- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Induction of Repeated Social Defeat Stress in Rats
Published: Vol 12, Iss 3, Feb 5, 2022 DOI: 10.21769/BioProtoc.4306 Views: 3618
Reviewed by: Supriya GhoshSubhi MarwariAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 80 Views
Abstract
Repeated social defeat stress (RSDS) is a model of chronic stress in rodents. There are several variants of social defeat procedures that exert robust effects in mice, but few published detailed protocols to produce a robust stress and altered immunological profile in rats. In this article, we describe the protocol for the induction of RSDS in adult male Sprague-Dawley rats. Using a resident-intruder paradigm, a physical component of stress is induced by direct attack from the resident aggressive retired breeder Long-Evans rats on the intruder experimental rats. A subsequent threat component is induced by the presence of the aggressor in the vicinity of the intruder, but with physical separation between them. The RSDS induced by this protocol produces robust immunological and behavioral changes in the experimental rats, as evidenced by development of anxiety-like behaviors in open field, social interaction, and elevated plus maze tests, as well as by changes in immune parameters (Munshi et al., 2020). This approach has been used as an ethologically relevant model of stressors that are potent enough to impact neural circuits that are similar to the neural circuits impacted in patients with depression and anxiety.
Background
Repeated social defeat stress (RSDS) is a robust model of chronic psychological stress in rodents (Rygula et al., 2005; Berton et al., 2006; Liu et al., 2017). It causes robust depression-like behavioral changes including anxiety, anhedonia, and social-avoidance (Rygula et al., 2005 and 2006; Berton et al., 2006). A unique aspect of social defeat stress that distinguishes it from other environmental stressors is that the subjects do not develop habituation of pituitary-adrenal axis activity over repeated social confrontations (Tornatzky and Miczek, 1993; Koolhaas et al., 1997), unlike many other repeated stressors, even though the subjects generate behavioral stress responses (Tidey and Miczek, 1997).
Additionally, RSDS is known to have a robust effect on immune parameters that affect anxiety behavior in rodents (Wohleb et al., 2013). Furthermore, RSDS induces anxiety-like behavior by acting via interleukin-1 type 1 receptor (IL-1R1) in the endothelial cells in the brain (Wohleb et al., 2011). RSDS-induced macrophage trafficking in the brain is required for the development of anxiety behavior, which in turn is dependent on microglia activation and recruitment of primed monocytes to the brain (Wohleb et al., 2013). Myeloid cells isolated from mice undergoing RSDS have enhanced production of interleukins (IL) and other cytokines, such as IL-1β, TNF-α, and IL-6, following toll-like receptor stimulation with the immunogen lipopolysaccharide (Stark et al., 2002; Bailey et al., 2009; Powell et al., 2009).
Social defeat procedures have been leveraged to understand the neurobiology of anxiety or depressive behaviors by examination of its effects on limbic regions. RSDS has also been shown to decrease the firing of dopaminergic neurons projecting from the ventral tegmental area (VTA) to the medial prefrontal cortex (mPFC) in mice (Chaudhury et al., 2013). In addition, this kind of stress is also known to cause a persistent neuronal adaptation in the basolateral amygdala (BLA), hippocampus, and PFC; for example, RSDS caused a decrease in apical dendritic spine density in CA1 hippocampal neurons but not in BLA neurons in rats; it also caused dendritic atrophy of CA1 basal dendrites, while increasing dendritic arborization in BLA pyramidal neurons (Patel et al., 2018). A similar stress paradigm, called chronic social defeat stress (CSDS), has been shown to decrease spine density from apical dendrites of PFC pyramidal neurons but increase BLA dendritic arborization after one month (long-term); however, it also increased BLA stellate neuronal spike density in the short-term (Colyn et al., 2019).
There is significant evidence that some variations of social defeat can preferentially produce effects on appetitive motivated behaviors (described as rodent models of anhedonia/depressive behaviors; Riga et al., 2015; Yoshida et al., 2021) versus effects on open field and maze exploration (described as rodent models of anxiety). In this context, social defeat has shown a degree of predictive validity for antidepressant drugs (Tsankova et al., 2006; Golden et al., 2011). Similar social defeat stress paradigms have recently been studied in adolescent transgenic mice to explore genotype by environment interactions resulting in the development of phenotypes in psychiatric diseases in the “two-hit” model. For example, the mice overexpressing Tcf4 developed cognitive impairments and novelty-induced hyperactivity when exposed to social defeat stress (Volkmann et al., 2021). Social defeat stress has also been used in mice to test the pharmacological effects of psychotropic drugs to investigate stress-induced immune dysregulation. For example, one study has shown that the anti-asthenic drug bromantane (Ladasten) decreased depressive-like behavior by preventing development of avoidance behavior and also by improving locomotor activity after stress. Similarly, Ladasten also prevented the stress-induced shift of CD4/CD8 T-cells towards T-cytotoxic cells and normalized their ratio in the thymus, spleen and blood of mice (Tallerova et al., 2014).
