- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation and Cultivation of Colonic and Small Intestinal Murine Organoids Including Analysis of Gene Expression and Organoid Viability
(*contributed equally to this work) Published: Vol 12, Iss 2, Jan 20, 2022 DOI: 10.21769/BioProtoc.4298 Views: 7453
Reviewed by: Takashi NishinaSalah Boudjadi

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3760 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 93 Views
Abstract
Organoids are complex three-dimensional structures, which contain different cell types and help to overcome many limitations of conventional 2D cell culture techniques. Here, we present a protocol for the cultivation of murine matched-pairs of small intestinal and colonic epithelial organoids, and colonic tumor organoids derived from the chemical colorectal cancer (CRC) AOM/DSS mouse model. Therefore, intestinal crypts or tumor tissue containing stem cells are isolated from the same donor mouse and cultivated in Matrigel®. The culture medium is supplemented with different growth factors to model the intestinal stem cell niche, allowing their self-renewal and differentiation. Matched-pair organoids enable the analysis of pharmacological effects and the tumor selectivity of drugs.
Graphic abstract:
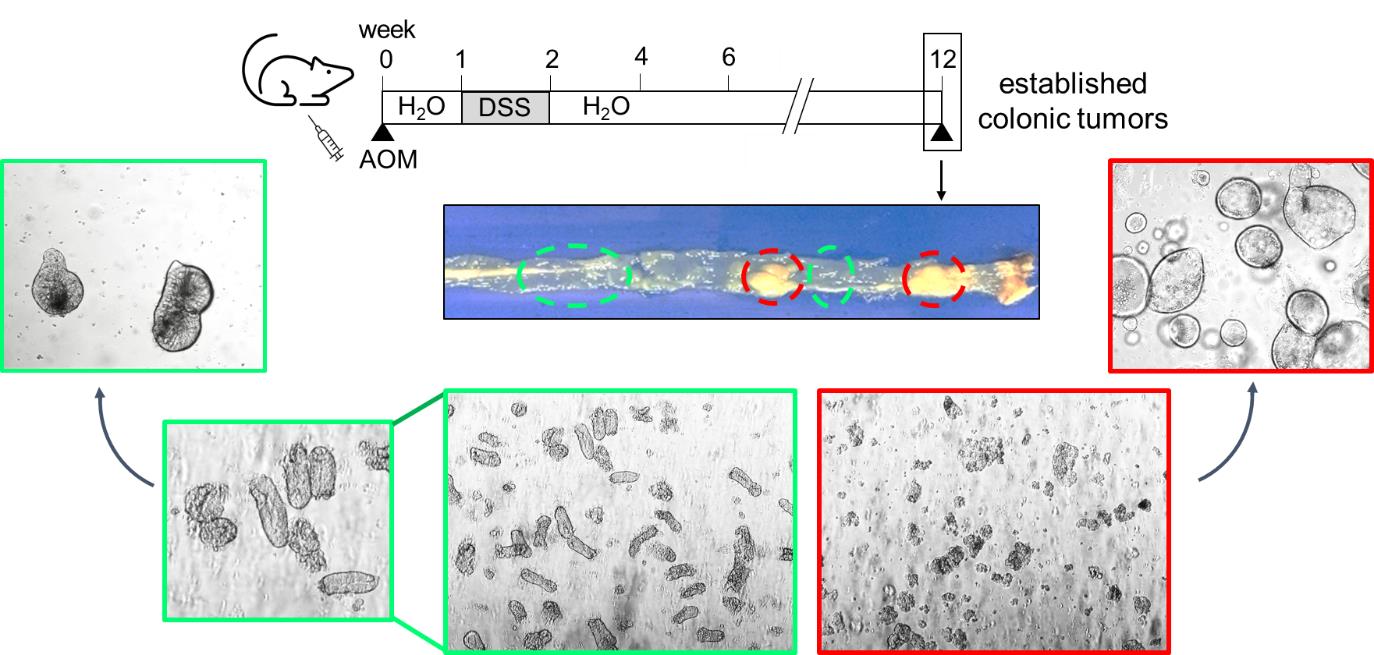
Schematic overview of colonic matched pair organoid preparation, generated from the chemical AOM/DSS colorectal cancer mouse model. Please note that normal colon-derived organoids (green) differ in their morphology from tumor-derived organoids (red). Normal colonic-derived organoids display a thicker and crypt-like epithelial layer, whereas tumor-derived organoids are round with a thin epithelial layer.
Background
Organoids are three dimensional structures, which are derived from pluripotent stem cells or organ restricted stem cells. They have the ability for self-organization and differentiation, mirroring key functional and structural properties of the respective organ (Clevers, 2016). Over the past decades, organoid culture became an important model in the fields of personalized and targeted therapies as well as for the prediction of drug responses (Lancaster and Knoblich, 2014; Clevers, 2016). Hence, organoids have become indispensable for many fields of biomedical and translational research (Wallach and Bayrer, 2017; Fair et al., 2018; Almeqdadi et al., 2019).
In the context of colorectal cancer (CRC), we provide a protocol to generate murine matched-pair organoids, to evaluate the cytotoxicity and tumor selectivity of certain cancer drugs.
Intestinal organoids were first established by Sato et al. (2009) from small intestinal stem cells for long-term culture in a collagen matrix. The technique was then further developed by the same research group, enabling organoid cultures from normal epithelium and tumor tissue from the colon (Sato et al., 2011).
Based on previously published protocols for intestinal organoids (O’Rourke et al., 2016; https://www.stemcell.com/technical-resources/intestinal-epithelial-organoid-culture-with-intesticult-organoid-growth-medium-mouse-lp.htmL), we summarize our modified step-by-step protocol for the generation of murine matched pair organoids. In contrast to previous protocols, we used three different cell lines to generate Wnt3a, Noggin, and Rspondin-1 conditioned medium. These components are essential for proper self-renewal and proliferation of the stem cell pool. Furthermore, we provide detailed protocols for further analysis of drug-treated organoids, such as RNA and protein isolation, morphological quantification, membrane permeability staining using propidium iodide/ Hoechst 33342, and an ATP-based cell viability assay (CellTiter-Glo® 3D).
