- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Swimming-based Assay to Determine the Exercise Capacity of Adult Zebrafish Cardiomyopathy Models
Published: Vol 11, Iss 15, Aug 5, 2021 DOI: 10.21769/BioProtoc.4114 Views: 3039
Reviewed by: William C. W. ChenJohn W PetersonAnonymous reviewer(s)
Abstract
Exercise capacity, measured by treadmill in humans and other mammals, is an important diagnostic and prognostic index for patients with cardiomyopathy and heart failure. The adult zebrafish is increasingly used as a vertebrate model to study human cardiomyopathy due to its conserved cardiovascular physiology, convenience for genetic manipulation, and amenability to high-throughput genetic and compound screening. Owing to the small size of its body and heart, new phenotyping assays are needed to unveil phenotypic traits of cardiomyopathy in adult zebrafish. Here, we describe a swimming-based functional assay that measures exercise capacity in an adult zebrafish doxorubicin-induced cardiomyopathy model. This protocol can be applied to any adult zebrafish model of acquired or inherited cardiomyopathy and potentially to other cardiovascular diseases.
Graphic abstract:
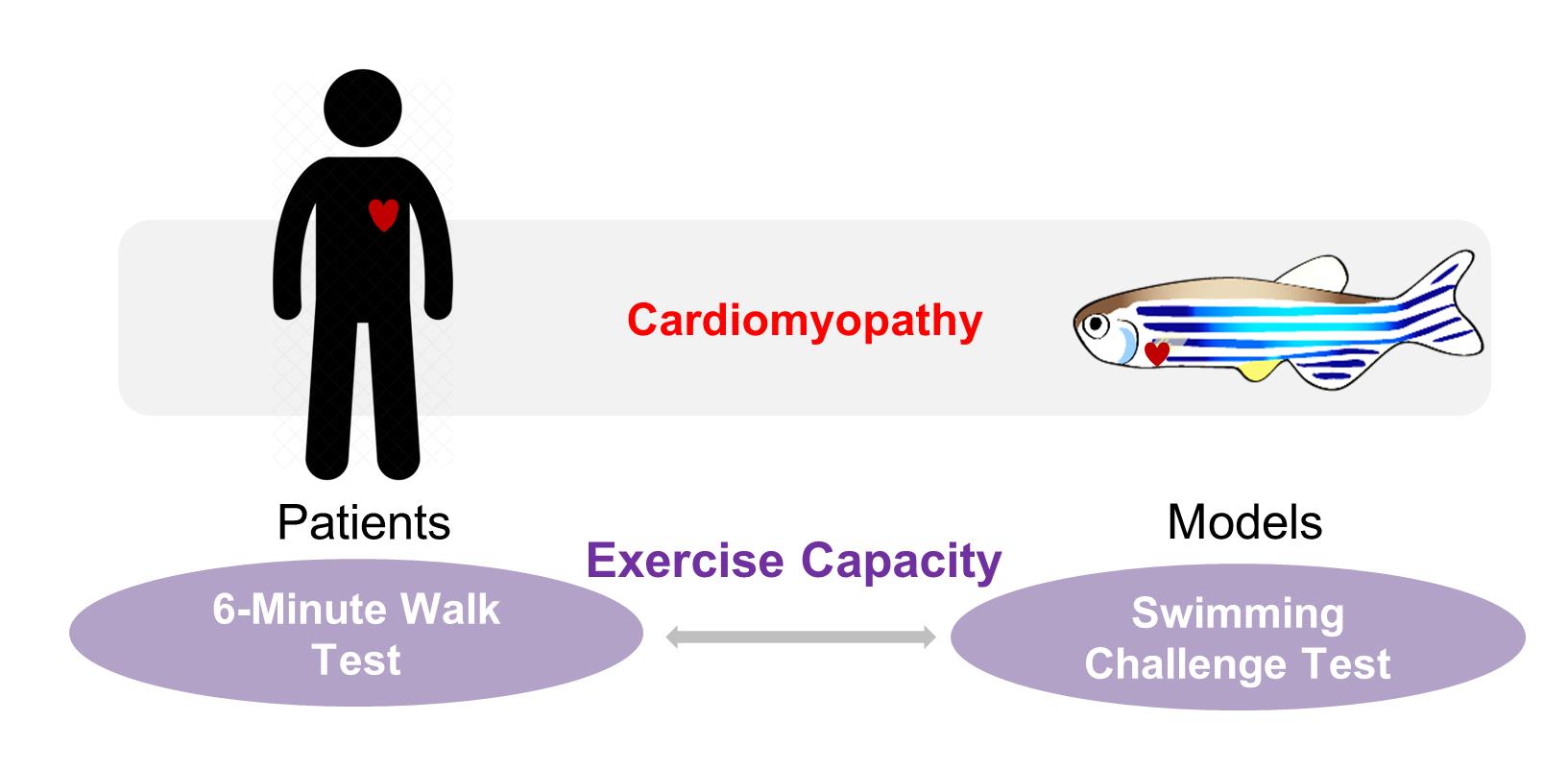
Clinical relevance of the swimming-based phenotyping assay in adult zebrafish cardiomyopathy models.