While there are published protocols for social defeat in mice, social defeat in rats, particularly repeated social defeat, has its own set of challenges. There are few detailed protocols for a repeated social defeat procedure in rats that can reliably produce a stress phenotype and altered peripheral immune function. One purpose of this article is to fill this need. The RSDS model leverages ongoing social dynamics, instead of imposing circumstances that are unusual, which provides a degree of face validity, and can potentially provide more translatable information than less naturalistic stressors for understanding several of the physiological and behavioral abnormalities induced by social stress between individuals. In our original study (Munshi et al., 2020), we used the RSDS model in adult male Sprague-Dawley rats, following a previously published protocol (Jaisinghani and Rosenkranz, 2015). This has been adapted and modified from an earlier published study using the social defeat model of stress (Berton et al., 2006).
Subjects and housing: Adult male Sprague-Dawley rats (Envigo, Indianapolis, IN) were obtained at post-natal day 59-63 and were housed 2-3 per cage in a climate-controlled facility at Rosalind Franklin University of Medicine and Science, with ad libitum access to standard rat chow diet and water. After habituating in the animal facility for at least four days, rats were subjected to RSDS or control handling. The body-weights of the Sprague-Dawley rats were 300-350 g at the start of the experiments. Lights in the housing room were on a reverse 12 h light/dark schedule (lights off: 07:00-19:00). Sprague-Dawley rats in typical laboratory conditions are fairly social and rarely attack other rats, outside of play fighting or dams that are still lactating. Therefore, an alternative is required to induce defeat. Adult male retired breeder Long-Evans rats (Envigo, Indianapolis, IN) were single-housed and used to induce social defeat stress in the Sprague-Dawley rats. Rats were randomly assigned to experimental groups, and experiments were performed in multiple cohorts. The social defeat experiments were performed in a separate procedure room within the animal facility that was dedicated for stress procedures. This separate room was approximately 8 meters away from the housing room. The experiments were performed during the dark-phase of the rats’ diurnal cycle, between 9 am and 2 pm. Residents and intruders were not housed in the same cages, as is done for some social defeat procedures in mice. In our procedure, residents and intruders only interacted during the single daily stress sessions.
Because these experiments involve stress, it was particularly important to establish humane exclusion criteria that go beyond standard humane endpoints in consultation with our institutional veterinarian. Rats were monitored daily. This included daily weighing of the rats, observation of behavior (lethargy, extreme avoidance of the experimenter), and observation of appearance (coat appearance, presence of porphyrin). If any rat displayed a combination of >15% reduction in weight, persistent porphyrin staining, lethargy, or indication of non-grooming (matted fur), it would be removed from the study. Long-Evans rats were also monitored over the course of the social defeat procedures for the same measures (observation of behavior and of appearance).