Materials and Reagents
6 well clear TC-treated multiple well plates (Costar, catalog number: 3516)
12 well clear TC-treated multiple well plates (Greiner, catalog number: 665180)
24 well clear TC-treated multiple well plates (Corning, catalog number: 353504)
96 well black/clear flat bottom TC-treated imaging microplate with Lid (Corning, catalog number: 353219)
150 mL bottle top vacuum filter, 0.22 µm pore (Corning, catalog number: 431161)
Cannulas, Braun, Sterican® Gr. 18, G 26 x 1""/ ø 0.45 x 25 mm
Cell culture flasks, 550 mL, 175 cm2 (Greiner, catalog number: 660175)
Cell strainer Nylon mesh 100 µm (BD Biosciences, catalog number: 352360)
Cover glasses (Merck, catalog number: C9802-1PAK)
Cryotube vials 1.8 mL (Thermo Scientific, catalog number: 377267PK)
Oral gavage needle (GLOMED Inc., catalog number: AFN2038S)
Cutting board or styrofoam board
Petri dish (Sarstedt, catalog number: 82.1472.001)
Pipette tip, 10 µL (Sarstedt, catalog number: 70.1130.600)
Pipette tip, 200 µL (Sarstedt, catalog number: 70.760.502)
Pipette tip, 1,000 µL (Heinemann, catalog number: PSB)
Portable Pipet-Aid® XP (Drummond, catalog number: 4-000-201)
Serological pipette, plugged, 10 mL (Sarstedt, catalog number: 86.1254.025)
Sterile single use bottle top filters (Thermo Scientific, catalog number: 596-4520)
Surgical blades (Swann Morton, catalog number: 0206)
Transfer pipette (Sarstedt, catalog number: 86.1171.010)
Tube, 50 mL (Labsolute, catalog number: 7696705)
Razor blades (Fisher Scientific, catalog number: 12-640)
Reaction vials, 1.5 mL (Roth, catalog number: 7080.1)
Reaction vials, 2 mL (Roth, catalog number: 7083.1)
Cultrex HA-R-Spondin1-Fc 293T Cells (R&D Sytems, catalog number: 3710-001-01)
HEK293T mWnt3a cells (kindly provided by Robyn Laura Kosinsky)
HEK293T mNoggin cells (kindly provided by Tiago De Oliveira; published in Farin et al., 2012)
A83-01 (Merck Millipore, Sigma-Aldrich, catalog number: SML0788)
Adenosine 5′-triphosphate disodium salt hydrate (ATP, Sigma-Aldrich, catalog number: A2383-1G)
Advanced DMEM/F-12 (Thermo Fisher Scientific, Gibco, catalog number: 12634010)
Azoxymethane (AOM, Sigma-Aldrich, catalog number: A5486)
Dextran sodium sulfate (MP Biomedicals, catalog number: 160110)
B-27TM supplement (Thermo Fisher Scientific, Gibco, catalog number: 17504044)
BCA protein assay (Pierce, catalog number: 23227)
Bovine serum albumin (Roth, catalog number: 8076.3)
Cell recovery solution (Corning, catalog number: 354253)
CellTiter-Glo® 3D cell viability assay (Promega, catalog number: G9682)
CHIR 99021 (Axon Medchem, catalog number: 1386)
Collagenase type I (Gibco, catalog number: 17018)
cOmpleteTM mini protease inhibitor cocktail (Roche, catalog number: 11836170001)
DMEM, high glucose, GlutaMAXTM (Thermo Fisher Scientific, Gibco, catalog number: 10566016)
DMSO (AppliChem, catalog number: A3672,0100)
Dual-Glo® Luciferase Assay System (Promega, catalog number: E2920)
M50 Super 8× TOPFlash (Addgene, catalog number: 12456)
M51 Super 8× FOPFlash (TOPFlash mutant) (Addgene, catalog number: 12457)
pRL-TK vector (Promega, catalog number: E2241)
EDTA (Roth, catalog number: 8040.3)
Ethanol (Roth, catalog number: 8388.3)
Fetal bovine serum (Anprotec, catalog number: AC-SM-0190)
GeneticinTM, G418 (InvivoGen, catalog number: ant-gn-1)
GlutaMAXTM supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
Goat anti-Human IgG (H+L) Secondary Antibody, HRP (Invitrogen, catalog number: 31410)
HEPES (Thermo Fisher Scientific, Gibco, catalog number: 15630080)
Hoechst 33342 Trihydrochloride Trihydrate (Invitrogen, catalog number: H3570)
Hydrochloric acid 25% (Merck, catalog number: 1003122500)
Imidazole (Roth, catalog number: 3899.2)
Immobilon Western Chemiluminescent HRP Substrate (ECL) (Merck Millipore, catalog number: WBKLS0500)
L-Glutamine (Gibco, catalog number: 25030123)
LipofectamineTM 3000 Transfection Reagent (Invitrogen, catalog number: L3000015)
Matrigel® Matrix GFR (Corning, catalog number: 354230)
N-2 supplement (Thermo Fisher Scientific, Gibco, catalog number: 17502048)
N-Acetyl-L-Cysteine (Merck Millipore, Sigma-Aldrich, catalog number: A9165)
Nicotinamid (Merck Millipore, Sigma-Aldrich, catalog number: N3376)
Nitrocellulose membrane (Merck, catalog number: GE10600001)
Powdered milk (Roth, catalog number: T145.4)
Normal mouse IgG1 (Santa Cruz, catalog number: SC-3877)
InVivoMAb human IgG1 isotype control (bxcell, catalog number: BE0297)
Peroxidase AffiniPure F(ab')2 Fragment Donkey Anti-Mouse IgG (H+L) (Jackson ImmunoResearch, catalog number: 715-036-150)
Paclitaxel (Sigma-Aldrich, catalog number: T7191)
PBS tablets (Gibco, catalog number: 18912014)
Penicillin-streptomycin (Gibco, catalog number: 15140122)
Propidium iodide solution (Sigma-Aldrich, catalog number: P4864-10ML)
rmEGF (Immunotools, catalog number: 12343407)
ROCK inhibitor Y-27632 (Merck Millipore, Sigma-Aldrich, catalog number: SCM075)
Sodium chloride (Roth, catalog number: 3957.2)
Sodium deoxycholate (Sigma-Aldrich, catalog number: 30970)
Sodium fluoride (Applichem, catalog number: A0401)
Sodium orthovanadate (Sigma-Aldrich, catalog number: S6508)
Sodium pyruvate (Thermo Fisher Scientific, Gibco, catalog number: 11360070)
Tris-HCl (Roth, catalog number: 4855.3)
TritonTM X-100 (AppliChem, catalog number: A1388)
TRIzolTM Reagent (Invitrogen, catalog number: 15596026)
Trypsin-EDTA (0.05%) (Gibco, catalog number: 25300096)
TrypLETM Express Enzyme (1X) (Thermo Fisher Scientific, catalog number: 12604013)
Tween® 20 (AppliChem, catalog number: A4974)
ZeocinTM (InvivoGen, catalog number: ant-zn-05)
TBS-T buffer (see Recipes)
RIPA buffer (see Recipes)
HEK293T Noggin cell culture medium (see Recipes)
HEK293T Rspondin-1 cell culture medium (see Recipes)
HEK293T Wnt3a cell culture medium (see Recipes)
Ad+ medium (see Recipes)
Colon normal and tumor organoid culture medium (see Recipes)
Small intestinal organoid culture medium (see Recipes)
Equipment
Forceps (Carl Roth™)
Micropipettes (Eppendorf)
Multichannel pipette (Eppendorf)
Surgical scissors (Braun, catalog number: 64069805)
Axiovert 40 CFL Microscope (Carl ZeissTM, model: 491202-0002-001)
Celigo S Imaging Cytometer (Nexcelom Bioscience)
Centro LB 960 Luminometer (Berthold Technologies, model: 38100-50)
HERAcell 150 Incubator (Heraeus, model: 2854)
Megafuge 1.0R Centrifuge (Heraeus, model: 75003060)
Thermomixer Comfort (Eppendorf, model: 5355 000.011)
Software
AxioVision 4.8.2 (Carl Zeiss MicroImaging GmbH)
Celigo 4 Channel Software Version 5.0.0.0 (Nexcelom Bioscience)
Fiji or ImageJ (http://fiji.sc/ or https://imagej.nih.gov/ij/)
GraphPad Prism 5, Version 5.04 (Graphpad Software Inc.)
Microsoft 365 (Microsoft)
MikroWin 2000, Version 4.37 (Mikrotek Laborsysteme GmbH)
Procedure
Part I: Preparation of conditioned medium (CM)
Cultivation of HEK293T cells to condition media with Noggin, Rspondin-1, and Wnt3a (Figure 1)
Note: HEK293T cells that have been genetically modified with mNoggin-Fc, mRspondin-1-Fc, or mWnt3a express the proteins of interest and secrete them into the medium. This conditioned medium is an important component of the medium used for organoid cultivation.
Thaw one aliquot of each HEK293T cell line, transfer into 10 mL of corresponding culture medium, and centrifuge at 500 × g for 5 min.
Carefully aspirate the supernatant to remove the DMSO present in the freezing medium.
Resuspend the pellet in 5 mL of culture medium and transfer the suspension into a T175 flasks containing 25 mL of growth medium (for a total of 30 mL).
Incubate cells at 37°C and 5% CO2 under humidified atmosphere.
