- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Electrophysiology of Murine Sympathetic Postganglionic Neurons in the Thoracic Paravertebral Ganglia
Published: Vol 11, Iss 20, Oct 20, 2021 DOI: 10.21769/BioProtoc.4189 Views: 4889
Reviewed by: Mary L. PhillipsAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Method for Extracellular Electrochemical Impedance Spectroscopy on Epithelial Cell Monolayers
Athena J. Chien [...] Craig R. Forest
Jun 20, 2025 3218 Views

In Vitro Co-culture of Bacterial and Mammalian Cells to Investigate Effects of Potential Probiotics on Intestinal Barrier Function
Ajitpal Purba [...] Dulantha Ulluwishewa
Jun 20, 2025 2515 Views

Whole-Mount Immunostaining for the Visual Separation of A- and C-Fibers in the Study of the Sciatic Nerve
Valeriia Ustymenko [...] Nana Voitenko
Dec 5, 2025 1421 Views
Abstract
The thoracic paravertebral sympathetic chain postganglionic neurons (tSPNs) represent the predominant sympathetic control of vascular function in the trunk and upper extremities. tSPNs cluster to form ganglia linked by an interganglionic nerve and receive multisegmental convergent and divergent synaptic input from cholinergic sympathetic preganglionic neurons of the spinal cord (Blackman and Purves, 1969; Lichtman et al., 1980). Studies in the past have focused on cervical and lumbar chain ganglia in multiple species, but few have examined the thoracic chain ganglia, whose location and diminutive size make them less conducive to experimentation. Seminal studies on the integrative properties of preganglionic axonal projections onto tSPNs were performed in guinea pig (Blackman and Purves, 1969; Lichtman et al., 1980), but as mice have become the accepted mammalian genetic model organism, there is need to reproduce and expand on these studies in this smaller model. We describe an ex vivo approach that enables electrophysiological, calcium imaging, and optogenetic assessment of convergence, divergence, and studies on pre- to postganglionic synaptic transmission, as well as whole-cell recordings from individual tSPNs. Preganglionic axonal connections from intact ventral roots and interganglionic nerves across multiple segments can be stimulated to evoke compound action potential responses in individual thoracic ganglia as recorded with suction electrodes. Chemical block of synaptic transmission differentiates spiking of preganglionic axons from synaptically-recruited tSPNs. Further dissection, including removal of the sympathetic chain, enables whole-cell patch clamp recordings from individual tSPNs for characterization of cellular and synaptic properties.
Keywords: MouseBackground
Thoracic sympathetic postganglionic neurons (tSPNs) are housed in bilateral paravertebral chain ganglia. Comprising a significant component of the final motor output of the sympathetic nervous system, paravertebral tSPNs contribute to autonomic homeostatic mechanisms by directly innervating effector tissues, including vasculature, adipose tissue, sweat glands, and piloerector muscles (Janig, 2006; Bartness et al., 2010). Thoracic sympathetic ganglia contain a distinct composition of genetically separable postganglionic neuron groups as compared to more rostral cervical and lower thoracic segments (Furlan et al., 2016). Their morphological and electrophysiological properties may also differ from sympathetic postganglionic neurons (SPNs) elsewhere (Jobling and Gibbins, 1999). Our understanding of the electrophysiological properties of paravertebral sympathetic chain postganglionic neurons has been largely derived from studies in cervical and lumbar ganglia (Eccles, 1935; Erulkar and Woodward, 1968; Lichtman et al., 1979; Purves and Wigston, 1983; Cassell et al., 1986; Li and Horn, 2006; Bratton et al., 2010) with intracellular recordings undertaken using sharp microelectrode approaches (Blackman and Purves, 1969; Lichtman et al., 1980; Jobling and Gibbins, 1999) (however, see Springer et al., 2015) with an impalement injury conductance that alters basic membrane properties (Staley et al., 1992; Rall, 2011; Springer et al., 2015).
Despite their physiological importance, our understanding of the function of paravertebral ganglia is based largely on work undertaken in larger mammalian models (Blackman and Purves, 1969; Lichtman et al., 1980; Rubin and Purves, 1980; Purves and Lichtman, 1985). Greater understanding of their operational properties has not benefited from powerful molecular toolkits provided by molecular genetic approaches in mouse models (Furlan et al., 2016). Unlike cervical and lumbar chain ganglia, tSPNs are situated in a protected paraspinal and subpleural location on the ventral wall of the thoracic cavity. This makes in vivo electrophysiological studies difficult and explains the need for ex vivo approaches (Blackman and Purves, 1969; Lichtman et al., 1980; Rubin and Purves, 1980). Moreover, as the largely transparent individual ganglia are very small (<200 μm in length) with the entire thoracic chain being <2 cm, dissection for study is challenging.
We also developed the ex vivo preparation to enable whole-cell recordings of tSPNs for assessment of membrane properties independent of impalement leak produced with sharp microelectrodes. Results revealed an order of magnitude higher membrane resistivity and associated amplified excitability, with greater intrinsic capacity for synaptic integration and the ability for maintained firing (McKinnon et al., 2019).
To undertake whole-cell patch recordings, the chain is removed entirely from the vertebral column using fine iridectomy microdissection scissors and glass probes (McKinnon et al., 2019). With this method, one can investigate multisegmental preganglionic actions in tSPNs in mice (cp Lichtman et al., 1980), including via optogenetic recruitment of preganglionic cholinergic axons using ChAT::CHR2 mice (Llewellyn et al., 2010). As there are several genetically distinct populations of sympathetic preganglionic neurons (Deuchars and Lall, 2015; Blum et al., 2020), various cre-based driver approaches can be leveraged to study multisegmental actions arising from different preganglionic populations. Capturing individual or population activity of genetically distinct tSPNs is also possible with the use of cre-based reporters or genetically-encoded Ca2+ indicators (Furlan et al., 2016). In summary, the described approach enables the use of mice for highly accessible ex vivo studies for electrophysiology, calcium imaging, optogenetics, and pharmacology for cellular and circuit studies on input-output relations of the thoracic paravertebral chain.