Materials and Reagents
Clean paper towels
Intruder (experimental) adult male Sprague-Dawley rats
Resident (aggressor) adult male retired breeder Long-Evans rats
Scoring sheet (Table 1)
70% ethanol
Disinfectant wipes for equipment (for example, Caviwipes Surface Disinfectant Wipes, Metrex item# 13-1100)
Table 1. Scoring sheet.
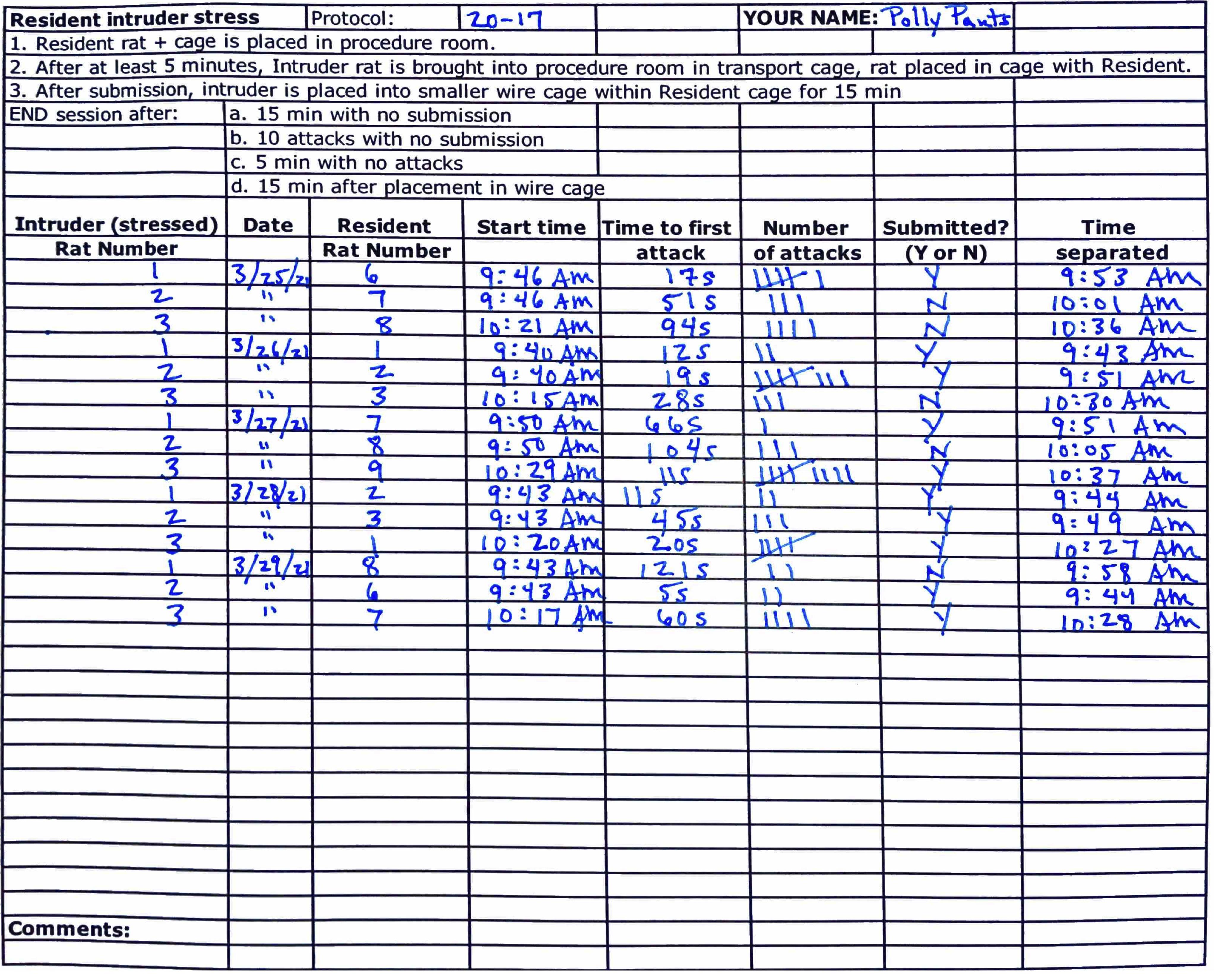
This is a standard scoring sheet that can be useful for the social defeat procedure. After submission, or if any criteria (a-c) are met, the Phase 1 session ends and the intruder rat is placed in the wire mesh enclosure. The Phase 2 session (separated by wire mesh enclosure) ends after 15 minutes. See completed sheet below for more details (Table 2).Resident Intruder Stress Protocol: Your Name: Resident rat + cage is placed in procedure room After at least 5 min, the Intruder rat is brought into the procedure room in a transport cage; the rat is placed in the cage with the Resident. Timer is started. After submission, the Intruder is placed into a smaller wire mesh enclosure within the Resident cage for 15 min END Phase 1 session after submission or:
a. 15 min with no submission
b. 10 attacks with no submission
c. 5 min with no attacks
END Phase 2 after:
a. 15 min after placement in wire mesh enclosureIntruder (Stressed) Rat Number Date Resident Rat Number Start Time Time to First Attack Number of Attacks Submitted? (Y or N) Time Separated
Comments:
Equipment
Resident rat housing cages: sanitized woodchip bedding, 21” × 11.5” × 8” height, clear polycarbonate, wire top
Rat transport cages: sanitized woodchip bedding, 12” × 6.5” × 5” height, clear polycarbonate, microisolator top
Smaller wire mesh enclosure (Figure 3): approximately 6” × 7” × 8” height, 3/4” square mesh, PVC coated
Digital stopwatch with time of day
Procedure
RSDS by the resident-intruder paradigm is a robust model of chronic psychological stress in rodents (Rygula et al., 2005; Berton et al., 2006; Liu et al., 2017). In our study, housing cages of rats were randomly assigned to the stressed and control groups.
Note: Most Long-Evans retired breeders are aggressive in our conditions, but some are not. You can screen these rats prior to experiments, using the same resident-intruder approach described below, to select more or less aggressive rats depending on your needs. If more aggressive resident behavior is required, pre-screening criteria may include short latency to attack and multiple attacks within the first minute of the session. In our experiments, the retired breeders displayed reduced attacks over the course of weeks-months. If a retired breeder displayed no attacks, it might be removed from the rotation of aggressive residents.
The housing cages of the resident aggressor Long-Evans rats, containing one Long-Evans rat per cage, were transported to a dimly lit procedure room free from noise within the animal facility on the morning of the experiments. They were left undisturbed for acclimation in the environment for 15-30 min.
Note: In our experience, a single experimenter can perform this procedure with up to 3 resident-intruder interactions at the same time (i.e., 3 resident cages in the procedure room). More than this becomes difficult to observe by one individual.