When cells are properly attached (usually after 1-2 days), add antibiotics for selection (sAB) if necessary (mNoggin-Fc cells = 500 µg/mL GeneticinTM; mRspo1-Fc cells = 300 µg/mL ZeocinTM; mWnt3a cells = no sAB).
When cells reach approximately 80% confluency, split all cells into new flasks (p1) by washing with PBS and further trypsinizing them until cells detach from the plate; dilute cells and plate them into new T175 flasks (1:3-1:4).
Add sAB to all flasks of mNoggin-Fc and mRspo-1-Fc cells.
When cells reach 80% confluency, split cells for passage 2 (p2) again at 1:3 or 1:4.
Note: Higher dilutions are also possible to obtain more cells.
For conditioned medium (CM), cells must reach 100% confluency.
IMPORTANT: DO NOT add sAB into flasks for conditioned medium.
Note: If cells for further cultivation are required, add antibiotic to those cells/flasks only.
When cells have reached 100% confluency, remove the culture media and add 50 mL of Ad+ medium (without selection antibiotics).
After at least 5 to 7 days incubation, pool the conditioned media (CM) of the corresponding cell lines to be filtered through 0.22 µm filters into sterile bottles and aliquot. To prevent the bottle top vacuum filters from clotting, CM can be transferred into 50 mL tubes and centrifuged at 800 × g for 3 min at 4°C (to pellet and remove non-adherent cells) before filtration.
Store the CM at -20°C for further use, avoid freezing and thawing.
The remaining cultivation flask can be used for at least 3-6 passages, to repeat this process and generate more conditioned medium.
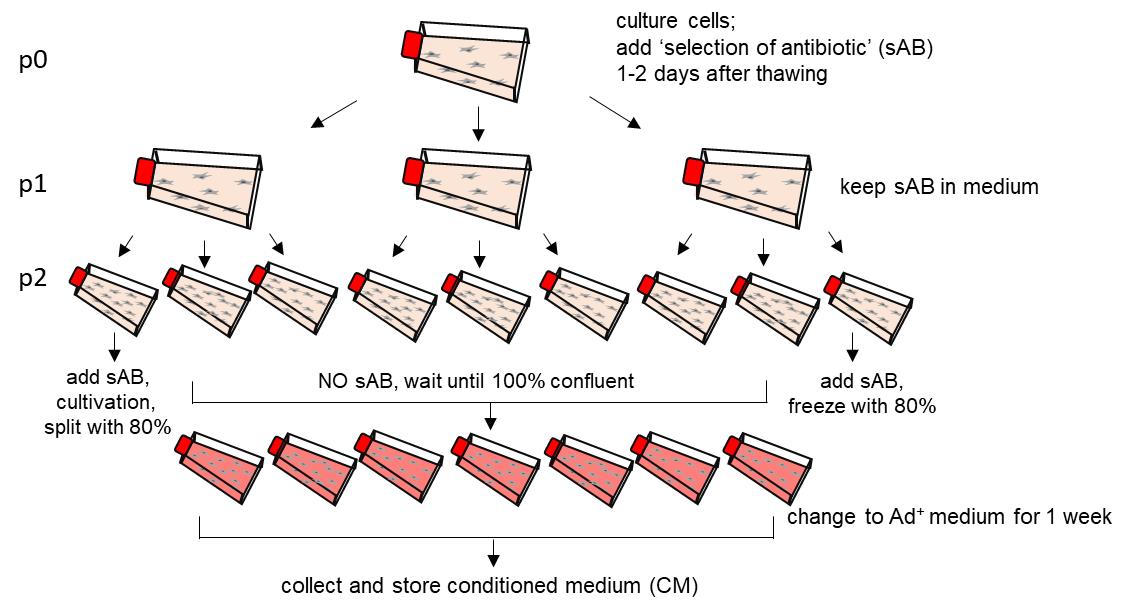
Figure 1. Graphical illustration of the medium conditioning procedure for one HEK293T cell line. sAB; selection antibiotics.Note: Some researchers cultivate passage 2 (p2) still with antibiotics (with sAB) until cells reach 100 % confluency. Importantly, Ad+ medium has to be free of selection antibiotics (NO sAB).
Performing dot blot analysis, to determine the quantity of Rspondin-1 and Noggin in conditioned media (CM) (Figure 2)
Note: In the HEK293T cells utilized, Noggin is fused to a human IgG1 Fc-fragment and Rspondin-1 is fused to a murine IgG1 Fc-fragment. Dot blots are performed to detect Fc-coupled proteins.
If needed, prepare a dilution series of the respective IgG standard for the dot blot. Standard for Rspondin-1: Normal mouse IgG1, Santa Cruz, catalog number: SC-3877. Standard for Noggin: InVivoMAb human IgG1 isotype control, bxcell, catalog number: BE0297.
Recommended dilution for the standard row (IgG Stock 0.2 mg/mL): From 0.2 to 0.025 mg/mL, in 5% BSA in TBS-T (0.1% Tween® 20).
Thaw an aliquot each of the conditioned Noggin and Rspondin-1 medium and prepare a dilution series. Dilute in 5% BSA in TBS-T: undiluted – 1:2 – 1:4 – 1:8 – 1:16.
Prepare two nitrocellulose membranes, by cutting an approximatelly 7 × 9 cm sized blot, which can be incubated in 50 mL tubes. Draw lines with a pencil, to indicate where the spots will be applied.
Spot 2 µL of samples or standards on the corresponding membranes. Minimize the area by applying the solution/dot very slowly. Spot all samples, standards, and negative controls.
Transfer sampled nitrocellulose membranes into a box or Petri dish, cover with a lid, and let membranes dry (for several hours or overnight).
Transfer each membrane into a 50 mL tube.
Block nonspecific sites by incubating membranes in blocking solution (5% milk in TBS-T) for 30-60 min at RT on a roller.
Remove blocking solution and incubate with the HRP-conjugated secondary antibody [anti-human HRP (mNoggin-Fc) or anti-mouse HRP (mRspo1-Fc), both 1:10,000 in blocking solution] for 30 min at RT on a roller.
Wash three times with TBS-T, for a minimum of 10 min each on the roller.
Use a Western Chemiluminescent HRP Substrate to develop the signal (usually, very strong signal is achieved).
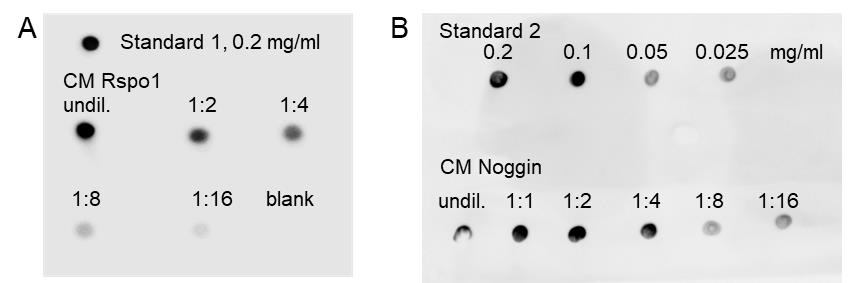
Figure 2. Representative dot blots of Rspondin-1 and Noggin conditioned medium. Dot blot analysis used to estimate the amount of Rspondin-1 (Rspo1) (A) and Noggin (B) in the conditioned medium (CM). Undiluted (undil.) and diluted CM were spotted and, for quantification, the respective standards were spotted on each membrane. Standard 1 for Rspo1: Normal mouse IgG1, Santa Cruz, material #71. Standard 2 for Noggin: human Fc fragment, InVivoMAb human IgG1 isotype control, material #72. Unconditioned Ad+ medium was used as blank.Note: The standards can also be used as a concentration series, to better quantify amounts as in (B).
Performing TopFlash assay, to determine the activity of Wnt3a conditioned medium (CM) (Figure 3)
Notes:
1) For the TOPFlash assay, a well-transfectable cell line, such as H1299 cells, can be used.