Materials and Reagents
2 mm glass probes made from stringer (Bullseye Glass, catalog number: 000147-0272-F-Tube)
½ ml Monoject Insulin Syringe, 29 G × ½” (Covidien, catalog number: 8881600350)
Needle 25 G (BD PrecisionGlide, catalog no: 14-826G)
1.5 ml MCT Graduated Mixed Tubes (Fisherbrand, catalog number: 05-408-137)
0.20 mm Stainless Steel Insect pins (Fine Science Tools, catalog number: 26002-20 )
0.22 μm Syringe Filter (Fisherbrand, catalog number: 09-719C)
100 mm × 15 mm Petri Dish (VWR, catalog number: 25384-070)
Square plastic weighing dish (Dyn-A-Med, catalog number: 80055)
Electrode Storage (World Precision Instruments, catalog number: E215)
VWR micro cover glass 24 × 50 mm (VWR, catalog number: 48393081)
Adult C57Blk/6 mice (6+ weeks old, alternative animal strains can be used)
Collagenase Type III (Worthington Biochemical Corporation, catalog number: LS004180)
Isoflurane, USP (Piramal Critical Care, catalog number: 400648037)
Urethane (Sigma-Aldrich, catalog number: U2500)
Ketamine (Henry Schein, catalog number: 056344)
Xylazine (Sigma-Aldrich, catalog number: X1251)
Custom-built Sylgard-coated dissecting dish (see Procedure D)
SYLGARDTM 170 Silicone Elastomer Kit (DOW Inc., catalog number: 4026157)
High vacuum grease (DOW Corning, catalog number: H051J89018)
Sodium chloride (Fisher Scientific, catalog number: S642-500)
Potassium chloride (Sigma-Aldrich, catalog number: P9541-1KG)
Magnesium sulfate Heptahydrate (Fisher Scientific, catalog number: BP213-1)
Calcium chloride dihydrate (Fisher Scientific, catalog number: C69-500)
Potassium phosphate monobasic (Sigma-Aldrich, catalog number: P5655-500G)
D-(+)-Glucose (Sigma-Aldrich, catalog number: G7528-1KG)
Sodium bicarbonate (Sigma-Aldrich, catalog number: S6297-1KG)
95% O2, 5% CO2 gas (Nexair, catalog number: UN3156)
Potassium D-gluconate, 99% (Alfa Aesar, catalog number: B25135)
EGTA (Sigma-Aldrich, catalog number: E-3889)
HEPES (Sigma-Aldrich, catalog number: H3375-250G)
ATP (Sigma-Aldrich, catalog number: A9187)
GTP (Sigma-Aldrich, catalog number: G9002)
Artificial Cerebrospinal Solution (aCSF) for Electrophysiological Recordings (see Recipes)
High Magnesium Low Calcium Microdissection Solution (see Recipes)
Patch Electrode Solution (see Recipes)
Equipment
Surgical tools
2.5 mm Vannas spring scissors (Fine Science Tools, catalog number: 15002-08)
Wagner scissors (Fine Science Tools, catalog number: 14068-12)
9 mm Castroviejo microdissecting spring scissors (Roboz, catalog number: Rs-5658)
Dumont #5 Forceps (Fine Science Tools, catalog number: 11251-20)
pH meter (Denver Instruments, model: UB-10)
Osmometer (Vapro, model: Model 5600)
Magnetic stir plate (IKA Works USA, model: CERAMAG Midi)
Magnetic stir bar (VWR, catalog number: 76006-400)
600 ml beaker (Pyrex, catalog number: CLS1000600)
Recording equipment for whole-cell recordings
Generally, there are multiple commercially available components that can be used to undertake visually guided whole-cell recordings from microdissected nervous tissue. We used an upright microscope containing 40× liquid immersion objectives with differential interference contrast and infrared imaging for image enhancement. Conventional electrophysiology recording methods were employed, including an experimental chamber with oxygenated aCSF, air table, micromanipulator with attached electrode holder, high impedance low noise patch clamp amplifier, A/D converter, and associated specialty software for data capture. Details are provided in various reviews. Those used by our lab are described in a recent publication (McKinnon et al., 2019) as listed below (Figure 1).
Upright DIC fluorescence microscope (Olympus, model: BX51WI) affixed with a low-light camera (Olympus, model: OLY-150)
Vertical Electrode Puller (Narishige, model: PP-83)
Micro-forge (Narshige, model: MF-9)
MultiClamp 700A and Digidata 1322A (Molecular Devices)
Custom Sylgard-coated recording chamber and perfusion system
Controllable blue light laser (custom built but are also commercially available; e.g., Thorlabs S1FC473MM Multimode Fiber-Coupled Laser)
Electrical stimulation can be provided by commercially available stimulators (e.g., A-M Systems, model: 2200 Analog Stimulus Isolator)
Gravity fed superfusion with Masterflex pump for recirculation (Cole Palmer, model: 77200-50)
Tubing for perfusion (Masterflex, catalog number: 96412-16)
1.5-mm outer diameter filamented, borosilicate glass capillaries (World Precision Instruments, catalog number: TW150F-4)
Coated silver wire (A-M Systems, catalog number: 787000)
Connector Socket 20-24AWG gold crimp (Digi-Key, catalog number: 205090-1)
Connector D-Sub Pin 20-24 AWG AU (Digi-key, catalog number: 205089-1)
Silicone tubing for electrode (Masterflex, catalog number: 96410-10)
Cotton Swab with plastic shaft (Just The Basics, catalog number: PINSB0300DLJB01)
Quick-Setting Epoxy Syringe (J-B Weld, catalog number: 50112)
4-Indent D-Sub Crimper 26-20 AWG (Paladin Tools, model: PA1440)
Multipurpose wire stripper and cutter (Klein Tools Inc., model: 1010)
Three-Way, Stopcock with Male Luer Lock, Non-Sterile (Cole-Palmer, catalog number: UX-30600-02)
5 ml disposable syringe with Luer Lock (BD, catalog number: 309646)
Electrical tape (VWR, catalog number: 470020-186)
Insulated shielded Copper Wire 20AWG 300V BLK 25' (CNC Tech, catalog number: 1430-20-1-0500-001-1-TS)
Ground electrode probe (WPI, model: Ep2)
Communication Cable (Belden, catalog number: 8441)
Double banana plug Connectors for amplifier (Pomona Electronics, catalog number: 1330)
Single banana Plug (Pomona Electronics, catalog number: 1325)
Precision screwdriver (Westward, model: 401L69)
Clorox bleach (VWR, catalog number: 89501-620)

Figure 1. Materials for manufacturing suction electrodes. (A) Three-way stopcock with Luer lock. (B) Blunt 25 G needle. (C) Silicone tubing. (D) Insulated copper wire. (E) Communication cable. (F-G) Coated silver wire. (H) Glass electrode. (I) Quick-setting epoxy syringe. (J) D-sub crimper. (K) Cotton swab with plastic shaft. (L) Double banana plug. (M) Single banana plug. (N) Connector socket. (O) Connector D-Sub pin. (P) Wire stripper and cutter.Recording equipment for studies involving multisegmental preganglionic and postganglionic compound action potentials
Stereo dissecting microscope with 0.8-5.6× zoom range and DFPL 0.5× objective (Olympus, model: SZX7)
Multichannel differential amplifier (custom-built but are also commercially available (e.g., A-M Systems, model: 1700 Differential AC Amplifier)
Constant current stimulator (custom built but are also commercially available; e.g., A-M Systems, model: 2200 Analog Stimulus Isolator)
Controllable blue light laser (custom built but are also commercially available; e.g., Thorlabs, S1FC473MM Multimode Fiber-Coupled Laser)
Magnetic stand with 3-axis manual micromanipulator control (Kanetec, model: MB-PSL)
Gravity fed superfusion with Masterflex pump for recirculation (Cole Palmer, catalog number: 77200-50)
Tubing for perfusion (Masterflex, catalog number: 96412-16)
Digidata 1322A (Molecular Devices)
1.5-mm outer diameter filamented, borosilicate glass capillaries (World Precision Instruments, catalog number: TW150F-4)
Coated silver wire (A-M Systems, catalog number: 787000).
