant impact on the measurement of DID-labeled MV uptake.
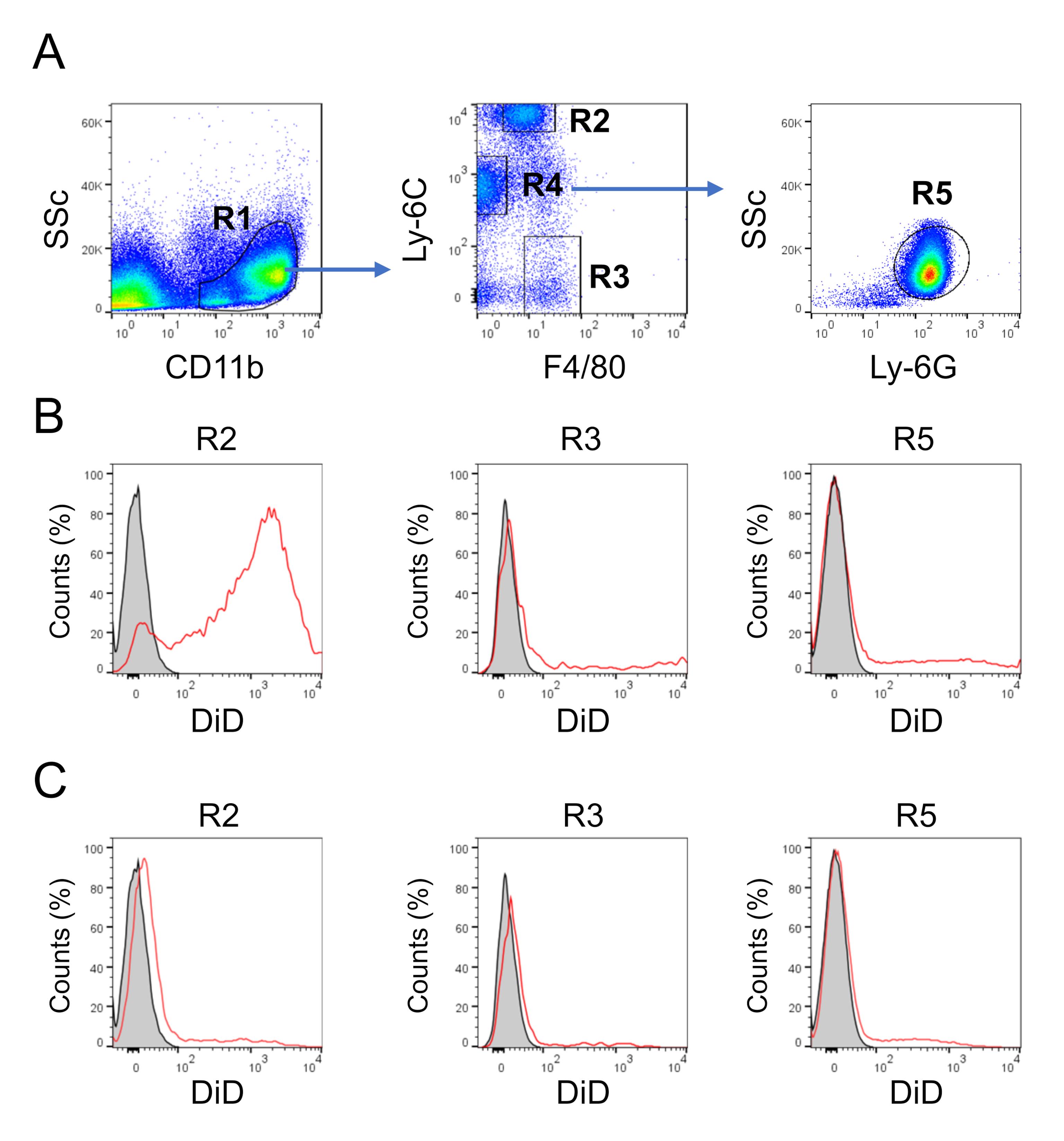
Figure 5. Flow cytometric analysis of MV uptake by myeloid perfusate cells. Perfusate cells were incubated with DiD-labeled MVs (25,000 FU/mL) for 1 h at 37°C with continuous mixing. A. Within CD11b+ gated events (R1), Ly-6Chigh monocytes were defined as F4/80+, Ly6C-high (R2), Ly-6Clow monocytes as F4/80+, Ly6C-low (R3), and neutrophils as F4/80-, Ly6C-medium (R4), Ly6G+ (R5). B. Representative histogram plots showing cell-associated DiD fluorescence for each cell population, after incubation alone (gray fill), or with DiD-labeled MVs (red line). C. Representative histogram plots showing cell-associated DiD fluorescence for each population, after incubation alone (gray fill) or with DiD prepared by incubation and centrifugation without MVs (red line). Note that an excess of the DiD only preparation was used (25,000 FU/mL) for comparison purposes, and that contamination of the DiD-labeled MV preparation with unbound DiD is normally <3% of the total fluorescence (O'Dea et al., 2020, Supplementary Figure S2C).This optimized assay allowed a detailed investigation of MV uptake mechanisms (O'Dea et al., 2020, Figure 6B and 6C) and elucidation of cell surface receptor-dependent uptake, using blocking reagents (O'Dea et al., 2020, Figure 7).