2) Wnt3a is not fused to a Fc-fragment, hence dot blot analysis as performed for Noggin and Rspondin-1 is not possible. Therefore, the TopFlash assay is performed to assess the activity of produced Wnt3a.
Culture a well-transfectable cell line.
Seed cells into a 6-well plate in 2 mL of culture medium.
After 24 h culturing, transfect cells with 500 ng TOP/200 ng Renilla or 500 ng FOP/200 ng Renilla plasmid, using LipofectamineTM 3000 according to the manufacturer’s guidelines.
After 24 h of incubation of cells with the transfection reagent, remove medium and add 2 mL of conditioned Wnt3a medium. Incubate for at least 16 h at 37°C.
After incubation with Wnt3a CM, a luciferase assay is performed using the Dual-Glo® Luciferase Assay System, according to the manufacturer’s guidelines.
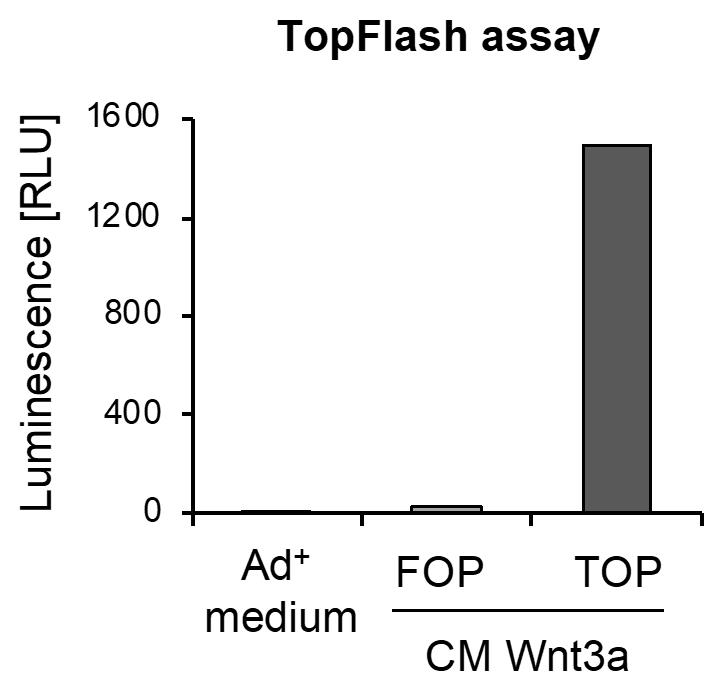
Figure 3. Representative result of TopFlash assay. Conditioned medium (CM) was used in the TopFlash assay, to estimate the activity of Wnt3a in the medium. Therefore, cells were transfected with TOP or FOP plasmids and incubated with CM Wnt3a. Unconditioned Ad+ medium was used as blank control.
Part II: Preparation of murine matched-pair organoids from one donor mouse
Note: Colonic matched-pair organoid preparation means the generation of tumor-derived and normal epithelial-derived organoids from the same recipient. Murine colorectal tumors were induced using the chemicals azoxymethane (AOM) and dextran sodium sulfate (DSS), as previously described (Tanaka et al., 2003; Becker et al., 2005; Klemke et al., 2021).
Preparation of organoids
Isolation of mouse intestinal epithelia and tumors
Notes:
1) For an optimal reduction of contamination risk, disinfect or sterilize all used tools before starting.
2) For an optimal reduction of contamination risk, PBS can be supplemented with antifungal drugs, such as amphotericin, antibiotics, such as penicillin/streptomycin, or combined drug solutions, such as antibiotic-antimycotic (e.g., from Thermo Fisher).
3) When a fresh vial of Matrigel® is used, it can be slowly thawed at 4°C on ice overnight, then aliquoted into 1.5 mL or 2 mL tubes. Before usage, thaw Matrigel® aliquots on ice for at least one hour.
4) Matrigel® should always be kept on ice. There are different possible ways to handle Matrigel® under the biosafety hood. For optimal sterility, disinfectable gel-filled cooling bags or cooling racks can be used for cooling. Ice-filled disinfectable buckets or boxes provide easier handling, but pose a higher risk of contamination.
5) Matrigel® can be diluted generally up to 15-20% in cold medium before usage, depending on protein concentration and stiffness of the Matrigel® lot. Attention should be paid for lot variations (protein concentrations should be higher than 9 mg/mL and endotoxin levels should be lower than 1.5 EU/mL).
6) Plates can be pre-warmed to facilitate Matrigel® solidification.
Euthanize and prepare a mouse using an appropriate method, according to local legislation and the respective animal experiment application.
Remove the intestinal tract completely or any intestinal segments individually.
Note: Optimal sterility at these steps minimizes contamination risks in culture. For easier handling, attach the mouse in a supine position to a sanitizable cutting board or a styrofoam board using needles or cannulas. Disinfect the fur before opening the skin with a pair of scissors. For optimal sterility, carefully separate the skin from the abdominal wall to prevent perforation of the peritoneum. Using fresh scissors, open the peritoneal cavity.
Remove the mesenteries with a forceps and cut the intestine into smaller easier to handle parts (e.g., roughly the diameter of a Petri dish). Place the intestinal parts into cold PBS, labelled appropriately for small intestine and large intestine (colon).
Gently flush the intestinal parts with cold PBS e.g., by using a rodent gavage needle.
Place the intestinal parts onto a Petri dish lid and open each intestinal part longitudinally, with the luminal surface upwards.
For tumor preparation from the colon, dissect several tumors, transfer them into ice-cold PBS, and cut them in small fragments.
For normal small intestinal and colonic epithelia preparation, very gently scrape off excess mucus or residual feces from the intestine with a cover slip.
Cut normal intestinal fragments out of the intestine. Be careful and avoid dissection of parts with tumorous epithelial tissue. Cut intestinal fragments into smaller pieces of approximately 2 mm × 2 mm. (For example, cut once longitudinally and then transversally, using a razor blade to guide and a scalpel to cut.)
Transfer the pieces into a 50 mL tube filled with approximately 15 mL of cold PBS. From now on, always work on ice.
Washing:
Normal small intestine:
Wash thoroughly. Use a pre-wet 10 mL serological pipette to pipette the pieces up and down (approximately 30 times). This step is crucial to remove the intestinal microflora adhered on the mucosa. To improve the preparation yield, pre-coating the pipettes with FCS can be recommended.
Let the pieces settle by gravity and gently aspirate the supernatant.
Add 15 mL of cold PBS and repeat the rinsing procedure.
Repeat these washing steps (i-iii) 10 times. The supernatant should be almost clear at the end of this procedure. Because of the high amount of mucous in the small intestine, washing steps must be carried out properly.
Normal colon:
Wash moderately. Use a pre-wet transfer pipette to pipette the pieces up and down (approximately 10 times). This step is crucial to remove the intestinal microflora adhered on the mucosa. To improve the preparation yield, pre-coating the pipettes with FCS can be recommended.
Let the pieces settle by gravity and gently aspirate the supernatant.
Add 15 mL of cold PBS and repeat the rinsing procedure.
Repeat these washing steps (i-iii) 3-5 times. The supernatant should be almost clear at the end of this procedure.
Colonic tumor:
Wash thoroughly. Use a pre-wet transfer pipette to pipette the pieces up and down (approximately 10 times). This step is crucial to remove the intestinal microflora adhered on the mucosa. To improve the preparation yield, pre-coating the pipettes with FCS can be recommended.
Let the pieces settle by gravity and gently aspirate the supernatant.
Add 15 mL of cold PBS and repeat the rinsing procedure.
Repeat these washing steps (i-iii) 5-7 times. The supernatant should be almost clear at the end of this procedure.
Note: The amount of washing steps might differ from person to person but, in any case, washes can be terminated when the supernatant is almost clear.