Background
Given its conserved cardiovascular physiology and genetic amenability, adult zebrafish is an emerging vertebrate model for cardiomyopathy studies (Gut et al., 2017). Since the first evidence of conserved cardiac remodeling processes in this low vertebrate model (Sun et al., 2009), a panel of acquired and inherited cardiomyopathy models has been established in adult zebrafish (Dvornikov et al., 2018). In comparison with rodent models, zebrafish models possess higher throughput that enables forward genetic screens to identify novel genetic modifiers (Ding et al., 2016; Ma et al., 2018) and chemical screens to rapidly assess therapeutic drug candidates for cardiomyopathy. However, phenotyping tools for adult zebrafish models of cardiomyopathy remain a bottleneck, partially due to technical challenges associated with the small size of adult zebrafish.
In human patients with cardiomyopathy and subsequent heart failure, exercise intolerance is an important pathophysiologic hallmark (Del Buono et al., 2019). For example, the stratification of heart failure patients following the New York Heart Association (NYHA) classification guidelines is based heavily on exercise capacity. The 6-min walk test, in particular, is a classic and commonly used quantitative method to determine exercise capacity in clinical settings, which has been used to provide important diagnostic and prognostic information in cardiomyopathy and heart failure patients (Pina et al., 2003; Del Buono et al., 2019). To develop a corresponding phenotyping tool for adult zebrafish models, we recently leveraged a swimming-based assay to evaluate the exercise capacity in a doxorubicin-induced cardiomyopathy (DIC) model (Ma et al., 2020). We found that the critical swimming speed is a useful non-invasive index to validate the successful establishment of the model and evaluate the benefits of therapeutic intervention. Indeed, the swimming assay has also been implemented in studies using zebrafish as models for a variety of purposes including tissue regeneration (Wang et al., 2011; Mokalled et al., 2016), skeletal muscle growth (Palstra et al., 2010), and aging (Gilbert et al., 2014). More recently, a general protocol to measure the critical swimming speed in adult zebrafish has been described in detail (Wakamatsu et al., 2020). Herein, we describe the protocol used in our program for phenotyping an adult zebrafish model of DIC (Ding et al., 2011; Ma et al., 2018). It is anticipated that this protocol can be extended to other adult zebrafish models of cardiovascular diseases (Ding et al., 2020).
Materials and Reagents
Zebrafish WIK strain (In house)
Zebrafish housing system water (In house)
Hank’s Balanced Salt Solution (ThermoFisher Scientific, catalog number: 14025092)
Doxorubicin hydrochloride (Sigma-Aldrich, catalog number: D1515)
Ethyl 3-aminobenzoate methanesulfonate, Tricaine (Sigma-Aldrich, catalog number: E10521)
Equipment
Swim tunnel (Loligo Systems, model: 10L 120V/60Hz, catalog number: SW10110)
Aquarium air pump (API, Rena Air Pump, model 400)
Metal sheets (11.2 × 9.5 cm; pore diameter: 0.25-0.50 cm; made of steel; see Figure 1A)
Water flow meter (Hontzsch, flowtherm NT)
Weight scale (Torbal, catalog number: AD520)
Timer (Fisher Scientific, Fisherbrand, catalog number: 14-649-17)
Software
Excel (Microsoft Office) or JMP (SAS Institude Inc.)
Prism (GraphPad)
Procedure
Establish DIC model in adult zebrafish
Raise WIK fish under controlled density (< 10 fish per liter) and growth conditions to > 3 months old.
(Optional) If studying inherited genetic models, raise mutant and wild-type fish under the same density and growth conditions to > 3 months old. Genotyping can be conducted at 3 months. Ensure that an equal number of mutant and wild-type fish are accommodated in their own tanks.
Randomly assign adult fish into control and model groups. Add additional model groups if testing any therapeutic interventions.
Dissolve doxorubicin powder in de-ionized water to a stock concentration of 5 mg/ml.
Intraperitoneally inject Hank’s Balanced Salt Solution (HBSS) (5 μl) or doxorubicin (5 μl, 20 mg/kg, prepared in HBSS) into individual fish in the control and model groups, respectively. The position for injection can be the midline between the pelvic fins or the lateral line above the pelvic fin. Detailed methods for doxorubicin injection can be found in a separate protocol (Ma et al., 2018).
Maintain fish for 4 weeks or longer in system water with regular feeding until the time of conducting the swimming challenge test. With a single bolus of high-dose doxorubicin, fish develop cardiomyopathy-like phenotypes 4 weeks post-injection.
