- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Tracking the Subcellular Localization of Surface Proteins in Staphylococcus aureus by Immunofluorescence Microscopy
(*contributed equally to this work) Published: Vol 11, Iss 10, May 20, 2021 DOI: 10.21769/BioProtoc.4038 Views: 7803
Reviewed by: Juan Facundo Rodriguez AyalaCristina Colomer-WinterFilipa Vaz

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Mădălina Mocăniță [...] Vanessa M. D'Costa
Mar 5, 2024 2895 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2087 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1655 Views
Abstract
Surface proteins of Staphylococcus aureus and other Gram-positive bacteria play essential roles in bacterial colonization and host-microbe interactions. Surface protein precursors containing a YSIRK/GXXS signal peptide are translocated across the septal membrane at mid-cell, anchored to the cell wall peptidoglycan at the cross-wall compartment, and presented on the new hemispheres of the daughter cells following cell division. After several generations of cell division, these surface proteins will eventually cover the entire cell surface. To understand how these proteins travel from the bacterial cytoplasm to the cell surface, we describe a series of immunofluorescence microscopy protocols designed to detect the stepwise subcellular localization of the surface protein precursors: surface display (protocol A), cross-wall localization (protocol B), and cytoplasmic/septal membrane localization (protocol C). Staphylococcal protein A (SpA) is the model protein used in this work. The protocols described here are readily adapted to study the localization of other surface proteins as well as other cytoplasmic or membrane proteins in S. aureus in general. Furthermore, the protocols can be modified and adapted for use in other Gram-positive bacteria.
Graphic abstract:
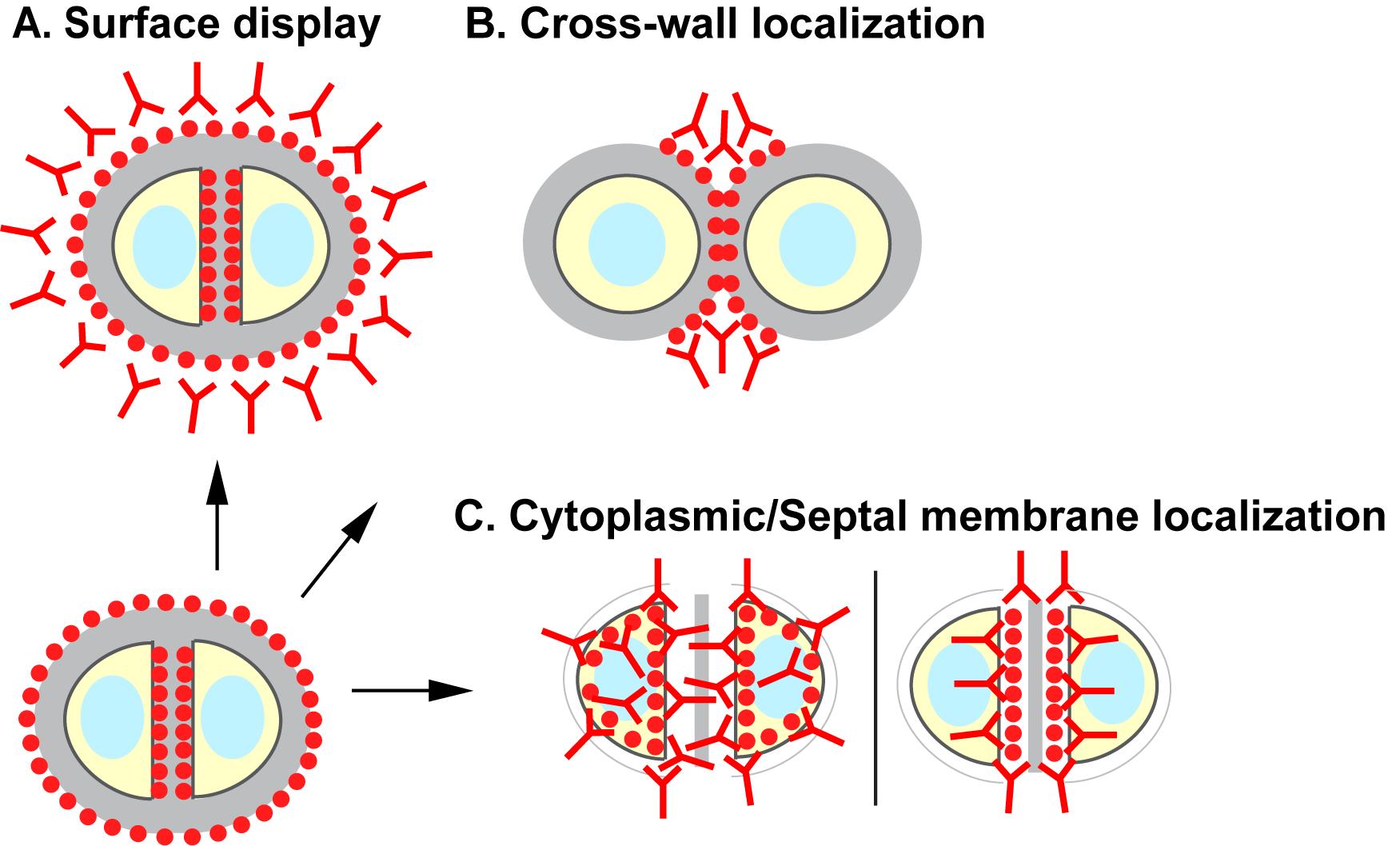
Tracking the subcellular localization of surface proteins in S. aureus
Background
Staphylococcus aureus is a Gram-positive bacterium and an opportunistic pathogen. It frequently colonizes human nares and skin and is a leading cause of both hospital- and community-acquired infections (von Eiff et al., 2001; Tong et al., 2015). The cell envelope of S. aureus consists of a cytoplasmic membrane and a thick cell wall peptidoglycan layer. To replicate, S. aureus undergoes binary fission by forming a division septum at the mid-cell. The cell wall biosynthesis machinery is recruited to the septum during cell division (Pinho and Errington, 2003). New cell wall peptidoglycan is synthesized to form a cross-wall ring and eventually a cross-wall disc coupled with the invagination of the septal membrane (Zhou et al., 2015). Once the cross-wall disc is fully synthesized, specific cell wall hydrolases cleave at the outer edges of the cross-wall to split the two daughter cells (Oshida et al., 1995; Sugai et al., 1995; Yamada et al., 1996; Kajimura et al., 2005). Due to high internal turgor pressure, the two daughter cells separate from each other and the newly synthesized cross-wall discs become the new hemispheres of the daughter cells (Monteiro et al., 2015; Zhou et al., 2015).