Crypt and tumor cell isolation
Normal small intestine:
Aspirate the supernatant. Equilibrate with approximately 15 mL of 5 mM EDTA solution, remove the supernatant, add 15 mL of fresh EDTA solution, and incubate for 30 min on a benchtop roller at 4°C.
Note: During incubation, checking for visible crypts in the supernatant, by pipetting a little drop onto a clean dish or microscope slide for examination under a phase-contrast microscope, can help to optimize incubation times at this step.
Prepare 50 mL tubes under a sterile cell culture hood, add a cell strainer (100 µm pore diameter), equilibrate the filter by rinsing it with 10 mL of PBS, and let the PBS stay in the tubes.
After 30 min EDTA incubation, use a sterile serological pipette to pipette up and down six times. Let the pieces settle by gravity and aspirate the supernatant as a first fraction.
Note: Working quickly at these steps reduces EDTA-mediated toxicity on isolated crypts. Check for characteristic crypts using a phase-contrast microscope. Depending on the number of crypts present in the fraction, the first fraction can be either discarded or filtered through the cell strainer into the 50 mL tube containing ice cold PBS, to directly dilute the EDTA solution.
Add 10 mL of ice-cold PBS to the intestinal pieces, pipette up and down several times, check for crypts using a microscope, and repeat filtering into the 50 mL tube for every fraction. Repeat until the tube is filled or until enough crypts have been isolated.
Normal colon:
Aspirate the supernatant. Equilibrate with approximately 10 mL of 4 mM EDTA solution, remove the supernatant, add 5 mL of fresh 4 mM EDTA solution, and incubate for 30 min on a benchtop roller at 4°C.
Notes:
1) For colonic crypts, we achieved proper yield in viable and well-isolated crypts using 4 mM EDTA. However, for small intestinal crypts, 5 mM EDTA was needed for a proper crypt isolation in our hands. Concentrations of EDTA might even be increased up to 10 mM, depending on the yield of isolated crypts (Lukovac and Roeselers, 2015). The EDTA concentration needed might not just depend on the respective part of the gut, but also on the strength of mechanical disruption.
2) During incubation, checking for visible crypts in the supernatant, by pipetting a little drop onto a clean dish or microscope slide for examination under a phase-contrast microscope, can help to optimize incubation times at this step.
Prepare 50 mL tubes under a sterile cell culture hood, add a cell strainer (100 µm pore diameter), and equilibrate the filter by rinsing it with 10 mL of PBS.
After 30 min EDTA incubation, pipette up and down vigorously (approx. 10 times), and check the supernatant for crypt numbers in the first fraction. Usually, the number of colonic crypts in the first fraction from the EDTA solution supernatant is quite low, so the supernatant can be discarded. However, if there are many crypts present in the EDTA supernatant, the first fraction can be used.
Add 10 mL of cold PBS to the intestinal pieces, pipette up and down vigorously (approximatelly 10-20 times), let the pieces settle by gravity, and aspirate the supernatant as a new fraction. Check for crypt numbers under the microscope. If an appropriate number of crypts is present, filter through a cell strainer under the cell culture hood into the fresh 50 mL tube containing 10 mL of cold PBS.
Repeat step iv until the tube is filled or until enough crypts have been isolated. In case more crypts can be isolated from the pieces, use a new 50 mL tube.
Colonic tumor:
Aspirate the supernatant.
Add 2 mL of 2 mg/mL Collagenase type I solution in Ad+ medium, incubate at 37°C for 20-30 min. Pipette the tissue pieces up and down every 10-15 min, using a transfer pipette to facilitate cell detachment.
After 30 min incubation, add 10 mL of PBS, and pipette up and down vigorously until almost no bigger tumor pieces are left.
Filter the suspension through a cell strainer into a new 50 mL tube.
Work under sterile conditions from now on.
In any case, if necessary, add up to 50 mL of ice-cold PBS to the tubes.
Centrifuge tubes at 700 × g for 3 min at 4°C.
Aspirate supernatants carefully.
Add 10 mL of cold Ad+ medium and carefully suspend pellets.
Centrifuge tubes again at 500 × g for 5 min at 4°C. Aspirate the complete supernatants carefully and place tubes on ice.
Seeding
Note: The volume [µL] per Matrigel® dome is an approximate value or recommendation for routine cultivation, and can be adjusted to the specific experimental needs (Table 1).
Table 1. Cell suspension with recommended Matrigel® and medium volumes for different plate formats.
Plate size Matrigel®domes per well number of drops × µL per drop (total volume Matrigel®)
Medium volume [mL] 96-well 1× 10 µL (10 µL) 0.1 24-well 1× 50 µL (50 µL) 0.5 12-well 3× 33 µL (100 µL) 1 6-well 8× 25 µL (200 µL) 2 Notes:
1) For an optimal reduction of contamination risk, disinfect or sterilize all used tools before starting.
2) For an optimal reduction of contamination risk, all organoid culture media can be additionally supplemented with antifungal drugs, such as amphotericin, or combined antifungal/antibacterial drug solutions such as antibiotic-antimycotic (e.g., from Thermo Fisher).
3) Matrigel® should always be kept on ice or otherwise cooled. To minimize Matrigel® loss, pipette tips can be pre-cooled at -20°C. Add an appropriate volume of Matrigel® (Table 1) to the pellets and gently mix until pellets are completely dispersed. Avoid generating air bubbles in the Matrigel®.
4) Usually, after preparation of one mouse, enough crypts and tumors cells were isolated to culture 1-2 wells in a 6-well plate for crypts, and 2-3 wells in a 6-well plate for tumor cells.
Seed 200 µL of the cell suspension per well in a pre-warmed 6-well plate (8 drops of 25 µL for one well). The number of wells depends on the isolation yield and the desired volume and cell density.
Incubate at 37°C for approximately 30 min until the Matrigel® has solidified.
Add 2 mL of pre-warmed organoid medium per well and cultivate under humidified atmosphere at 37°C with 5% CO2.
Exchange medium every 2-3 days.
Cultivate and treat according to your experimental needs.
Note: Keep in mind that Matrigel® may retain treatment solutions. Medium and treatment traces in the domes cannot easily be washed away. Therefore, wash-out of treatment must be done thoroughly: After aspiration of the supernatant, perform an initial washing step with pre-warmed PBS. Add medium and incubate at 37°C for at least 30 min. Then, change medium again. Change medium again the next day, to further reduce treatment traces.
Special considerations for seeding in 96-well plates:
Prepare 96-well flat bottom culture plates by prefilling the outer row of wells with PBS and pre-warming the plate in an incubator at 37°C.
Dispense organoid suspension well (when passaging from a 50 mL tube, gentle shaking is sufficient).
Continue with seeding. Seed 10 µL of organoid suspension per well. Seed the droplet into the center of the well carefully, to not touch the wall of the well. For beginners, precision can be improved by tapping the loaded pipette on the bottom of the well once without ejection for depth-orientation before seeding.
Note: More volume will increase the number of organoids that can be used for experiments, but will also increase the risk of the droplet adhering to the wall of the well, which must then be excluded from analysis.
Repeat steps 2 and 3 for every well, dispensing the suspension after every seeded well, to prevent unequal seeding densities.
Carefully transfer plate into an incubator for 30 min to solidify the Matrigel®.
Add 100 µL of culture medium per well (using a multi-channel pipette is recommended).
Cultivate and treat according to your experimental needs.
Notes: For aspiration of the supernatants in 96-well format, the following can be recommended:
1) Use the lowest power setting on the vacuum pump.
2) If the suction still damages the Matrigel® domes, the power can be further reduced by stacking plastic pipette tips.
Passaging
Note: Splitting is recommended after approximately 4-7 days, for small intestinal organoid culture, or after 6-8 days, for colonic and tumor organoid culture, with a splitting ration of 1:2 or 1:3, depending on seeding density and on the physical disruption before seeding.
Aspirate media carefully from wells.