Experimental Sprague-Dawley rats were weighed daily in their housing room and their body weight was noted. Animal condition was also noted, with attention to porphyrin around the eyes, nose, or fur.
After weighing, the Sprague-Dawley rats were placed in clean transport cages.
Control rats (housed together) remained in transport cages for 30 min, then they were returned to their home cage.
Rats in the stress group were transported to the procedure room in clean transport cages separate from the controls.
PHASE 1 (Direct physical contact): Individual Sprague-Dawley rats were transferred to the housing cage of a resident Long-Evans rat in the procedure room and the lid of the cage was secured tightly. This allowed the intruder rat to stay in direct physical contact with the resident rat (Figure 1). The time of this event was noted. The rats were continuously observed for the entire duration of the interaction.
All scoring of a cohort of rats was performed by one trained experimenter. The experimenter was positioned at a distance that allows clear observation of the rats and rapid intervention, but far enough that rats do not direct attention towards the experimenter (approximately 1-2 meters). The measures can be reliably obtained by one experimenter, and we therefore did not routinely video sessions.
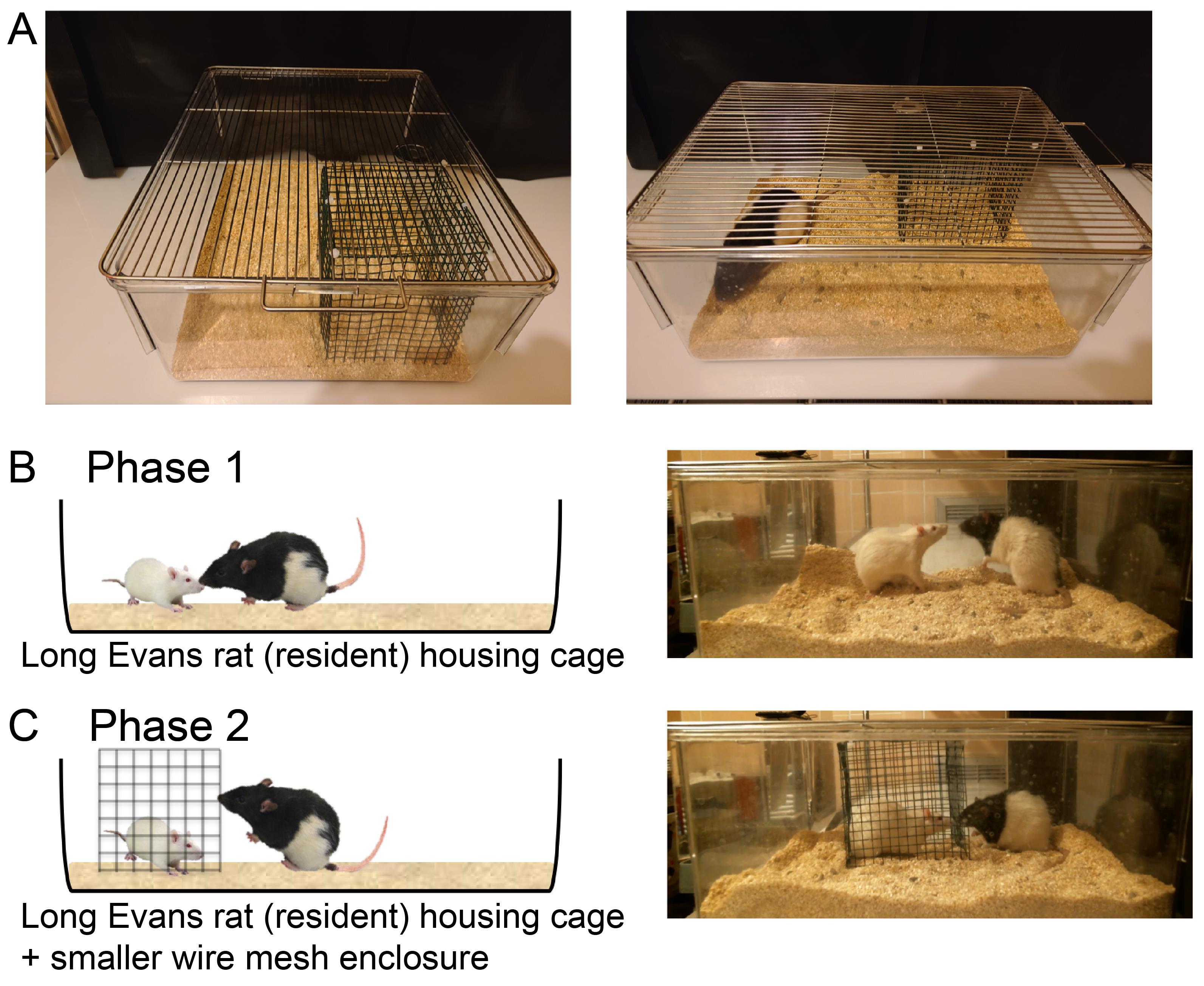
Figure 1. Setting up the resident intruder procedure. A. Photographs of the resident cage with the wire mesh enclosure (green) placed inside (left). Also shown with resident present (right) for scale. B. Phase 1 of the stress procedure, when resident and intruder can have direct physical contact. C. Phase 2 of the stress procedure, when resident and intruder are separated by the wire mesh enclosure.During the period of direct physical contact, every attack made by the aggressor rat on the intruder was scored manually by a trained observer, including noting down the time of first attack (Table 2). A defeat was scored when the intruder rat submitted to the attack of the aggressor by lying down and exposing the ventral surface (abdomen; Figure 2C).