Connector Socket 20-24AWG gold crimp (Digi-Key, catalog number: 205090-1).
Connector D-Sub Pin 20-24 AWG AU (Digi-key, catalog number: 205089-1).
Ground electrode probe (WPI, model: Ep2)
Communication Cable (Belden, catalog number: 8441)
Double banana plug Connectors for amplifier (Pomona Electronics, catalog number: 1330)
Single banana Plug (Pomona Electronics, catalog number: 1325)
Image Capture Equipment for Calcium Imaging
AC/DC differential amplifier (A-M Systems, model: 51249)
Master 8 system and an ISO-Flex stimulus isolator (A.M.P.I, model: 1955)
Inverted microscope (Olympus, model: IX70)
Xenon lamp housing (Olympus, model: U-ULS75XE)
Power supply (Olympus, model: AH2-RX-T)
Neutral density filter (Olympus, catalog number: Chroma ND-50)
Uniblitz shutter (Vincent Associates, model: VCM-D1)
400 nm dichroic mirror (Olympus, model: Chroma NC474265)
490-520nm Emission filter (Olympus, model: Chroma U-MF2)
CCD camera (Stanford Photonics, model: XR/ABF with XR/M camera)
Trinitron color video monitor (Sony, model: PVM-135MD)
Software
Clampex software (Molecular Devices, RRID: SCR_011323)
PCI software (Hamamatsu, Sunayama-cho, Naka-ku, Hamamatsu City, Shizuoka, Japan)
Fiji (ImageJ from NIH)
Procedure
We describe the methodology developed for an ex vivo mouse model for physiologic characterization of their properties. Procedures and all experiments presented were undertaken in adult (8+ weeks) C57Bl/6 mice, but the approach has been used in mice as young as postnatal day 7. Thoracic chain ganglia in continuity with communicating rami, spinal nerves, and ventral roots are not dissected from surrounding tissue. Rather, they are left adherent to the ribcage to prevent nerve injury. Access to communicating rami in mice is difficult, so recruitment of preganglionic axons is instead achieved by stimulating ventral roots. Specialty fabrication of tight-fitting glass suction electrodes enables stable extracellular recordings of compound action potentials.
The anatomical organization of preganglionic projections to tSPNs is shown and described in Figure 2. To access the sympathetic ganglia and connections to the ventral root for recording, the following series of procedures are important: (1) the peritoneum must be removed; (2) to facilitate recordings, the chain should be carefully separated from embedded brown adipose tissue, and care is required as separation can easily sever the interganglionic nerve or rami; (3) as the ganglia themselves are shrouded in a collagen sheath, with individual tSPNs further encased in a glial covering, preparations that desire access to individual neurons for whole-cell recordings require both preincubation in collagenase and mechanical disruption of the glial casing using patch electrode shaped capillary tubes attached to a micromanipulator or glass probes.
Preparation for experimental studies first involves dissecting the vertebral column by making lateral incisions through the ribcage. Using a combination of iridectomy microdissection scissors, forceps, and fine-tipped glass probes, the peritoneum is peeled away. While collagenase is required for whole-cell recordings, we have undertaken population studies without it (Blum et al., 2020). Nonetheless, seal quality and signal resolution of population suction electrode recordings are superior following collagenase treatment. If collagenase is used, the vertebral column is allowed to incubate in a heated chamber filled with continuously oxygenated artificial cerebrospinal fluid (aCSF) and collagenase solution (Blum et al., 2020). The chain is cleaned of remaining fat and connective tissue. A ventral vertebrectomy exposes the spinal cord and ventral roots.
An ex vivo approach offers the ability to superfuse pharmacological agents to assess actions at known doses without concern for drug access (Blackman and Purves, 1969; Lichtman et al., 1980; Thorne and Horn, 1997; Ireland et al., 1998). This is shown in Figure 3 with blocked recruitment of tSPNs following application of hexamethonium, a ganglionic nicotinic receptor antagonist (Li and Horn, 2006; Wehrwein et al., 2016). For studies on recruitment of segmental preganglionic axons, the spinal cord and dorsal roots are removed, leaving behind only intact ventral root connections.

Figure 2. Anatomical organization of preganglionic projections to tSPNs. (A) Ex vivo dissection and identification of chain ganglia. Transparent chain ganglia are shown in the right panel (arrows). (B-C) Overview of anatomical organization of connectivity between sympathetic preganglionic and postganglionic neurons. Preganglionic axons exit the ventral root and enter the sympathetic chain via the white ramus. Those innervating paravertebral postganglionic neurons (tSPNs) do so within several chain ganglia via rostrocaudally projecting collaterals in the interganglionic nerve. Other preganglionics axons do not innervate tSPNs within chain ganglia but may travel along the interganglionic nerve before exiting to innervate prevertebral ganglia (not shown).