(Optional) Other adult zebrafish models of cardiomyopathy can be leveraged to substitute the DIC model.
Set up the swim tunnel
Follow the manufacturer’s instructions to assemble the different parts of the swim tunnel.
Choose the proper metal sheet for zebrafish and assemble it into the swim tunnel (Figure 1B). The reason for using metal sheets with a small pore size (pore diameter: 0.25-0.5 cm) is to prevent fish from entering the circulatory water system of the swim tunnel, which avoids damage to the fish.
Fill the swim tunnel with zebrafish housing system water (pH = 7.9) to the maximum level (Figure 1C). Attach the air pump to the swim tunnel and place the nozzle at the corner of the tunnel (Figure 1D) to provide constant oxygen.
Use the flow meter to measure flow speed (Figure 1E). Increase the water flow in the swim tunnel stepwise from low to high by adjusting the motor controller. Under each water flow condition, record the revolutions per minute (RPM) shown on the motor controller.
Establish the linear correlation between flow speed and RPM, and use the universal equation to allow conversion of RPM to flow speed for future use (Figure 1F).
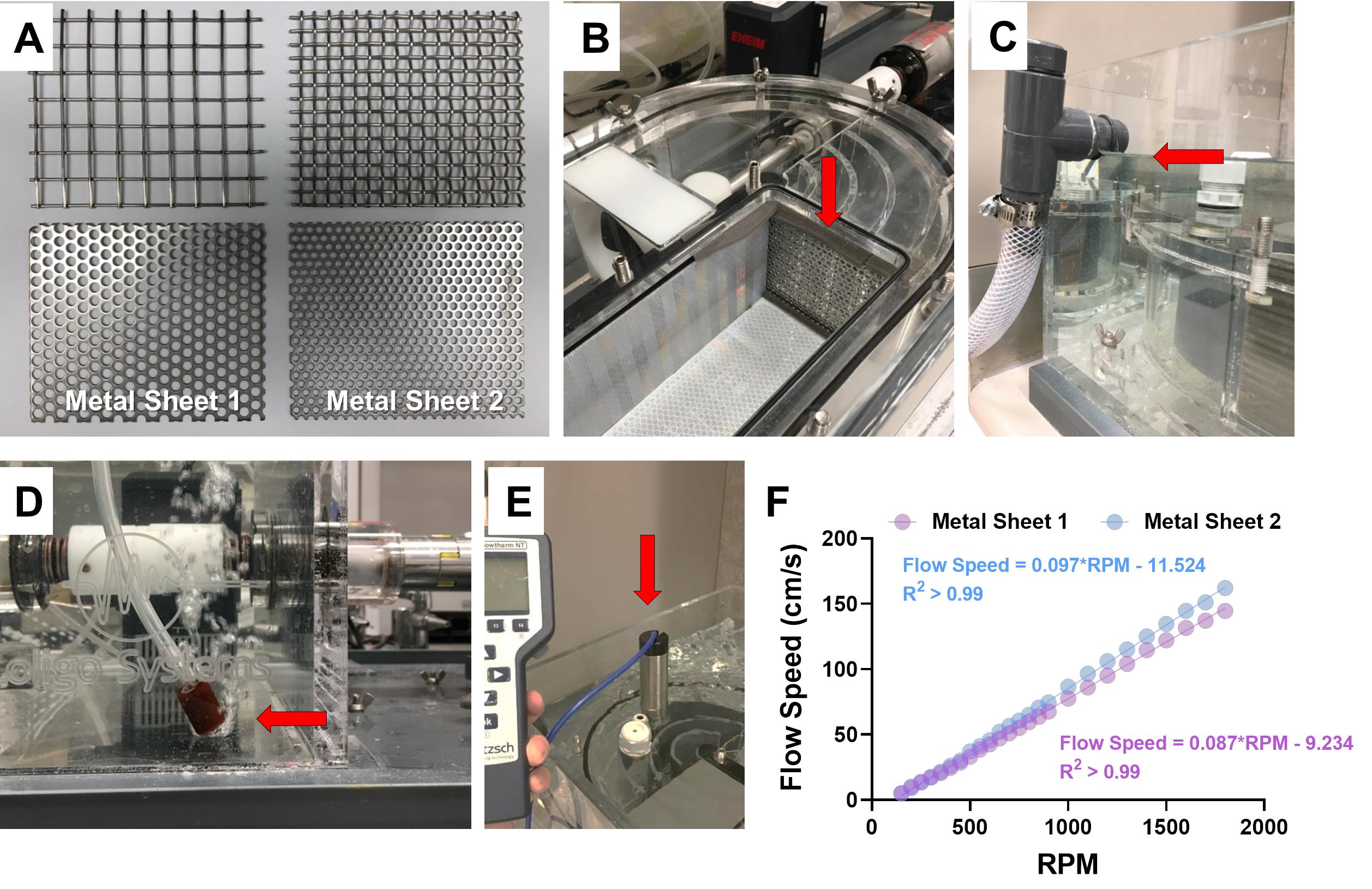
Figure 1. The setup of the swim tunnel and water flow speeds. A. Different metal sheets can be used as the downstream screen to prevent exhausted fish from entering the water circulation. The two at the bottom (referred to as Metal Sheet 1 and Metal Sheet 2) are suitable for zebrafish studies. Pore diameter of Metal Sheet 1 is 0.5 cm and that of Metal Sheet 2 is 0.25 cm. B. The assembly of the metal sheet (indicated by the red arrow) in the swim tunnel. C. Water level (indicated by the red arrow) in the swim tunnel for all experiments. This is also the maximum capacity for this tunnel (about 40 L water). D. A picture showing where to place the air pump nozzle (indicated by the red arrow) in the tunnel. E. A picture showing where to place the flow meter to measure water speed (indicated by the red arrow). F. The linear correlation between water flow speed and revolutions per minute (RPM) of the motor when using either of the metal sheets shown in (A).