Dissolve Matrigel® domes by adding ice-cold PBS to each well.
Transfer the suspension to a 50 mL tube containing 5-10 mL of cold PBS. Rinse wells with ice-cold PBS again, if there is still Matrigel® with organoids left.
Add up to 50 mL of ice-cold PBS.
Centrifuge tubes at 500 × g for 5 min at 4°C.
Aspirate supernatants carefully.
Add 3 mL of ice-cold PBS, pipette up and down vigorously 20 times. For longer splitting intervals or for smaller fragments, pipette up and down 40 times with a 1 mL pipette. For optimal mechanical disruption with minimal destruction, place the blue pipette tip diagonally to the side of the tube bottom, not directly into the center. Alternatively, a 200 µL pipette tip attached onto a 5 mL serological pipette can be used for mechanical dissociation.
Notes:
1) To enhance Matrigel® removal, incubation in 2 mL of cell recovery solution on ice for 10 min can be recommended after step 6. Centrifuge tubes at 500 × g for 5 min at 4°C and aspirate supernatants.
2) If organoid fragments are still too big after step 7, organoids can also be dissociated by incubation in 4 mM EDTA solution for colon and 5 mM EDTA for small intestine, for 30 min on a roller at 4°C, followed by additional mechanical disruption.
Add up to 50 mL of ice-cold PBS.
Centrifuge tubes at 500 × g for 5 min at 4°C.
Aspirate supernatants carefully and place tubes on ice.
Add 10 mL of cold Ad+ medium and carefully suspend pellets.
Centrifuge tubes again at 500 × g for 5 min at 4°C. Aspirate the complete supernatants carefully and place tubes with the cell pellet on ice.
Continue with seeding as described in section B.
Note: To verify that normal colon-derived organoids are not contaminated with tumor-derived organoids, one well of normal colon organoids can be seeded in parallel and cultured in the related organoid medium without Wnt3a conditioned medium. Whereas tumor organoids can grow without Wnt3a, normal colon organoids do not, which serves as a functional control. Furthermore, normal colon-derived organoids differ in their morphology from tumor-derived organoids. As seen in the graphical abstract, normal colonic-derived organoids (green) display a thicker and crypt-like epithelial layer, whereas tumor-derived organoids (red) are round with a thin epithelial layer.
Data analysis
Protein and RNA isolation from organoids
Protein isolation
Notes:
1) To have enough protein for immunoblot analysis, organoids are seeded in 6-well plates.
2) To prevent sample degradation, proteinase and phosphatase inhibitors can be added to all solutions before sample lysis in RIPA buffer.
Place plate on ice and aspirate the medium.
Add 1 mL of ice-cold PBS (for 6-well format), and slowly pipette up and down in the well to carefully disrupt the Matrigel® domes.
Transfer into 5 mL tubes on ice and fill up to 5 mL with ice-cold PBS.
Centrifuge tubes at 500-1,000 × g for 3 min at 4°C.
Remove supernatant carefully.
Depending on the amount of remaining Matrigel® (cloudy phase on top of the pellet) add 200-300 µL of ice-cold cell recovery solution (CRS) to the pellet.
Incubate for 10-15 min in CRS on ice.
Add up to 5 mL of cold PBS.
Centrifuge tubes at 500-1,000 × g for 3 min at 4°C.
Carefully remove as much supernatant as possible. If there is still Matrigel® present, repeat incubation steps with CRS (steps 6-10).
Add 80 µL of RIPA buffer to the pellet. Suspend and transfer the suspension into a 1.5 mL tube.
Measure the protein concentration with a BCA protein assay according to the manufacturer’s guidelines, to ensure equal protein amounts for further immunoblot experiments, and/or freeze at -80°C for further usage.
Note: Alternatively, the first PBS washing and centrifugation step can be skipped, and organoids can be harvested directly from the plates using cold CRS. This increases the yield but makes the use of larger volumes of CRS necessary, as organoids should then be incubated with approximately 1 mL of CRS per well (200 µL Matrigel®) for 10-15 min on ice. Then, continue with PBS dilution and centrifugation (step 8).
RNA isolation
For quantitative real-time polymerase chain reaction (qRT-PCR), the amount of RNA when seeding organoids in 12-well plates is usually sufficient.
Two ways in which RNA isolation can be used:
RNA isolation directly from the well:
Aspirate media and wash once with pre-warmed PBS. Aspirate PBS solution.
Add 1 mL of TRIzolTM directly to wells and incubate for 10 min.
Carefully pipette the samples up and down several times to homogenize the solution.
Transfer the suspension into a 1.5 mL tube.
RNA isolation after removal of Matrigel®
The procedure is similar to the protocol described for protein isolation (Protein isolation part).
Aspirate media and wash once with pre-warmed PBS. Aspirate PBS solution.
Place plate on ice and add ice-cold PBS to each well. Disrupt Matrigel® domes by pipetting up and down.
Transfer into 5 mL tubes and fill up to 5 mL with ice-cold PBS.
Centrifuge at 1,000 × g for 3 min at 4°C.
Remove as much supernatant as possible.
In case of remaining Matrigel® (cloudy substance on top of the pellet), add 200-300 µL of CRS (Corning) to the pellet, and incubate on ice for 10-15 min.
Fill up to 5 mL with ice-cold PBS.
Centrifuge at 1,000 × g for 3 min at 4°C.
Remove supernatant and add 1 mL of TRIzolTM reagent to the pellet.
Incubate for 10 min before transferring the suspension into a 1.5 mL tube.
In both cases, isolate RNA in TRIzolTM reagent according to the manufacturer’s guidelines.
Note: In any case, it is highly recommended to check both methods side by side, to exclude any primer-specific interference with the Matrigel®. Isolation of RNA directly out of the well might have the advantage of higher yield and a fast stop of biological processes, however, some primers might be able to interfere with components of the Matrigel®.
Quantification of organoid morphology and viability
Morphological quantification from brightfield images
Morphological quantification can be determined according to a modified description from Grabinger et al. (2014).
However, for non-spheroidal growth morphologies, such as small intestinal organoids showing budding growth architectures, this might not be that easily adaptable, and we additionally recommend other assays, as described below.
Notes: Based on morphology, organoids can be subdivided into three groups (Figure 4):
1) Healthy (green arrowhead): Normal morphology, with very little accumulation of dead cell in the lumen of the organoids.
2) Stressed (yellow arrowhead): Moderate to massive accumulation of dead cells in the lumen, outer epithelial barrier is still intact.
3) Dead (red arrowhead): Partial or complete disruption of the organoids into clumps of dead cells, loss of outer epithelial barrier.
Healthy and stressed organoids can be considered as alive and as such could be summarized into one group.
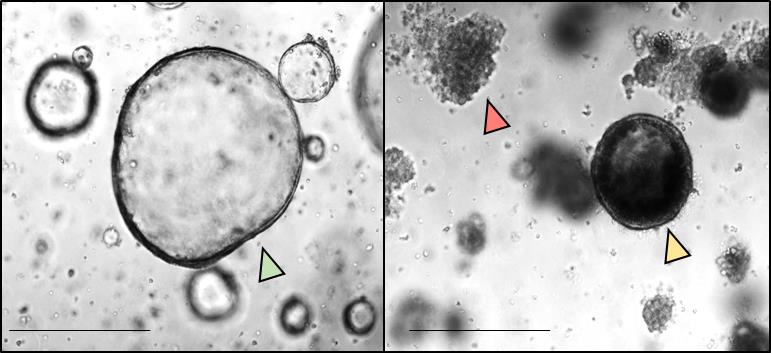
Figure 4. Morphological quantification of colonic tumor organoids. Colonic tumor organoids were seeded in 6-well plates and treated with HSP90 inhibitor (right) or DMSO control (left) for 24 h. Morphologically, all imaged organoids were subdivided into healthy (green arrowhead), stressed (yellow arrowhead), or dead (red arrowhead). The number of organoids per group was counted using the ‘cell counter’ function of ImageJ. Scale bars, 200 µm.After culturing or treatment of organoids based on the experimental set up, take images by light microscopy. Usually, with a 4x magnification, take one image per gel domes.