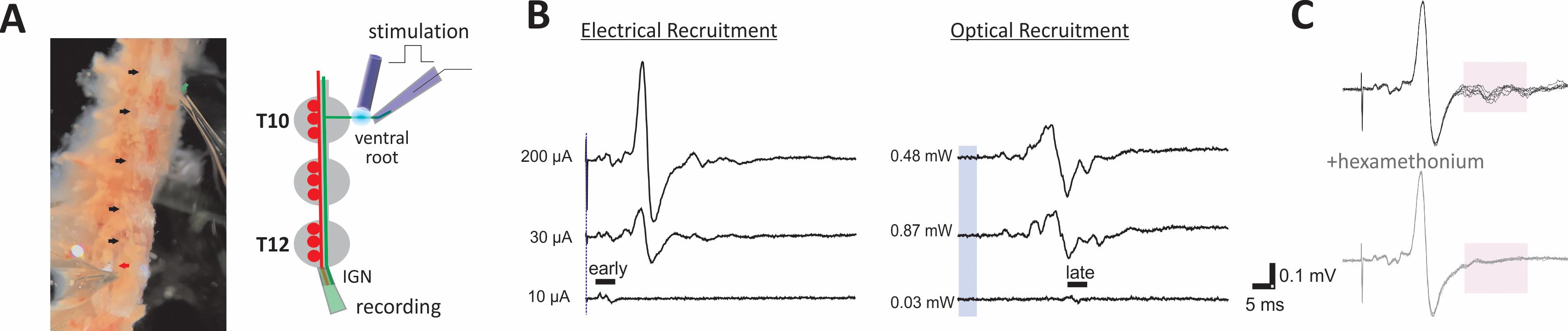
Figure 3. Ex vivo dissection enables the study of multisegmental preganglionic actions. (A) Experimental configuration. Trumpet-shaped glass electrodes placed on the ventral root (green arrow) and IGN (red arrow) enable stable extracellular recordings (left). Ganglia between sites of stimulation and recording are indicated by black arrows. Scheme of the experimental configuration for panels B and C shown on the right. (B) Recruitment of preganglionic axons and evoked synaptic tSPN responses following ventral root stimulation. Examples of electrical (left) and optogenetic (blue laser light; right) intensity-dependent recruitment of preganglionic axons from the the T10 ventral root in ChAT::CHR2 transgenic mice. Here, the recording suction electrode captured direct and synaptically-evoked population responses from the cut interganglionic nerve immediately caudal to the T12 chain ganglion. Stimulation intensity differences in recruitment are shown with duration kept constant (200 ms for electrical; 5 ms for optical). Blue vertical line and bar represent respective stimulus timing. Waveforms are averages of three episodes at labeled intensities. Shown is preferential intensity-dependent recruitment of the fastest conducting axons with electrical (early) and slowest conducting (late) axons with optogenetic stimulation, respectively. Recruitment profiles were undertaken in collagenase-treated tissue from a ChAT::ChR2 mouse. (C) Use of ganglionic blockers to discriminate preganglionic from postganglionic activity. Block of synaptically-recruited postganglionic (tSPN) activity following application of the nicotinic receptor antagonist hexamethonium (100 µM) is shown in the lower panel. The shaded area in both panels highlights this loss.
See Video 1 for instructions on tissue preparation. Video time stamps of individual steps are provided in the text.
Ex vivo mouse dissection for experiments recording multisegmental pre and postganglionic compound action potentials.
A1. Tissue PreparationAnesthetize mice with inhaled isoflurane. Maintain with urethane (intraperitoneal injection, at 2 g/kg for in vitro electrophysiology). Confirm anesthetic depth via lack of pinch-evoked foot withdrawal reflex and eye blink reflex.
Use 25 G needles to pin animal down with dorsal side facing up in a dissection dish.
Pinch the skin near the tail and use scissors to cut up midline to remove dorsal skin and expose underlying dorsal surface of the vertebral column. The visible dorsal surface includes muscle overlying the vertebral column and dorsal spinal processes. Use forceps to remove excess or loose hair (Video 1. Sympathetic Chain Dissection 0:00-1:18).
To remove thoracic vertebral column and attached chain, begin lateral incisions 5 mm away from the midline near the L3 vertebrae. Cut through muscle and ribcage rostrally to the T1 vertebrae ensuring the scissors run parallel to the midline. Repeat incision on the opposite side. Pinch the column and make a transverse cut below T3. Lift vertebral column to separate and cut viscera. Make a transverse cut above T1 to remove thoracic vertebral column (Video 1. Sympathetic Chain Dissection 1:20-2:40).
Quickly rinse isolated tissue in oxygenated high-Mg2+ (6.5 mM)/low-Ca2+ (1.2 mM) aCSF (see Recipe 1) to remove excess blood and fat.
Perform collagenase treatment (if desired). Place tissue in a 1.5 ml tube or dish filled with a continuously oxygenated solution of 20 mg-Type III Collagenase per 1-ml aCSF (see Recipe 2), maintained at 36°C for 1 h. If necessary, trim tissue to fit collagenase treatment tube (Video 1. Sympathetic Chain Dissection 3:13-3:24).
A2. Tissue Stabilization and Electrode Placement for Compound Action Potential RecordingsIf collagenase treatment was used, remove tissue from 1.5 ml tube and quickly rinse in oxygenated aCSF (see Recipe 2) to remove digested tissue.
Pin vertebral column dorsal up in perfusion dissection dish filled with oxygenated high magnesium low calcium microdissection fluid at room temperature. Using scissors, remove excess fat and muscle to expose midline (Video 1. Sympathetic Chain Dissection 3:26-3:46).
Using scissors, cut the vertebral column along midline from rostral to caudal end (Video 1. Sympathetic Chain Dissection 4:08-5:08).
Flip tissue and pin such that dorsal side is facing down. Using microdissecting spring scissors, cut vertebral column along midline to expose the spinal cord (Video 1. Sympathetic Chain Dissection 5:09-7:30).
Carefully separate two halves of the tissue to expose spinal roots. Using Vannas microscissors, cut ventral roots close to the spinal cord. Cut dorsal roots close to the vertebral column. Once all roots have been cut, remove spinal cord with attached dorsal roots and discard (Video 1. Sympathetic Chain Dissection 7:59-11:23).
Before electrode placement, ensure the tissue is securely pinned. Using glass probes or forceps, carefully peel away any remaining peritoneum and allow the tissue to rest for 30 min. The time required to set up and position electrodes is the ideal time to allow the tissue to rest (Video 1. Sympathetic Chain Dissection 11:24-12:12).
At this point, individual ganglia of the paravertebral chain should be visible. Glass suction electrodes can be placed on ventral roots and ganglia of interest. To place electrodes, move the electrode manipulator close to the recording dish. Use the manipulator’s dials to move the electrode close to the intended nerve or ventral root such that the tip of the electrode is touching the cut end of the nerve or ventral root. Apply mild suction to stabilize initially. Once all electrodes are similarly placed, apply more suction to stabilize.
The sample can provide stable recordings for at least 12 h if the bath is continuously oxygenated and the bath temperature is maintained at 22°C. Viability at elevated temperatures has not been explored systematically.
The electrophysiology rig and perfusion system are detailed in Figure 4.