This direct physical contact was allowed for a maximum of 15 min each session. The experimental rat was separated with the wire mesh enclosure prior to 15 min if any of the following criteria was met: (i) submission of the intruder; (ii) ten attacks with no submission; (iii) 5 min with no attack; or (iv) any attack that severely wounded the experimental rat. Thus, it was possible for a rat to be removed without demonstrating submission each day. Parameters can be modified if experimental design calls for daily defeat.
PHASE 2 (Physical separation): The intruder was separated within the resident rat’s cage using a smaller wire mesh enclosure for an additional 15 min (Figure 1C, Figure 2C). The time of physical separation was noted.
Note: The wire mesh enclosure used for physical separation was spacious enough not to cause any form of physical restraint to the movements of either the intruder rat inside or the resident rat outside the mesh enclosure. This was done in order to remove only the physical component of the social stress in Phase 2, without affecting the sensory (visual, auditory, and olfactory) components associated with the stressors (aggressor rats).
All surfaces were thoroughly cleaned with 70% ethanol between experiments, including wire mesh enclosures.
This same procedure was repeated for five consecutive days, once per day for each intruder rat. Resident-intruder pairings were cycled such that each intruder experienced a novel resident each day.
Notes:
a. Resident rats were limited to three daily sessions. After three daily sessions, or after extended periods of time (weeks to months), resident retired breeder rats tend to display less aggression. It has also been noted that resident rats tend to be less aggressive on the day of changes to a clean housing cage.
b. If a rat were to get badly wounded during an attack (eye damaged, large wound) it would be removed and humanely euthanized. In some instances, a rat might receive a wound that is not severe (e.g. <2 cm to flank, without apparent muscle damage). If this occurs, the wound should be flushed with sterile saline followed by application of antibiotic+analgesic cream (e.g. Neosporin dual action cream). The rat should be monitored daily. If the wound does not close or otherwise show significant improvement within two days, the rat should be euthanized.