Measure fish length and weight
Fast fish in the control and model groups for 24 h prior to the swimming challenge test. This step is performed to synchronize the status of different fish with a view to reducing potential variations associated with food consumption and metabolic processes for ATP production.
Anesthetize the fish in 0.16 mg/ml tricaine.
Measure the body length of each fish (do not include the tail fin) with a ruler (Figure 2A).
Pat the fish dry and measure the weight of each fish. Use a spoon to move the fish to a weighing dish on top of a scale (Figure 2B).
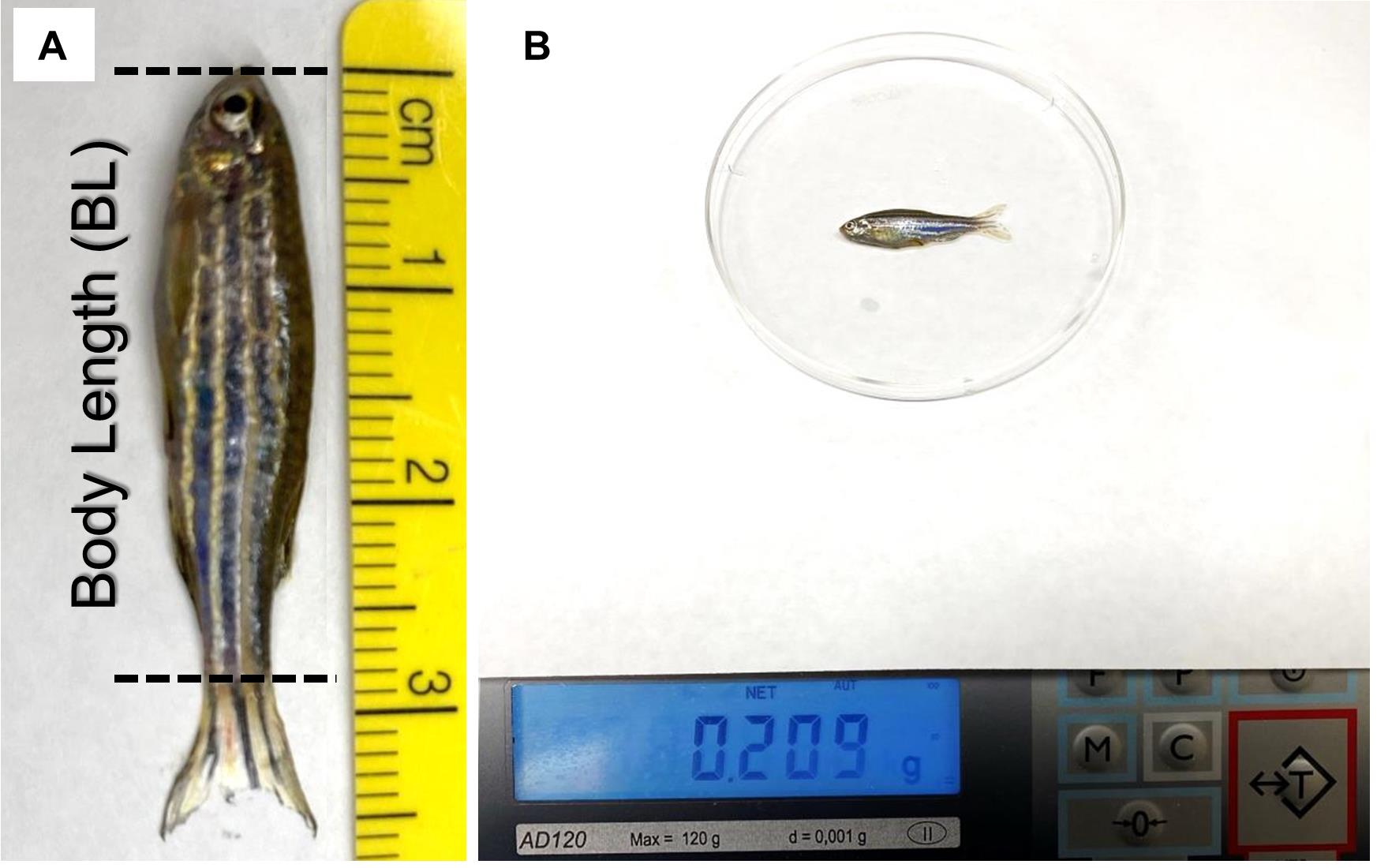
Figure 2. Representative images showing the measurement of the body length (A) and weight (B) of adult zebrafish. The length of the tail fin is not included when measuring the body length.Place fish back in a tank with system water for recovery. Typically, fish should not be left out of water for more than 5 min to avoid potential injury or death.