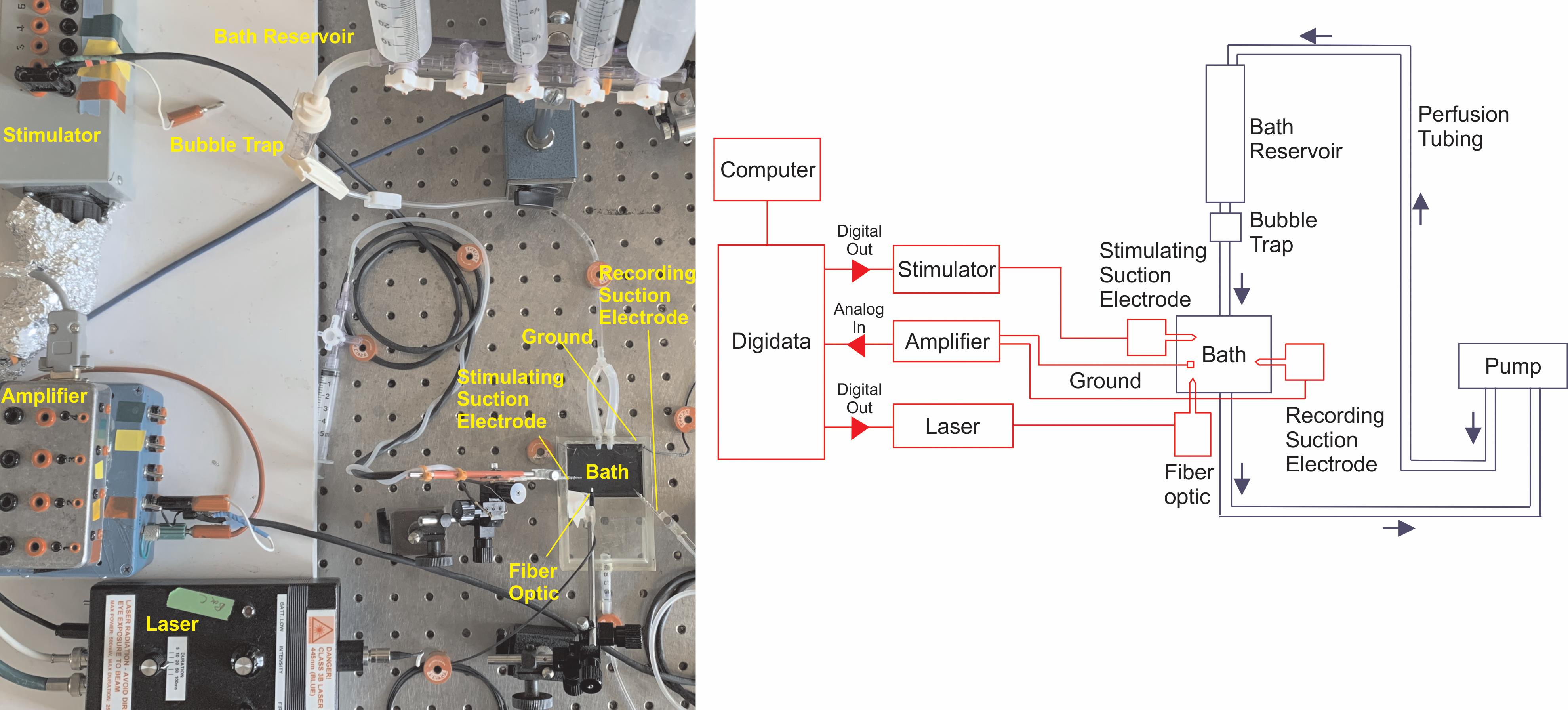
Figure 4. Overall set-up for experiments recording extracellular population potentials in paravertebral sympathetic chain ganglia. Left: Picture of the electrophysiology rig. Right: Schematic of electrophysiology rig shown in photograph. The wiring diagram is shown in red. The bath perfusion system is shown in blue.
Ex vivo mouse dissection for whole-cell patch clamp recordings or calcium imaging
B1. Tissue Preparation and Collagenase TreatmentAnesthetize mice as described in Procedure A1.1.
Pin animal down with dorsal-side facing up in a dissection dish.
Use scissors to remove dorsal skin and expose underlying vertebral column.
To remove thoracic vertebral column and attached chain, begin lateral incisions 5 mm away from the midline near the L3 vertebrae. Cut through muscle and ribcage rostrally to the T1 vertebrae ensuring the scissors run parallel to the midline. Repeat incision on the opposite side. Make transverse cuts above T1 and L3 to remove thoracic vertebral column.
Halve the spinal column by cutting along midline of vertebral column on dorsal and ventral sides. Remove the spinal cord and reserve both halves of the vertebral column for the next step.
Quickly rinse isolated vertebral columns in oxygenated high magnesium low calcium microdissection fluid (see Recipe 1) to remove excess blood and fat.
Place tissue in a 1.5 ml tube or dish filled with a continuously oxygenated solution of 20 mg-Type III Collagenase per 1-ml aCSF (see Recipe 2) maintained at 36°C for 1 h.
B2. Preparation for Whole-Cell Patch Clamp Recordings or Calcium ImagingRemove tissue from 1.5 ml tube and quickly rinse in oxygenated aCSF (see Recipe 2) to remove digested tissue and residual collagenase.
Pin vertebral column ventral up in perfusion dissection dish filled with oxygenated high magnesium low calcium microdissection solution (see Recipe 1) at room temperature.
Using glass probes or forceps, carefully peel away any remaining peritoneum.
Remove the chain by using microscissors to sever rami (Video 1. Sympathetic Chain Dissection 12:36-15:20).
Using forceps and insect pins, pin the chain down onto a clear Sylgaard recording dish through which recirculating, oxygenated aCSF (see Recipe 2) is continuously perfused. If the tissue is being used for calcium imaging place, the chain in a recording chamber fitted with a glass cover slip on the bottom. Take some high vacuum grease on a cotton swab and apply it to the bottom surface of the recording chamber. Press the glass cover slip onto the grease to secure the glass to the recording chamber and create a waterproof seal.
Before any electrode placement, allow the tissue to rest for 30 min. The time required to set up and position electrodes is the ideal time to allow the tissue to rest.
For calcium imaging, cells are imaged from below using an inverted Olympus scope. For whole-cell patch clamp, cells can be identified using an upright microscope affixed with a low-light camera. Differential interference contrast (DIC) microscopy can be used to visualize tSPNs, while appropriate epifluorescent illumination can be used to identify fluorescently labeled tSPNs (e.g., Cre-dependent reporter mice). See Figure 5 for representative examples of the field of view for whole-cell patch clamp (Figure 5B) and calcium imaging (Figure 5C) experiments.