The total amount of images is dependent on size and amount of gel domes.
Assess number of organoids per group using the ‘cell counter’ function of ImageJ.
Calculate the percentage of each subgroup relative to the total amount of counted organoids per image.
After morphological quantification, organoids can be further used for protein or RNA isolation (Section A).
Note: Detailed data processing and analysis, and statistical calculations for this part (B.Quantification of organoid morphology) are provided in the corresponding open-access article Klemke et al. (2021).
Staining for cell viability, visualization using double staining with propidium iodide and Hoechst 33342 (Figure 5).
PI is a red fluorescent DNA intercalating dye, which does not permeate unfixed living cells, hence staining only dead cells in this context. Hoechst 33342 is a cell-permanent DNA intercalating nuclear reagent, and is used as a counterstaining for nuclei, allowing the morphological examination of the organoids. Therefore, double staining can be used to visualize live and dead cells in organoids (Jabs et al., 2017) (Figure 5).
Perform passaging and seeding as described in sections B. Seeding and C. Passaging into a 96-well plate, and cultivate and treat according to your experimental needs. For best results, use black/clear flat bottom 96-well plates.
Add propidium iodide to a final concentration of 1 µg/mL and Hoechst 33342 to a final concentration of 10 µg/mL to the medium. (To reduce fluorescence background, phenol-red-free medium can be used.)
Incubate at 37°C for 35 min. (Shorter incubation times might prevent the solutions from reaching the inner parts of the Matrigel® domes, longer incubation times might increase toxicities.)
Image using a plate reader, a fluorescence microscope, or an automatized imaging system, like a Celigo Image Cytometer (Nexcelom).
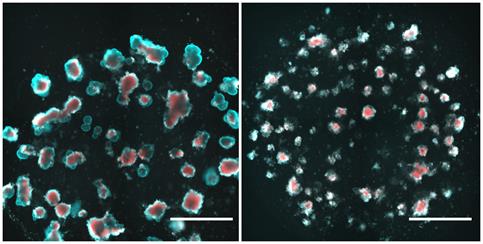
Figure 5. Exemplary data of propidium iodide/Hoechst 33342 fluorescence staining of small intestinal organoids. Two days post-seeding, murine small intestinal organoids were treated with DMSO (left) and 1 mM paclitaxel (right) for 24 h followed by a media change. After 4 days of washout, organoids were incubated with propidium iodide and Hoechst 33342 according to the protocol and imaged in a Celigo Image Cytometer. Scale bars: 1,000 µm.Cell viability assay with CellTiter-Glo® 3D
Note: The manufacturer of the CellTiter-Glo® 3D recommends using white non-transparent wells. However, these plates do not allow microscopic observation of organoids and, therefore, uneven seeding densities might not be detected. It is possible to perform CellTiter-Glo® 3D in 96-well transparent-clear flat bottom culture plates, which we would recommend. The CellTiter-Glo® 3D protocol is derived from the manufacturer’s guidelines.
ATP standard curve (Figure 6).
Note: It is good scientific practice to perform an ATP standard curve for a cell viability assay, and to exclude all experimental data not derived from the linear section of that standard curve, or derived from significantly different plates, or different experimental conditions.
Dilute a range of ATP concentrations in PBS. A range from approximately 50 nM to 500 µM can be recommended (see Figure 6).
Add 100 µL of the ATP solution per well, then add 100 µL of CellTiter-Glo® 3D solution and proceed with the incubation and measurement steps equal to your experiments.
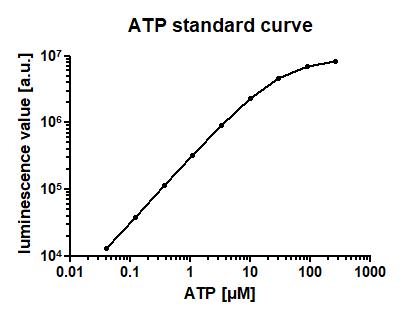
Figure 6. ATP standard curve for the CellTiter-Glo® 3D assay. Indicated concentrations of ATP solution in PBS were incubated for 45 min at room temperature, and luminescence was measured using the CellTiter-Glo® 3D assay. Mean of n=3 technical in-plate replicates ± SEM.CellTiter-Glo® 3D assay (Figure 7)
Perform passaging and seeding as described in sections B. Seeding and C. Passaging into a 96-well plate, and cultivate and treat according to your experimental needs.
Note: Equal seeding densities of organoids must be achieved (also see Special considerations for seeding in 96-well plates) above. For beginners, this might prove difficult. Therefore, we recommend to image all wells before treatment, to ensure equal organoid densities at the beginning of any experiment.
Let the CellTiter-Glo® 3D reagent solution equilibrate to room temperature.
Add 100 µL of CellTiter-Glo® 3D reagent per well directly into the medium.
Incubate at room temperature on a shaker at 450 rpm for at least 30 min. (Higher shaking velocities might eject the solution from the wells. Before the first experiment, test the right speed with water in a 96-well plate.)
Measure the luminescence signal with a plate reader. When organoids are stuck to the wall of a well, exclude it from analysis, as it disturbs the signal intensity.
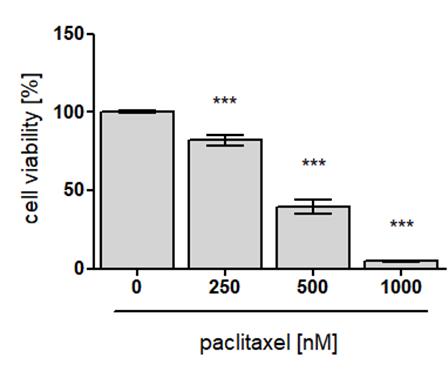
Figure 7. Exemplary data of CellTiter-Glo® 3D assay results of intestinal organoids. Murine small intestinal organoids, seeded in five technical in-plate replicates (n=5), were treated with indicated concentrations of paclitaxel or control (DMSO) for 24 h. After four days of washout, luminescence was measured after PI/Hoechst treatment, using the CellTiter-Glo® 3D assay and normalized to control (also see step B4). Data are presented as mean ± standard error of the mean (SEM). Differences between the groups were determined with an unpaired two-tailed Student’s t-test. ***P < 0.001, compared to control.
Combined PI/Hoechst staining and CellTiter-Glo® 3D cell viability assay
It is possible to combine the advantages of good visualization and morphological characterization of the PI/Hoechst staining with the advantages of the accurate quantification of cell viability using the CellTiter-Glo® 3D assay, by performing them successively without relevant impact on cell viability (Figure 8). We recommend using black/clear flat bottom 96-well plates.
Let the CellTiter-Glo® 3D reagent solution equilibrate to room temperature.
Perform step B2.
Aspirate the supernatant.
Add 100 µL of pre-warmed PBS per well.
Perform step B3b.iii.
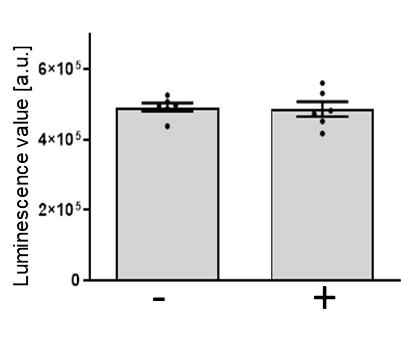
Figure 8. Propidium iodide/Hoechst 33342 (PI/Hoechst) staining has no influence on cell viability. Murine small intestinal organoids were cultivated for 3 days and step B4 was performed. Comparison of unstained (-) and PI/Hoechst stained (+) organoids, according to the protocol ‘2. Cell permeability staining for cell viability visualization using double staining with propidium iodide and Hoechst 33342 staining’. Luminescence was measured after PI/Hoechst treatment and images were acquired using the CellTiter-Glo® 3D assay (step B3b). Mean of six technical in-plate replicates (n=6) ± SEM.