Swimming challenge test
In a swim tunnel filled with system water, load 5-10 fish at a time to the swim chamber inside the tunnel. Seal the chamber with cap and screws.
Turn on the swim tunnel and air pump. Set the motor speed to 200 RPM (about 8-9 cm/s in our case) in an anti-clockwise direction. Use a timer to record the time starting from this step. Allow fish to acclimate to this low speed of water flow for 20 min (Figure 3A).
Increase the RPM of the motor in steps of 100 (i.e., 300 RPM, 400 RPM, 500 RPM, etc. This increases the water flow by 8.66 cm/s each time in our case). At each speed, allow fish to swim for 150 s (Figures 3B and 3C).
Closely monitor the fish and document each exhausted fish that fails to resume swimming from the downstream metal sheet (Figure 3D; Video 1). For each fish, record the highest water speed completed during the 150-s periods (Uii) and the swimming duration in the last 150-s period (Ti).
Turn off the tunnel when all fish have been exhausted.
Allow fish to recover for ~5 min and then transfer them back to the housing system.
Repeat Steps D1-D6 to complete the swimming challenge test for every fish in the control and model groups.
When finished, turn off the air pump, remove the water from the tunnel, clean it with towels, and cover the tunnel for maintenance.
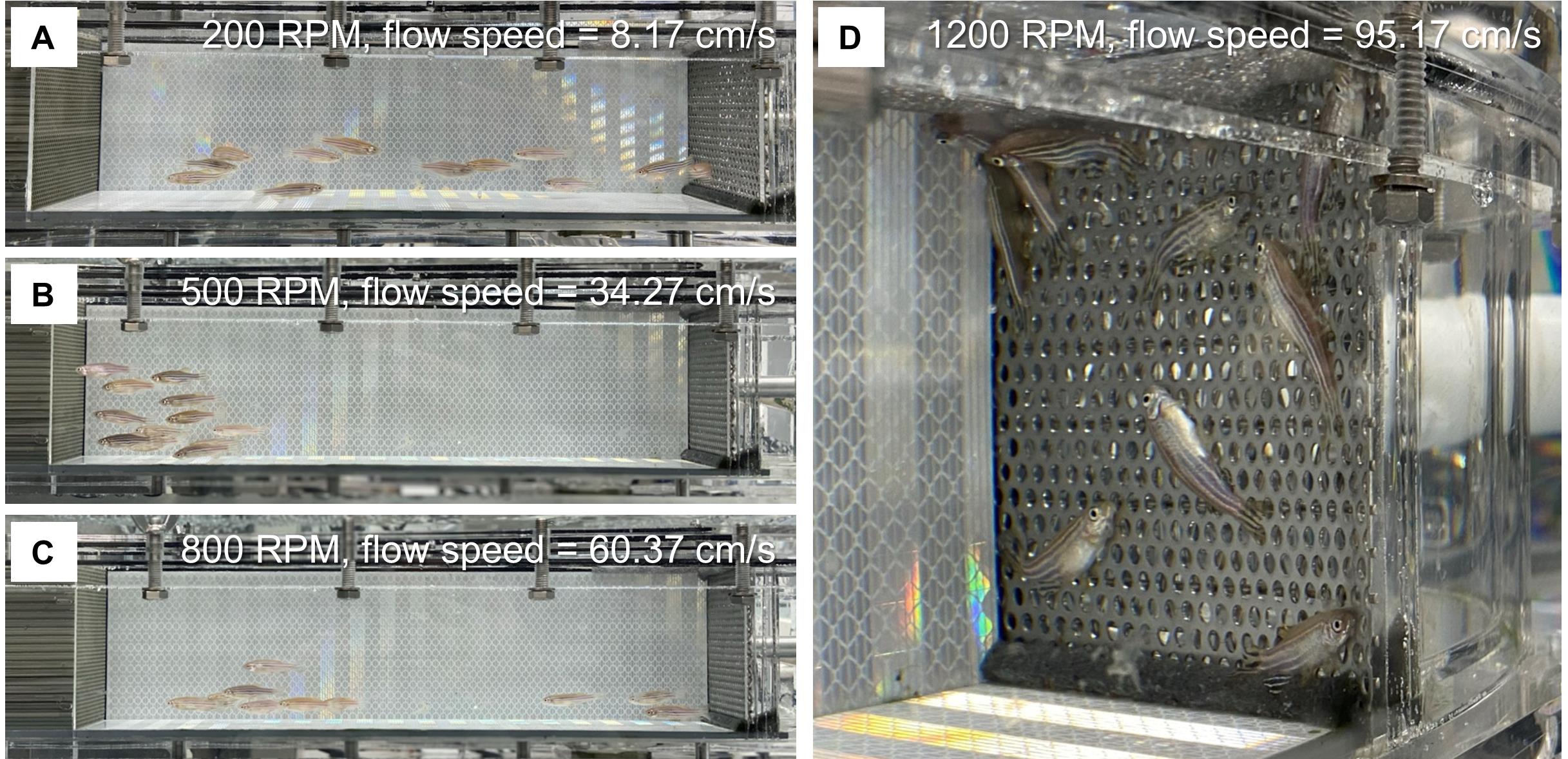
Figure 3. The process of the swim challenge. Representative images showing adult zebrafish in the swim channel at the motor speeds of 200 RPM (A), 500 RPM (B), and 800 RPM (C). D. The exhausted fish are attached to the metal sheet at a motor speed of 1,200 RPM. Metal sheet 2 was used in this experiment.Video 1. Representative video showing the process of fish being exhausted during the swim challenge
Data analysis
Compare fish length between the control and model groups
Calculate the mean and standard deviation of body length in each group. Use a t-test (or non-parametric test when indicated) for two-group comparison (Figure 4A) and use one-way ANOVA (or non-parametric test when appropriate) followed by multiple comparison methods for comparison among three and more groups.