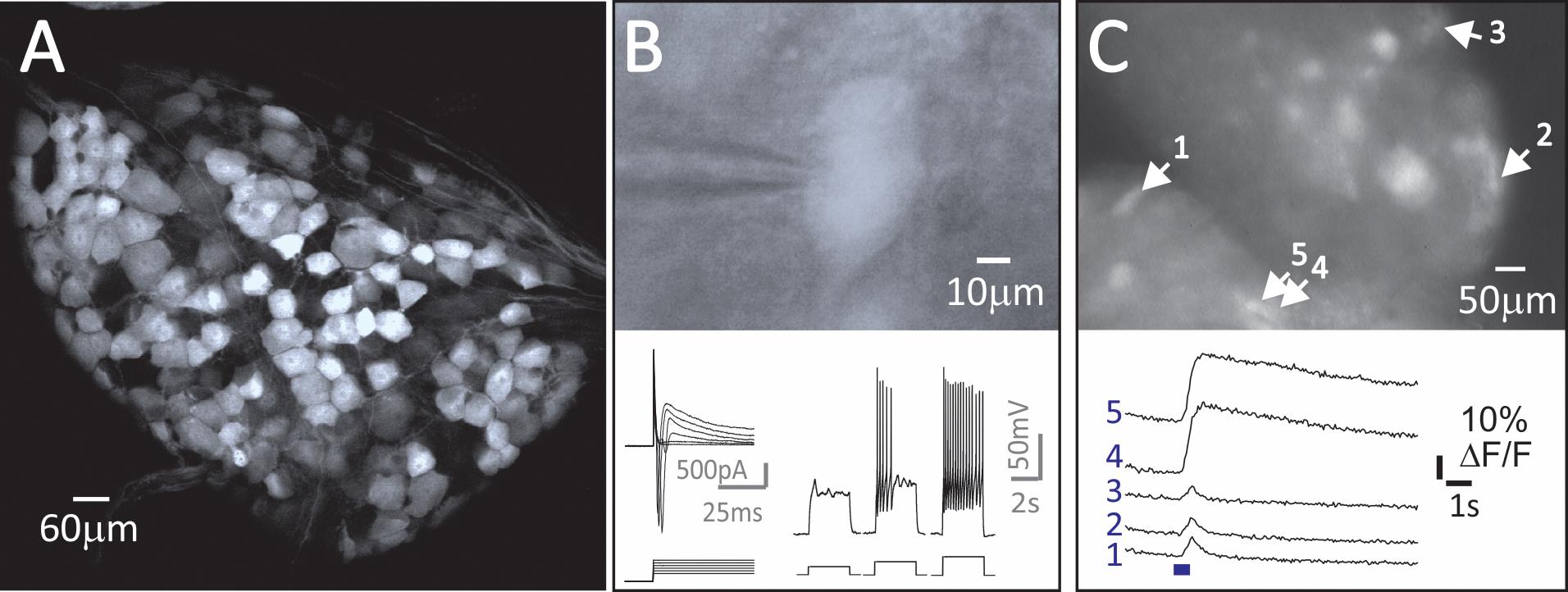
Figure 5. Genetic approaches enable the study of molecularly distinct tSPN subpopulations. (A) A large fraction of tSPNs express Neuropeptide Y (NPY), as shown in NPY::tdTomato mice (T8 sympathetic ganglion in 6 wk old mouse). (B-C) Studies on activity in NPY+ tSPNs. (B). Whole-cell recording from a fluorescently-identified NPY+ tSPN in a NYP::tdTomato mouse shows neuronal response properties to a series of depolarizing voltage or current steps. (C) Ca2+ signals in NPY::GCaMP6f tSPNs in T7-T8 whole thoracic ganglia evoked by stimulation of the interganglionic nerve. Shown are changes in activity following electrical stimulation with a 10Hz, 5-pulse train.After identifying a tSPN, gently clean the membrane by using an electrode filled with aCSF flushing the area lightly. Then replace the electrode with one filled with intracellular solution and gradually approach the tSPN with 0.2cc positive pressure, subsequently reduced to 0.05-0.1cc when the electrode is close to contacting the cell membrane.
Once the electrode contacts the membrane, release the positive pressure and apply a 0.1cc negative pressure and hold the electrode at -80mV. This allows the membrane to form a GOhM seal around the electrode tip. After the resistance is stable at GOhm range with ~-10 nA injected current, quickly apply 1cc syringe suction or a mouth suction pulse to break through the membrane.
The sample can provide stable recordings for at least 12 h if the bath is continuously oxygenated and temperature is maintained at 22°C.
Manufacture of Trumpet-Shaped Tips of Glass Suction Electrodes and Patch Electrodes (Figure 6)
Position glass capillary tubes on a vertical puller (Narishige, PP-83). Heat center and allow for two ends of capillary tube to be drawn apart 1-2 mm without breaking.
Allow the stretched capillary tube to come to room temperature. Without adjusting heating coil, heat capillary tube until two halves separate. Keep half with the bulbous tip.
Score electrode underneath the bulbous tip using the edge of a glass slide. Tap electrode tip against glass slide to remove section above the score mark.
Place electrode in a microforge (e.g., Narishige MF-9 Microforge) fitted with a curved filament. The electrode should be placed such that the neck of the electrode tip is equidistant from the curved edges of the filament.
Heat filament using the medium heat setting to trumpet the neck of the electrode. The trumpet shape promotes tight-fitting, stable recordings. The large-diameter mouth of the electrode allows the whole nerve (and sometimes ganglion) to enter the electrode, while the small-diameter neck locks the nerve in place.
Pull patch electrodes from borosilicate glass capillaries (1.5-mm outer diameter, 1.2-mm inside diameter, filamented, borosilicate glass capillaries on the vertical puller) for a target resistance of 4-7 MΩ. Fill electrodes with patch electrode solution (see Recipe 3).