Statistical quantification
For step B1 (Data analysis section), it can be recommended to take images of at least three Matrigel domes from each condition per independent experiment. The number of necessary images depends on size and culture density. The percentage of dead organoids was calculated relative to the total amount of organoids per image.
For steps B2 and B3 (Data analysis section), it can be recommended to perform at least three independent biological replicates. To reduce the impact of seeding variances on the results, it can be recommended to perform at least three in-plate technical replicates. Data can be analyzed and graphically illustrated using GraphPad Prism software. Experimental data can be normalized to the mean of control-treated organoids. Differences between the groups compared to control treatment can be determined with an unpaired two-tailed student’s t-test. For more complex statistical testing, correction for multiple testing and other methods of statistical analysis are recommended.
Recipes
TBS-T buffer
Autoclaved MilliQ water
50 mM Tris
150 mM sodium chloride
Adjust to pH 7.5 (HCl)
0.1% Tween® 20
RIPA buffer
Autoclaved MilliQ water
1% sodium deoxycholate
10 mM EDTA
1% TritonTM X-100
0.1% SDS
150 mM sodium chloride
20 mM Tris-HCl pH 7.5
CompleteTM mini protease inhibitor cocktail
Phosphatase inhibitor mix consisting of 2 mM Imidazol, 1 mM sodium orthovanadate, and 1 mM sodium fluoride
HEK293T Noggin cell culture medium
DMEM (Materials and Reagents #42) supplemented with:
10% FCS
1 mM Na-Pyruvate
15,000 units/L Penicillin/15,000 µg/L Streptomycin
Selection Antibiotics, sAB: 500 µg/mL GeneticinTM
HEK293T Rspondin-1 cell culture medium
DMEM (Materials and Reagents #42) supplemented with:
10% FCS
1 mM Na-Pyruvate
15,000 units/L Penicillin/15,000 µg/L Streptomycin
Selection Antibiotics, sAB: 300 µg/mL ZeocinTM
HEK293T Wnt3a cell culture medium
DMEM (Materials and Reagents #42) supplemented with:
10% FCS
1 mM Na-Pyruvate
15,000 units/L Penicillin/15,000 µg/L Streptomycin
No selection Antibiotics (no sAB)
Ad+ medium
Advanced DMEM-F12 supplemented with:
1× GlutaMAXTM supplement
10 mM HEPES
15,000 units/L Penicillin/15,000 µg/L Streptomycin
Colon normal and tumor organoid culture medium
Ad+ medium supplemented with:
50% CM Wnt3a
20% CM Noggin
10% CM Rspondin-1
1× N-2, 1× B-27TM
3.4 μg/mL ROCK inhibitor
5 μM CHIR 99021
500 nM A83-01
10 mM Nicotinamide
80 µM N-Acetyl-L-Cysteine
200 ng/mL rmEGF
Note: After p1, the ROCK inhibitor still needs to be added right after splitting, but can be removed from medium at the next medium exchange at day 2 after splitting.
Small intestinal organoid culture medium
Ad+ medium supplemented with:
20% CM Noggin
10% CM Rspondin-1
1× N-2, 1× B-27TM
80 µM N-Acetyl-L-Cysteine
50 ng/mL rmEGF
Acknowledgments
R. S.-H. and L. K. are supported by the Deutsche Forschungsgemeinschaft (DFG) (SCHUH-3160/3-1). J. P. B. was supported by scholarships of the Göttingen Promotionskolleg für Medizinstudierende (Else Kröner-Promotionskolleg für Genomdynamik und Epigenomik) and the Studienstiftung des deutschen Volkes. T. D. O. is supported by Universitätsmedizin Göttingen Forschungsförderungs-program 2020 (K-1403640). We thank Tamara Isermann (Molecular Oncology, Göttingen) for her technical assistance and Robyn Laura Kosinsky (Clinic for General, Visceral and Pediatric Surgery, Göttingen) for providing the HEK293T mWnt3a cells.
The original research paper published in an open-access journal where this protocol was derived from is Klemke L, De Oliveira T, Witt D, Winkler N, Bohnenberger H, Bucala R, Conradi L-C, Schulz-Heddergott R. Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis. Feb 4;12(2):155. 2021 (PMID: 33542244).
Competing interests
The authors declare to have no competing interests.
Ethics
Mouse experiments were approved by state (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, LAVES, Germany) and institutional (Göttingen University Medical Center) committees, which ensured that all experiments conformed to the relevant regulatory standards. Mice were housed and handled under pathogen-free barrier conditions.
References
- Almeqdadi, M., Mana, M. D., Roper, J. and Yilmaz, O. H. (2019). Gut organoids: mini-tissues in culture to study intestinal physiology and disease. Am J Physiol Cell Physiol 317(3): C405-C419.
- Becker, C., Fantini, M. C., Wirtz, S., Nikolaev, A., Kiesslich, R., Lehr, H. A., Galle, P. R. and Neurath, M. F. (2005). In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54(7): 950-954.
- Clevers, H. (2016). Modeling Development and Disease with Organoids. Cell 165(7): 1586-1597.
- Fair, K. L., Colquhoun, J. and Hannan, N. R. F. (2018). Intestinal organoids for modelling intestinal development and disease. Philos Trans R Soc Lond B Biol Sci 373(1750): 20170217.
- Grabinger, T., Luks, L., Kostadinova, F., Zimberlin, C., Medema, J. P., Leist, M. and Brunner, T. (2014). Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis 5: e1228.
- Jabs, J., Zickgraf, F. M., Park, J., Wagner, S., Jiang, X., Jechow, K., Kleinheinz, K., Toprak, U. H., Schneider, M. A., Meister, M., et al. (2017). Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol Syst Biol 13(11): 955.
- Klemke, L., De Oliveira, T., Witt, D., Winkler, N., Bohnenberger, H., Bucala, R., Conradi, L. C. and Schulz-Heddergott, R. (2021). Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis 12(2): 155.
- Lancaster, M. A. and Knoblich, J. A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345(6194): 1247125.
- Lukovac, S. andRoeselers, G. (2015). Intestinal Crypt Organoids as Experimental Models. In: Verhoeckx, K., Cotter, P., López-Expósito, I., et al. (Eds.). The Impact of Food Bioactives on Health: In vitro and ex vivo models (pp. 245-253). Springer International Publishing.
- O’Rourke, K., Ackerman, S., Dow, L. and Lowe, S. (2016). Isolation, Culture, and Maintenance of Mouse Intestinal Stem Cells. Bio-protocol 6(4): e1733.
- Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., Van Es, J. H., Van den Brink, S., Van Houdt, W. J., Pronk, A., Van Gorp, J., Siersema, P. D., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141(5): 1762-1772.
- Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., van Es, J. H., Abo, A., Kujala, P., Peters, P. J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262-265.
- Tanaka, T., Kohno, H., Suzuki, R., Yamada, Y., Sugie, S. and Mori, H. (2003). A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci 94(11): 965-973.
- Wallach, T. E. and Bayrer, J. R. (2017). Intestinal Organoids: New Frontiers in the Study of Intestinal Disease and Physiology. J Pediatr Gastroenterol Nutr 64(2): 180-185.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Klemke, L., Blume, J. P., De Oliveira, T. and Schulz-Heddergott, R. (2022). Preparation and Cultivation of Colonic and Small Intestinal Murine Organoids Including Analysis of Gene Expression and Organoid Viability. Bio-protocol 12(2): e4298. DOI: 10.21769/BioProtoc.4298.
Category
Cancer Biology > Invasion & metastasis > Cancer therapy
Cell Biology > Cell-based analysis
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link