Calculate the critical swimming speed for individual fish
The critical swimming speed is calculated using the following formula: Ucrit = Ui + Uii × (Ti/Tii), where Ucrit stands for critical swimming speed; Ui is the highest water speed that the fish reached during all 150-s periods; Uii is the water speed at which the fish became exhausted; Ti is the duration that the fish swam at the speed of Uii; and Tii is the time at each water speed (150 s in our case).
Compare the critical swimming speed among different groups
Representative data collected using a fish model of DIC and its control are presented in Figure 4, reporting the swimming duration, absolute critical swimming speed, and critical swimming speed normalized to body length in n = 15 DIC adult fish and controls. Typically, the values for critical swimming speed in each group follow a left-skewed distribution (Figures 4C and 4D). Comparison can be performed using the Mann-Whitney test (non-parametric) for two groups and the Kruskal-Wallis test (non-parametric) followed by multiple comparison methods for three or more groups. We used the Prism software for data visualization.
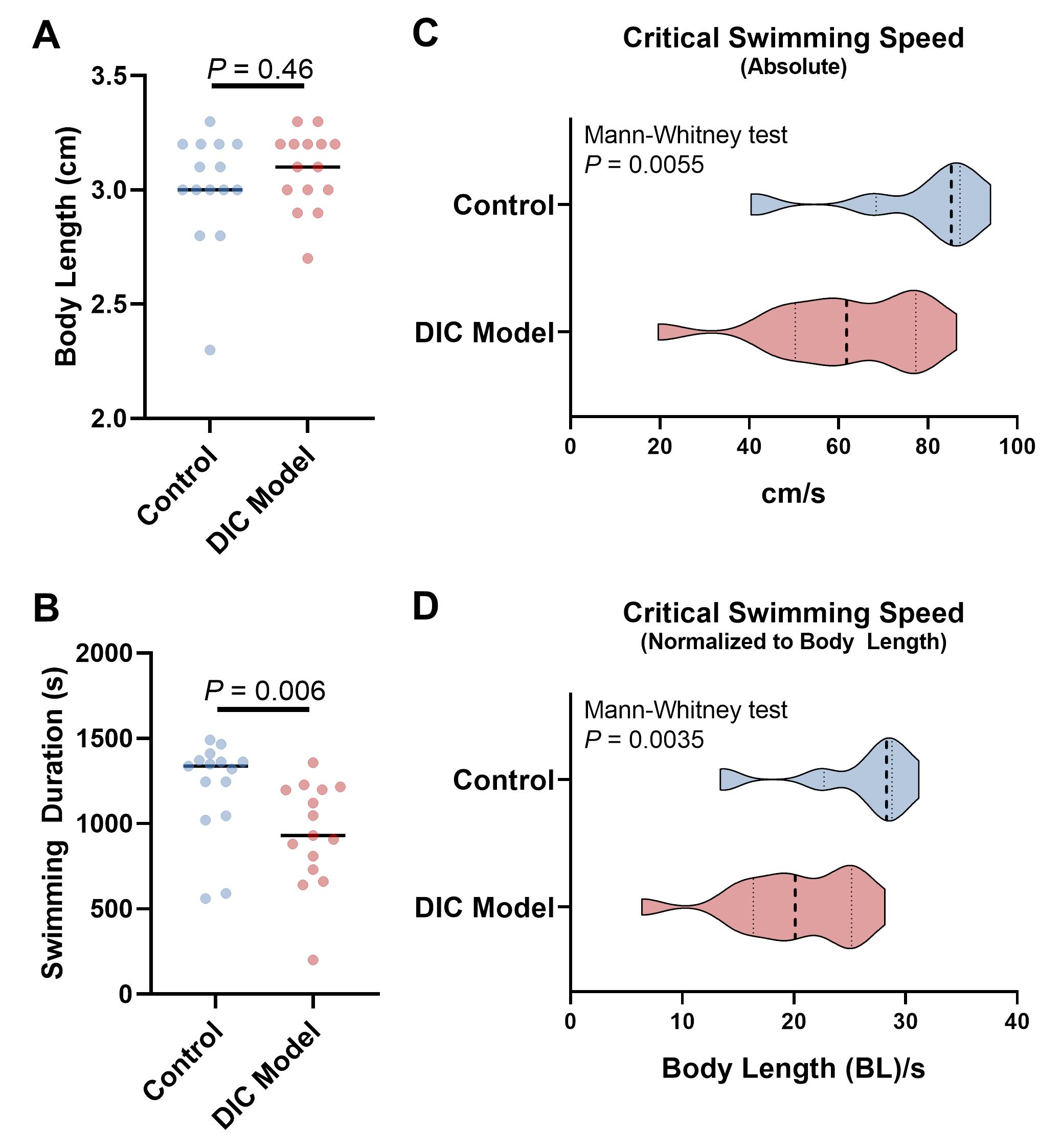
Figure 4. Representative data comparing the swimming indices between doxorubicin-induced cardiomyopathy (DIC) model and control adult zebrafish. A. Comparison of body length. B. Comparison of total swimming time in the tunnel. This does not include the 20-min acclimation time at 200 RPM. C. Comparison of the absolute critical swimming speed. D. Comparison of the critical swimming speed normalized to body length. BL/s represents the folds of body length per second. In C and D, the violins show the distribution of critical swimming speeds from 15 fish in each group. Dashed lines indicate the median (thicker) and 25th and 75th quartiles.
Notes