Recipes
Perfusion buffer
HBSS (without Ca2+/Mg2+)
4% HAS
Assay buffer
HBSS (with Ca2+/Mg2+) 0.5% HSA
Penicillin (100 units/mL)-streptomycin (10 µg/mL)
Flow cytometry buffer
Phosphate buffered saline (1×, prepared from a 10× stock)
2% FBS
0.1 mM sodium azide
2 mM EDTA
Acknowledgments
This work was supported by grants from the Chelsea and Westminster Health Charity, London, UK (Grant Number P67555) and the Medical Research Council/British Journal of Anaesthesia (Grant Number P54008).
This protocol was developed as part of the published article: O'Dea KP, Tan YY, Shah S, B VP, K CT, Wilson MR, Soni S, Takata M. Monocytes mediate homing of circulating microvesicles to the pulmonary vasculature during low-grade systemic inflammation. J Extracell Vesicles. 2020;9(1):1706708.
Competing interests
The authors declare no competing interests.
Ethics
All protocols were reviewed and approved by the UK Home Office in accordance with the Animals (Scientific Procedures) Act 1986, UK.
References
- Doherty, D. E. and Worthen, G. S. (1994). Lipopolysaccharide-induced monocyte retention in the lungs of rabbits. Role of cell stiffness and the CD11/CD18 leukocyte adhesion complex. Chest 105(3 Suppl): 108S.
- Hamon, P., Loyher, P. L., Baudesson de Chanville, C., Licata, F., Combadiere, C. and Boissonnas, A. (2017). CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood 129(10): 1296-1307.
- Joop, K., Berckmans, R. J., Nieuwland, R., Berkhout, J., Romijn, F. P., Hack, C. E. and Sturk, A. (2001). Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost 85(5): 810-820.
- Kuebler, W. M. and Goetz, A. E. (2002). The marginated pool. Eur Surg Res 34(1-2): 92-100.
- Nieuwland, R., Berckmans, R. J., McGregor, S., Boing, A. N., Romijn, F. P., Westendorp, R. G., Hack, C. E. and Sturk, A. (2000). Cellular origin and procoagulant properties of microparticles in meningococcal sepsis.Blood 95(3): 930-935.
- O'Dea, K. P., Young, A. J., Yamamoto, H., Robotham, J. L., Brennan, F. M. and Takata, M. (2005). Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am J Respir Crit Care Med 172(9): 1119-1127.
- O'Dea, K. P., Wilson, M. R., Dokpesi, J. O., Wakabayashi, K., Tatton, L., van Rooijen, N. and Takata, M. (2009). Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol 182(2): 1155-1166.
- O'Dea, K. P., Porter, J. R., Tirlapur, N., Katbeh, U., Singh, S., Handy, J. M. and Takata, M. (2016). Circulating microvesicles are elevated acutely following major burns injury and associated with clinical severity. PLoS One 11(12): e0167801.
- O'Dea, K. P., Tan, Y. Y., Shah, S., B, V. P., K, C. T., Wilson, M. R., Soni, S. and Takata, M. (2020). Monocytes mediate homing of circulating microvesicles to the pulmonary vasculature during low-grade systemic inflammation. J Extracell Vesicles 9(1): 1706708.
- Shi, C., Jia, T., Mendez-Ferrer, S., Hohl, T. M., Serbina, N. V., Lipuma, L., Leiner, I., Li, M. O., Frenette, P. S. and Pamer, E. G. (2011). Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34(4): 590-601.
- van Eeden, S. F., Kitagawa, Y., Klut, M. E., Lawrence, E. and Hogg, J. C. (1997). Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation 4(3): 369-380.
- Willekens, F. L., Werre, J. M., Kruijt, J. K., Roerdinkholder-Stoelwinder, B., Groenen-Dopp, Y. A., van den Bos, A. G., Bosman, G. J. and van Berkel, T. J. (2005). Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 105(5): 2141-2145.
- Wiklander, O. P., Nordin, J. Z., O'Loughlin, A., Gustafsson, Y., Corso, G., Mager, I., Vader, P., Lee, Y., Sork, H., Seow, Y., et al. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316.
- Yanez-Mo, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borras, F. E., Buzas, E. I., Buzas, K., Casal, E., Cappello, F., Carvalho, J., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066.
- Yipp, B. G., Kim, J. H., Lima, R., Zbytnuik, L. D., Petri, B., Swanlund, N., Ho, M., Szeto, V. G., Tak, T., Koenderman, L., et al. (2017). The Lung is a Host Defense Niche for Immediate Neutrophil-Mediated Vascular Protection. Science immunology 2(10).
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tan, Y. Y., O'Dea, K. P., Patel, B. V. and Takata, M. (2022). Investigation of Microvesicle Uptake by Mouse Lung-marginated Monocytes in vitro. Bio-protocol 12(3): e4307. DOI: 10.21769/BioProtoc.4307.
Category
Immunology > Immune cell isolation > Myeloid cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