c. If your endpoint includes immunological measures, you might consider removal of any rat that has been scratched, and pre-screening resident rats for those that do not tend to cause any wounds.
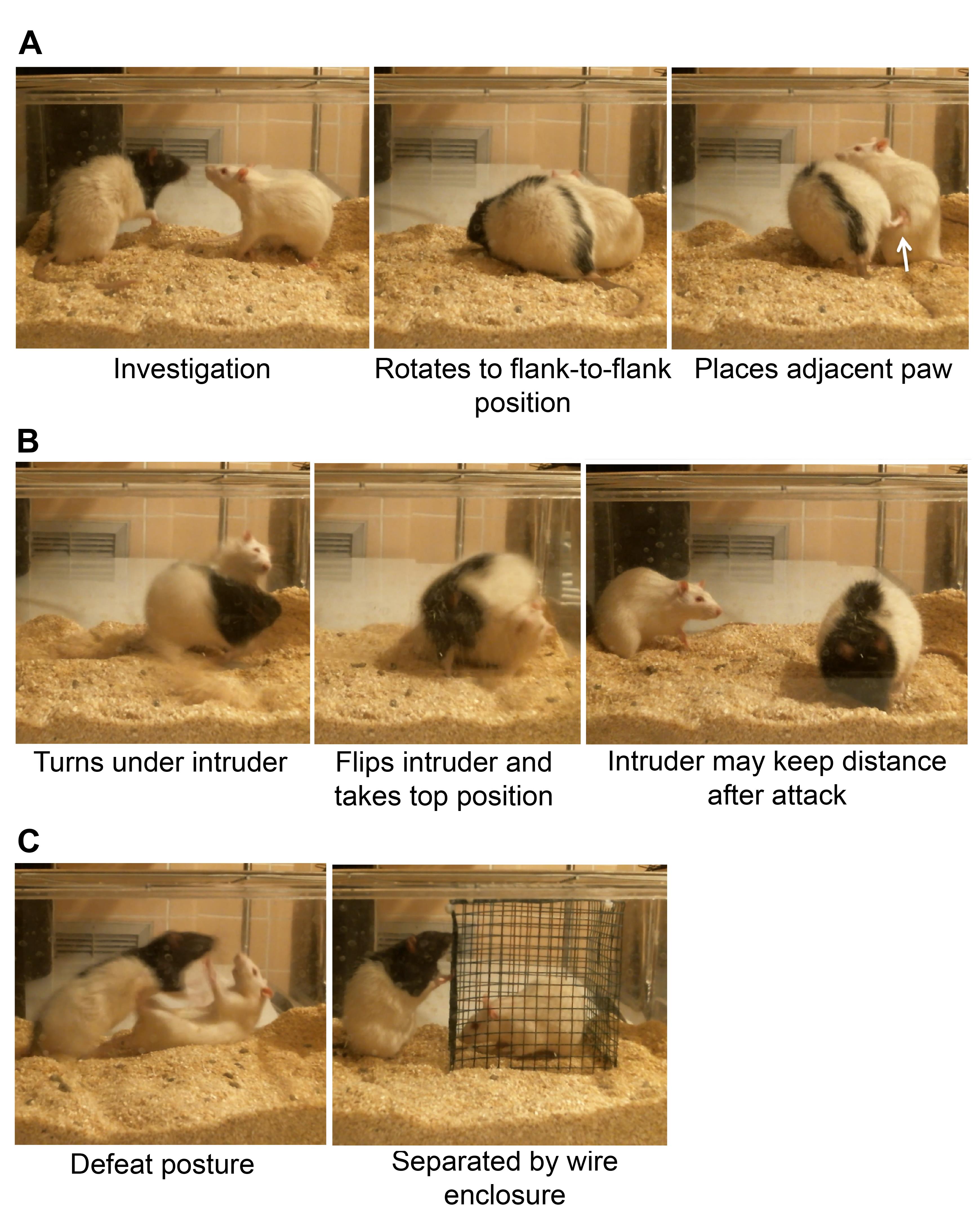
Figure 2. Postures during attack and defeat. A. Examples of social interaction, followed by a common posture that signals an imminent attack: the resident rat rotates so it is flank-to-flank with the intruder and then lifts the hind leg adjacent to the intruder, to maintain position before it enters the next step. B. The next steps that escalate into the most common attack. The resident rat twists its body while keeping the hind leg close to the intruder, and then turns under the intruder. This flips the intruder over, with the resident on top. In other less common instances, the resident rat may initiate and continue an attack from the top position, for instance when both rats are in an upright boxing stance (not shown). After attacks, the intruder may avoid the resident rat. C. After the experience, the intruder rat shows a typical defeat posture. At this point in the protocol, the rats are separated with a wire mesh enclosure.Table 2. Example of a completed scoring sheet for a five day repeated social defeat protocol of three intruder rats (rats 1-3) that were exposed to a rotation of resident rats (rats 1-3, and 6-9).
Instructions to make the wire mesh enclosure
There are commercially available enclosures for pets that may be suitable. We have never tried those options and have instead made our enclosures from supplies available at most hardware stores. There are a range of suitable options for materials to make a wire mesh enclosure. We chose a PVC-coated steel mesh, because it is easy to cut, bend to the desired shape, and can be readily cleaned. A mesh opening of 0.5-1 inch is suitable for our purposes, because this is too small for the resident rat to effectively attack through. We chose a mesh of 16 gauge because of its durability and weight. The final dimensions are approximately 6 × 7 × 8 inches.
Materials
Heavy duty wire cutters
Pliers
Coated wire mesh (for example, item 9259T11 from McMaster-Carr; www.mcmaster.com)
Zip ties (for example, item 6705K35 from McMaster-Carr; www.mcmaster.com)
Steps:
1. Cut the wire mesh to pattern (Figure 3).
2. Bend where indicated to form an open cube.
3. Fasten corners together with zip ties. Trim the ends of zip ties.
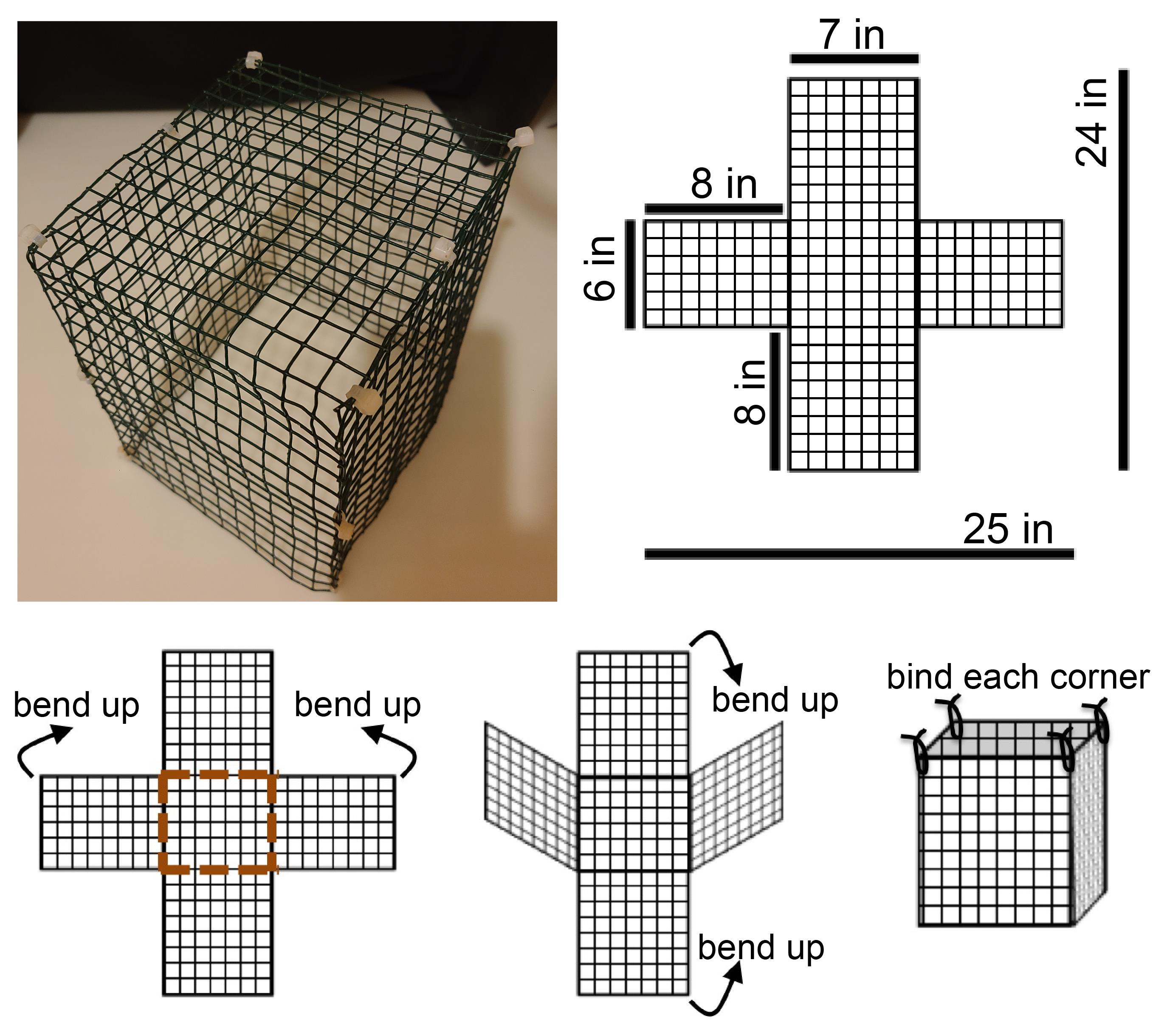
Figure 3. Photograph of completed wire mesh enclosure. This enclosure has lasted eight years of use with minimal damage. A template and instructions are shown here.
Data analysis
Exclusion criteria: The intensity of the repeated stress experience can be varied, depending on the experimental needs. In our studies, rats had to exhibit at least one instance of defeat, demonstrated by a passive defeat posture (see Figure 2). Experimental rats were excluded if they received a severe injury (see Step 10 Notes, under the “Procedure” section). Resident rats were excluded if they exhibited two sessions without attacks, or if they consistently attacked with a severity that produced injury.
During the period of direct physical contact, every attack made by the aggressor rat on the intruder was scored manually by a trained observer, including noting down the time of first attack and the time of physical separation (Table 2). The following parameters were analyzed: number of attacks made by the aggressor on the intruder rats, whether rats submitted, and if so, time of submission by the intruder rat (i.e., latency to submit, which will be the same as the “Time separated” in our score sheet), and the number of rats that submitted daily.
Conclusion
In this article, we discussed the protocol for the induction of RSDS in rats that we used in our study, showing that RSDS is able to induce robust immunological and behavioral changes in rats (Munshi et al., 2020). This builds on many prior studies that have successfully achieved social defeat in rats and mice.
Acknowledgments
The authors gratefully thank Matthew Anagnostopoulos and his team at the Biological Resource Facility at Rosalind Franklin University of Medicine and Science for taking care of the animals used in the study. The study was supported by the National Institutes of Health grants MH084970 and MH109484. The funding body had no role in the design of the study, collection and analysis of data, or decision to publish. The protocol is based on the original research article by Munshi et al. (2020) published in Brain, Behavior, and Immunity.
Competing interests
The authors declare no competing interests.
Ethics
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Rosalind Franklin University of Medicine and Science and were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
References
- Berton, O., McClung, C. A., Dileone, R. J., Krishnan, V., Renthal, W., Russo, S. J., Graham, D., Tsankova, N. M., Bolanos, C. A., Rios, M., Monteggia, L. M., Self, D. W. and Nestler, E. J. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762): 864-868.