In this described protocol, control and DIC groups used different zebrafish cohorts; thus, reducing variation between different fish cohorts is necessary. While the correlation between body length and swimming speed needs to be established, we recommend normalizing the critical swimming speed to body length for interpretation. Nevertheless, fish in different groups should be maintained at similar lengths and weights whenever possible for comparison of their swimming capacity. Outlier fish with either exceedingly high or low body weight were discarded.
Alternatively, studies using the DIC model can be conducted in the same fish cohort, and swimming capacity can be determined before and after treatment with doxorubicin. In this way, the effects of doxorubicin stress on swimming can be evaluated regardless of potential confounding factors.
Fish may learn to perform better in the swim tunnel over time. For example, the critical swimming speed has been shown to increase after fish were trained over days or weeks (Ding et al., 2019). Therefore, to reduce intergroup variation, it is recommended to measure the swimming capacity for fish that have a similar experience with a swim tunnel.
In our protocol, we always use the same amount of water in the swimming tunnel by filling it to maximum capacity (about 40 L) to reduce potential variations. The experimental temperature is set to be consistently 20°C, and the pH of the water is set at 7.9 (Leveque et al., 2016). Additionally, the oxygen level may affect the swimming capacity of zebrafish, which is not quantitated in this protocol.
Acknowledgments
The authors acknowledge support from U.S. NIH R01 grants (HL81753, HL107304, HL111437, and GM63904) and Mayo Foundation to X.X. This protocol was adapted with minor modification from a previous study published by Ma et al. (2018).
Competing interests
The authors declare no competing interests.
Ethics
All protocols were approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee.
References
- Del Buono, M. G., Arena, R., Borlaug, B. A., Carbone, S., Canada, J. M., Kirkman, D. L., Garten, R., Rodriguez-Miguelez, P., Guazzi, M., Lavie, C. J. and Abbate, A. (2019). Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol 73(17): 2209-2225.
- Ding, Y., Bu, H. and Xu, X. (2020). Modeling Inherited Cardiomyopathies in Adult Zebrafish for Precision Medicine. Front Physiol 11: 599244.