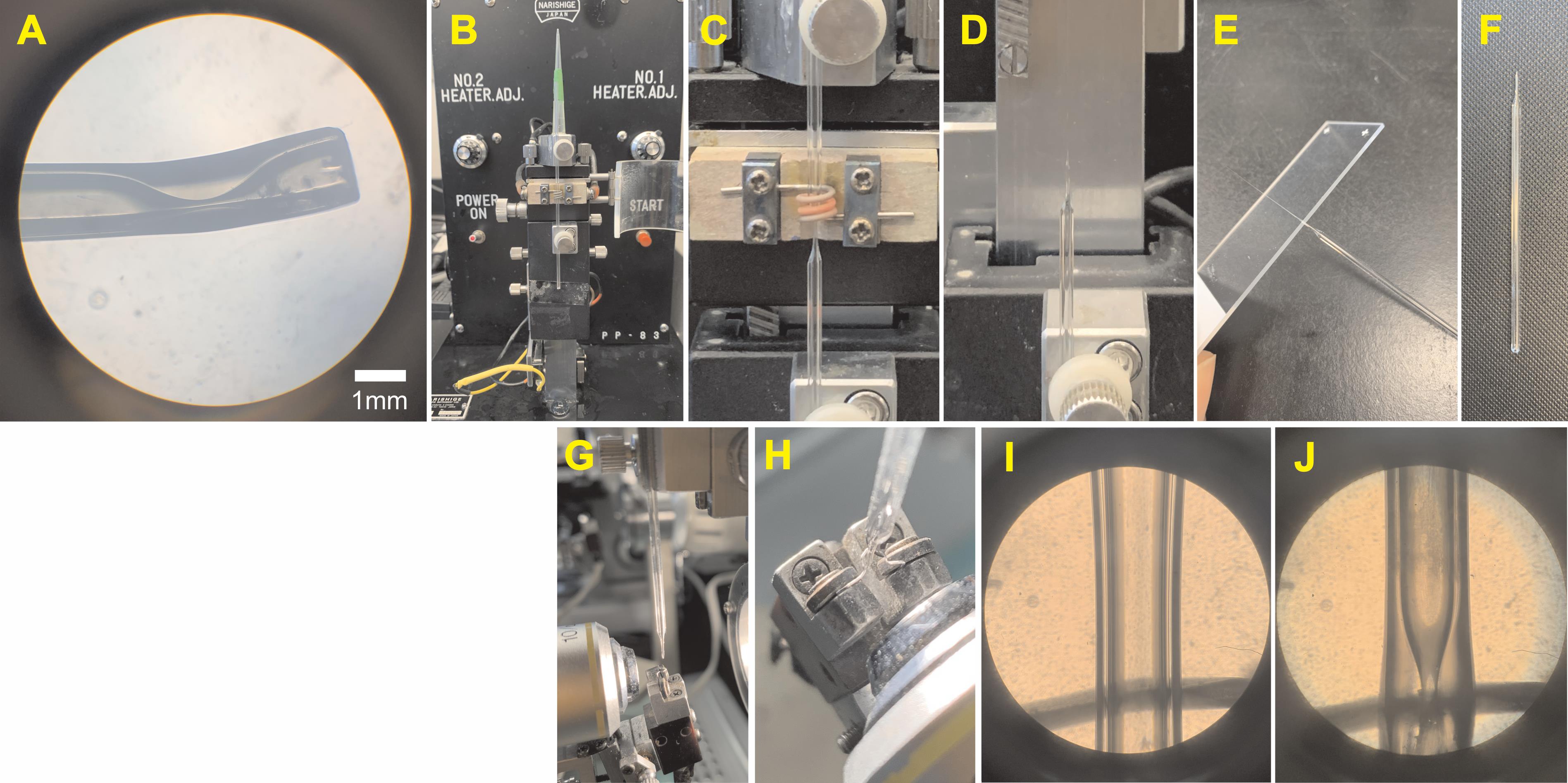
Figure 6. Trumpet-Shaped Glass Suction Electrodes. (A) Magnified view of the electrode tip used for recording and stimulating. (B-J) Manufacture of suction electrodes. For detailed instructions, see “Procedure C.” (B) Glass capillary tube positioned on vertical puller. (C) Tube heated to form 1-2mm neck. (D) Tube heated to separate halves, keeping bottom half with the bulbous tip. (E-F) Glass slide used to score underneath the bulbous tip and break the tip. (G) Electrode placed in Microforge with a u-shaped filament. (H) Tip positioned at the center of the filament. (I-J) View of electrode in the Microforge before and after heating.
Manufacture of Sylgard-coated dissecting dish
Take the bottom half of a 100mm plastic Petri dish and set it on a flat surface.
Follow instructions found in the SYLGARDTM 170 Silicone Elastomer Kit to mix 40 ml of encapsulant in a disposable plastic weighing dish.
Slowly pour encapsulant into Petri dish until the dish is filled halfway up (roughly 35 ml of encapsulate). Quickly pouring creates bubbles and an uneven surface for dissection.
Gently tap Petri dish on a benchtop to release bubbles trapped in the encapsulant.
Set the dish aside in an undisturbed location to allow the encapsulant to cure for 24 h at room temperature.
Manufacture of suction electrodes (Figure 1)
Cut a section of insulated shielded two-wire cable to the desired length. The length will vary depending on how far the amplifier is from the recording dish. Extra length will help the electrode cable to be slack and prevent tugging issues during recording.
Use the wire stripper to strip 1.5 inches of the outer PVC jacket at each cut end to reveal the braided shielding.
Cut away the braided shielding at one end to reveal the insulated copper cables (typically black and red). At the other end, unbraid the shield wire material from the pair of copper cables and re-twist into a point. Set cable aside.
Cut to free 5 inches of insulated copper wire. Use the wire stripper to strip 0.5 inches of insulation on both ends.
Splice together one end of the copper wire from Step E4 with the pointed shielding in step 3. Secure and insulate the spliced region with electrical tape.
Secure the other end of the copper to the single banana plug using a precision screwdriver to loosen the screw clamp in the plug to enable the copper wire to enter, then tighten to secure the wire.
Staying on the same side of the communication cable, use the wire stripper to strip 0.5 inches of insulation from the red and black wires. Secure the red and black wires to the double banana plug using the same procedure in Step E6.
Return to the side of the communication cable where the braided shielding was cut. Now use the wire stripper to strip 0.5 inches of insulation from the red and black wires. Using the D-sub crimping tool, crimp one D-Sub pin to each wire. The input cable is now complete and can be set aside.
To build the electrode holder, start by cutting a 1.5 inch piece from the plastic shaft of a cotton swab. Using a 25 G needle, gently poke a hole into the middle of the shaft, taking care not to poke through the opposite end.
Cut 4 inches of coated silver wire, thread it through the hole in the plastic shaft from the outside, and feed 3 inches through. Use epoxy to seal the hole and secure the wire tightly. Take care not to fill the shaft with epoxy as this will prevent you from applying suction to the electrode.
After the epoxy is set, cut 1 inch of silicone tubing and connect it to the end of the plastic shaft where the silver wire is protruding. The glass electrode will be placed on this end of the electrode holder.
Next, cut 1 foot of silicone tubing and connect it to the other end of the plastic shaft. Connect a blunted 25 G needle to the end of the silicone tubing and seal any gaps with epoxy. Connect a three-way stopcock to the 25 G needle. Connect a 5ml disposable syringe to the end of the stopcock.
Cut 4 inches of coated silver wire. Using a lighter, burn off 0.5 inches of the plastic coating on either end. Do the same for the epoxied silver wire in step 10. Coated wires that are bare at the top provide more stable recordings if DC-like events are acquired. For example, artifacts caused by minor changes in bath volume can be minimized.
Use the crimping tool to crimp a connector socket to one end of the cut coated silver wire from Step E13 and coated silver wire from Step E10.
Chemically chloride the uncrimped ends of the silver wires by dipping the ends into Clorox bleach for 24 h.
Notes
Signals were amplified using a MultiClamp 700A and digitized at 10 kHz using a Digidata 1322A and Clampex software (Molecular Devices, RRID: SCR_011323).
Recipes
High-Mg2+/Low-Ca2+ Microdissection Solution
127.99 mM NaCl
1.90 mM KCl
6.5 mM MgSO4·7H2O
0.85 mM CaCl2·2H2O
1.20 mM KH2PO4
9.99 mM glucose
26.04 mM NaHCO3
The pH is adjusted to 7.4 after saturation with gas (95% O2, 5% CO2) at room temperature.
The recommended volume to prepare for this protocol is 250 ml.