- Bailey, M. T., Kinsey, S. G., Padgett, D. A., Sheridan, J. F. and Leblebicioglu, B. (2009). Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav 98(3): 351-358.
- Chaudhury, D., Walsh, J. J., Friedman, A. K., Juarez, B., Ku, S. M., Koo, J. W., Ferguson, D., Tsai, H. C., Pomeranz, L., Christoffel, D. J., et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493(7433): 532-536.
- Colyn, L., Venzala, E., Marco, S., Perez-Otano, I. and Tordera, R. M. (2019). Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res 373: 112079.
- Golden, S. A., Covington, H. E., 3rd, Berton, O. and Russo, S. J. (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8): 1183-1191.
- Jaisinghani, S. and Rosenkranz, J. A. (2015). Repeated social defeat stress enhances the anxiogenic effect of bright light on operant reward-seeking behavior in rats. Behav Brain Res 290: 172-179.
- Koolhaas, J. M., De Boer, S. F., De Rutter, A. J., Meerlo, P. and Sgoifo, A. (1997). Social stress in rats and mice. Acta Physiol Scand Suppl 640: 69-72.
- Liu, Y. Y., Zhou, X. Y., Yang, L. N., Wang, H. Y., Zhang, Y. Q., Pu, J. C., Liu, L. X., Gui, S. W., Zeng, L., Chen, J. J., et al. (2017). Social defeat stress causes depression-like behavior with metabolite changes in the prefrontal cortex of rats. PLoS One 12(4): e0176725.
- Munshi, S., Loh, M. K., Ferrara, N., DeJoseph, M. R., Ritger, A., Padival, M., Record, M. J., Urban, J. H. and Rosenkranz, J. A. (2020). Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav Immun 84: 180-199.
- Powell, N. D., Bailey, M. T., Mays, J. W., Stiner-Jones, L. M., Hanke, M. L., Padgett, D. A. and Sheridan, J. F. (2009). Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav Immun 23(2): 225-231.
- Patel, D., Anilkumar, S., Chattarji, S. and Buwalda, B. (2018). Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav Brain Res 347: 314-324.
- Riga, D., Theijs, J. T., De Vries, T. J., Smit, A. B. and Spijker, S. (2015). Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior. Front Behav Neurosci 9: 195.
- Rygula, R., Abumaria, N., Flugge, G., Fuchs, E., Ruther, E. and Havemann-Reinecke, U. (2005). Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162(1): 127-134.
- Rygula, R., Abumaria, N., Domenici, E., Hiemke, C. and Fuchs, E. (2006). Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res 174(1): 188-192.
- Stark, J. L., Avitsur, R., Hunzeker, J., Padgett, D. A. and Sheridan, J. F. (2002). Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol 124(1-2): 9-15.
- Tallerova A. V.,Kovalenko L. P., Tsorin I. B., Durney A. D. and Seredenin S. B. (2014). Effects of the Novel Anti-Asthenic Drug Ladasten on Behavior and T-Cell Subsets Alterations in a Social Defeat Animal Model of Depression.Pharmacol Pharm 5(1): 4-10.
- Tornatzky, W. and Miczek, K. A. (1993). Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav 53(5): 983-993.
- Tidey, J. W. and Miczek, K. A. (1997). Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 130(3): 203-212.
- Tsankova, N. M., Berton, O., Renthal, W., Kumar, A., Neve, R. L. and Nestler, E. J. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9(4): 519-525.
- Volkmann, P., Stephan, M., Krackow, S., Jensen, N. and Rossner, M. J. (2020). PsyCoP - A Platform for Systematic Semi-Automated Behavioral and Cognitive Profiling Reveals Gene and Environment Dependent Impairments of Tcf4 Transgenic Mice Subjected to Social Defeat. Front Behav Neurosci 14: 618180.
- Wohleb, E. S., Hanke, M. L., Corona, A. W., Powell, N. D., Stiner, L. M., Bailey, M. T., Nelson, R. J., Godbout, J. P. and Sheridan, J. F. (2011). β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31(17): 6277-6288.
- Wohleb, E. S., Powell, N. D., Godbout, J. P. and Sheridan, J. F. (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33(34): 13820-13833.
- Yoshida, K., Drew, M. R., Kono, A., Mimura, M., Takata, N. and Tanaka, K. F. (2021). Chronic social defeat stress impairs goal-directed behavior through dysregulation of ventral hippocampal activity in male mice. Neuropsychopharmacology 46(9): 1606-1616.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Munshi, S., Ritger, A. and Rosenkranz, J. A. (2022). Induction of Repeated Social Defeat Stress in Rats . Bio-protocol 12(3): e4306. DOI: 10.21769/BioProtoc.4306.
Category
Neuroscience > Nervous system disorders > Animal model
Biological Sciences > Biological techniques
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