- Ding, Y., Dvornikov, A. V., Ma, X., Zhang, H., Wang, Y., Lowerison, M., Packard, R. R., Wang, L., Chen, J., Zhang, Y., Hsiai, T., Lin, X. and Xu, X. (2019). Haploinsufficiency of mechanistic target of rapamycin ameliorates bag3 cardiomyopathy in adult zebrafish. Dis Model Mech 12(10).
- Ding, Y., Long, P. A., Bos, J. M., Shih, Y. H., Ma, X., Sundsbak, R. S., Chen, J., Jiang, Y., Zhao, L., Hu, X., Wang, J., Shi, Y., Ackerman, M. J., Lin, X., Ekker, S. C., Redfield, M. M., Olson, T. M. and Xu, X. (2016). A modifier screen identifies DNAJB6 as a cardiomyopathy susceptibility gene. JCI Insight 1(14).
- Ding, Y., Sun, X., Huang, W., Hoage, T., Redfield, M., Kushwaha, S., Sivasubbu, S., Lin, X., Ekker, S. and Xu, X. (2011). Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res 109(6): 658-669.
- Dvornikov, A. V., de Tombe, P. P. and Xu, X. (2018). Phenotyping cardiomyopathy in adult zebrafish. Prog Biophys Mol Biol 138: 116-125.
- Gilbert, M. J., Zerulla, T. C. and Tierney, K. B. (2014). Zebrafish (Danio rerio) as a model for the study of aging and exercise: physical ability and trainability decrease with age. Exp Gerontol 50: 106-113.
- Gut, P., Reischauer, S., Stainier, D. Y. R. and Arnaout, R. (2017). Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol Rev 97(3): 889-938.
- Leveque, R. E., Clark, K. J. and Ekker, S. C. (2016). Mayo Clinic Zebrafish Facility Overview. Zebrafish 13 Suppl 1: S44-46.
- Ma, X., Ding, Y., Wang, Y. and Xu, X. (2018). A Doxorubicin-induced Cardiomyopathy Model in Adult Zebrafish. J Vis Exp(136).
- Ma, X., Zhu, P., Ding, Y., Zhang, H., Qiu, Q., Dvornikov, A. V., Wang, Z., Kim, M., Wang, Y., Lowerison, M., Yu, Y., Norton, N., Herrmann, J., Ekker, S. C., Hsiai, T. K., Lin, X. and Xu, X. (2020). Retinoid X receptor alpha is a spatiotemporally predominant therapeutic target for anthracycline-induced cardiotoxicity. Sci Adv 6(5): eaay2939.
- Mokalled, M. H., Patra, C., Dickson, A. L., Endo, T., Stainier, D. Y. and Poss, K. D. (2016). Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354(6312): 630-634.
- Palstra, A. P., Tudorache, C., Rovira, M., Brittijn, S. A., Burgerhout, E., van den Thillart, G. E., Spaink, H. P. and Planas, J. V. (2010). Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One 5(12): e14483.
- Pina, I. L., Apstein, C. S., Balady, G. J., Belardinelli, R., Chaitman, B. R., Duscha, B. D., Fletcher, B. J., Fleg, J. L., Myers, J. N., Sullivan, M. J., American Heart Association Committee on exercise, r. and prevention (2003). Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107(8): 1210-1225.
- Sun, X., Hoage, T., Bai, P., Ding, Y., Chen, Z., Zhang, R., Huang, W., Jahangir, A., Paw, B., Li, Y. G. and Xu, X. (2009). Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One 4(8): e6596.
- Wakamatsu, Y., Kashima, M. and Hirata, H. (2020). A Reproducible Protocol to Measure the Critical Swimming Speed of Adult Zebrafish. Bio-protocol 10(16): e3712.
- Wang, J., Panakova, D., Kikuchi, K., Holdway, J. E., Gemberling, M., Burris, J. S., Singh, S. P., Dickson, A. L., Lin, Y. F., Sabeh, M. K., Werdich, A. A., Yelon, D., Macrae, C. A. and Poss, K. D. (2011). The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138(16): 3421-3430.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ma, X. and Xu, X. (2021). A Swimming-based Assay to Determine the Exercise Capacity of Adult Zebrafish Cardiomyopathy Models . Bio-protocol 11(15): e4114. DOI: 10.21769/BioProtoc.4114.
- Ma, X., Zhu, P., Ding, Y., Zhang, H., Qiu, Q., Dvornikov, A. V., Wang, Z., Kim, M., Wang, Y., Lowerison, M., Yu, Y., Norton, N., Herrmann, J., Ekker, S. C., Hsiai, T. K., Lin, X. and Xu, X. (2020). Retinoid X receptor alpha is a spatiotemporally predominant therapeutic target for anthracycline-induced cardiotoxicity. Sci Adv 6(5): eaay2939.
Category
Medicine > Cardiovascular system
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