Artificial Cerebrospinal Solution (aCSF) for Electrophysiological Recordings
127.99 mM NaCl
1.90 mM KCl
1.30 mM MgSO4·7H2O
2.40 mM CaCl2·2H2O
1.20 mM KH2PO4
9.99 mM glucose
26.04 mM NaHCO3
The pH is adjusted to 7.4 after saturation with gas (95% O2, 5% CO2) at room temperature. aCSF may be buffered with HEPES or bicarbonate.
The recommended volume to prepare for this protocol is 500 ml.
Patch Electrode Solution
140.0 mM K-gluconate
11.0 mM EGTA
10 mM HEPES
1.32 mM CaCl2
The pH is adjusted to 7.3 using KOH. Target osmolarity was 290 mOsm. In most recordings (25/39 cells), a support solution was added consisting of 4.0 mM ATP and 1.0 mM GTP.
The recommended volume to prepare for this protocol is 3 ml.
Acknowledgments
This work was supported by the National Institutes of Health Grant 5R01NS102871 and the Department of Defense Grant SCI-30225.
Procedures associated with whole-cell recordings are as described in McKinnon et al. (2019). "Dramatically Amplified Thoracic Sympathetic Postganglionic Excitability and Integrative Capacity Revealed with Whole-Cell Patch-Clamp Recordings." eNeuro 6(2): ENEURO.0433-0418.2019.
Competing interests
No competing interests.
Ethics
All animal procedures were performed in accordance with the Emory University Institutional Animal Care and Use Committee’s regulations and conformed to the Guide for the Care and Use of Laboratory Animals under protocol # PROTO201700855 (exp 3/26/2023).
References
- Bartness, T. J., Vaughan, C. H. and Song, C. K. (2010). Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 34 Suppl 1: S36-42.
- Blackman, J. G. and Purves, R. D. (1969). Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol 203(1): 173-198.
- Blum, J. A., Klemm, S., Nakayama, L., Kathiria, A., Guttenplan, K. A., Hoang, P. T., Shadrach, J. L., Kaltschmidt, J. A., Greenleaf, W. J. and Gitler, A. D. (2020). Single-cell transcriptomic analysis of the adult mouse spinal cord. bioRxiv: 2020.2003.2016.992958.
- Bratton, B., Davies, P., Janig, W. and McAllen, R. (2010). Ganglionic transmission in a vasomotor pathway studied in vivo. J Physiol 588(Pt 9): 1647-1659.
- Cassell, J. F., Clark, A. L. and McLachlan, E. M. (1986). Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol 372: 457-483.
- Deuchars, S. A. and Lall, V. K. (2015). Sympathetic preganglionic neurons: properties and inputs. Compr Physiol 5(2): 829-869.
- Eccles, J. C. (1935). The action potential of the superior cervical ganglion. J Physiol 85(2): 179-206 172.
- Erulkar, S. D. and Woodward, J. K. (1968). Intracellular recording from mammalian superior cervical ganglion in situ. J Physiol 199(1): 189-203.
- Furlan, A., La Manno, G., Lubke, M., Haring, M., Abdo, H., Hochgerner, H., Kupari, J., Usoskin, D., Airaksinen, M. S., Oliver, G., Linnarsson, S. and Ernfors, P. (2016). Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control. Nat Neurosci 19(10): 1331-1340.
- Ireland, D. R., Davies, P. J. and McLachlan, E. M. (1998). The role of N-type Ca2+ channels in regulating excitability of guinea-pig sympathetic neurones. J Auton Nerv Syst 73(2-3): 109-114.
- Janig, W. (2006). The Integrative Action of the Autonomic Nervous System, Cambridge University Press.
- Jobling, P. and Gibbins, I. L. (1999). Electrophysiological and morphological diversity of mouse sympathetic neurons. J Neurophysiol 82(5): 2747-2764.
- Li, C. and Horn, J. P. (2006). Physiological classification of sympathetic neurons in the rat superior cervical ganglion. J Neurophysiol 95(1): 187-195.
- Lichtman, J. W., Purves, D. and Yip, J. W. (1979). On the purpose of selective innervation of guinea-pig superior cervical ganglion cells. J Physiol 292: 69-84.
- Lichtman, J. W., Purves, D. and Yip, J. W. (1980). Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol 298: 285-299.
- Llewellyn, M. E., Thompson, K. R., Deisseroth, K. and Delp, S. L. (2010). Orderly recruitment of motor units under optical control in vivo. Nat Med 16(10): 1161-1165.
- McKinnon, M. L., Tian, K., Li, Y., Sokoloff, A. J., Galvin, M. L., Choi, M. H., Prinz, A. and Hochman, S. (2019). Dramatically Amplified Thoracic Sympathetic Postganglionic Excitability and Integrative Capacity Revealed with Whole-Cell Patch-Clamp Recordings. eNeuro 6(2): ENEURO.0433-0418.2019.
- Purves, D. and Lichtman, J. W. (1985). Geometrical differences among homologous neurons in mammals. Science 228(4697): 298-302.
- Purves, D. and Wigston, D. J. (1983). Neural units in the superior cervical ganglion of the guinea-pig. J Physiol 334(1): 169-178.
- Rall, W. (2011). Core Conductor Theory and Cable Properties of Neurons. Comprehensive Physiology.
- Rubin, E. and Purves, D. (1980). Segmental organization of sympathetic preganglionic neurons in the mammalian spinal cord.J Comp Neurol 192(1): 163-174.
- Springer, M. G., Kullmann, P. H. and Horn, J. P. (2015). Virtual leak channels modulate firing dynamics and synaptic integration in rat sympathetic neurons: implications for ganglionic transmission in vivo. J Physiol 593(4): 803-823.
- Staley, K. J., Otis, T. S. and Mody, I. (1992). Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol 67(5): 1346-1358.
- Thorne, R. and Horn, J. P. (1997). Role of ganglionic cotransmission in sympathetic control of the isolated bullfrog aorta. J Physiol 498(1): 201-214.
- Wehrwein, E. A., Orer, H. S. and Barman, S. M. (2016). Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol 6(3): 1239-1278.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Halder, M., McKinnon, M. L., Li, Y., Wenner, P. and Hochman, S. (2021). Isolation and Electrophysiology of Murine Sympathetic Postganglionic Neurons in the Thoracic Paravertebral Ganglia. Bio-protocol 11(20): e4189. DOI: 10.21769/BioProtoc.4189.
- McKinnon, M. L., Tian, K., Li, Y., Sokoloff, A. J., Galvin, M. L., Choi, M. H., Prinz, A. and Hochman, S. (2019). Dramatically Amplified Thoracic Sympathetic Postganglionic Excitability and Integrative Capacity Revealed with Whole-Cell Patch-Clamp Recordings.eNeuro 6(2): ENEURO.0433-0418.2019.
Category
Neuroscience > Peripheral nervous system > Sciatic nerve
Cell Biology > Cell-based analysis > Electrophysiological technique
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