Cell wall peptidoglycan-anchored surface proteins are key components of the Gram-positive bacterial cell envelope. Many of them perform virulence functions in S. aureus, such as adhesion, biofilm formation, nutrient acquisition, antibiotic resistance, and immune evasion (Foster et al., 2014; Schneewind and Missiakas, 2019). Many surface protein precursors contain a specific N-terminal signal peptide with a highly conserved YSIRK/GXXS motif (Rosenstein and Götz, 2000; Tettelin et al., 2001). The secretion, cell wall anchoring, and surface display of YSIRK/GXXS proteins are tightly coupled with the bacterial cell cycle (Carlsson et al., 2006; Raz et al., 2012; Yu et al., 2018). In the early stages, the YSIRK/GXXS signal peptide promotes localized protein secretion at the division septum (Carlsson et al., 2006; DeDent et al., 2008). Subsequently, septal secreted surface proteins are covalently anchored to the cross-wall peptidoglycan by sortase A (Mazmanian et al., 1999). Upon cell division and separation, cross-wall-anchored surface proteins are displayed on the surface of the new hemisphere of the daughter cells (Cole and Hahn, 1962; Swanson et al., 1969; Raz et al., 2012; Yu et al., 2018). Eventually, surface proteins are displayed on the entire cell surface after several generations of cell division (DeDent et al., 2008; Raz et al., 2012; Yu et al., 2018).
Proper imaging methods are essential in revealing the subcellular localization of proteins. Here, we describe a series of protocols to track the subcellular localization of surface proteins. While it is straightforward to localize proteins on bacterial cell surface (protocol A, Figure 1), a “pulse-chase” type of method is used to reveal the localization of newly anchored surface proteins. In their classical paper, Cole and Hahn (1962) described an immunofluorescence staining method in which streptococcal cells were incubated with fluorescently labeled surface protein M antibody and subsequently with non-fluorescent antibody. In another classical study, streptococci were trypsin-treated to digest the existing M protein on the bacterial surface; new surface-deposited M protein was observed after re-incubating the bacteria in fresh medium without trypsin (Swanson et al., 1969). The method of trypsinization followed by regeneration has subsequently been used to localize newly anchored surface proteins on the cell surface of both streptococci and staphylococci (Carlsson et al., 2006; DeDent et al., 2008; Raz et al., 2012; Yu et al., 2018). Here, we provide a detailed description of the protocol that is specifically tailored to S. aureus (protocol B, Figure 1). The model protein we use is protein A (SpA), one of the major staphylococcal surface proteins that binds to host immunoglobulin and disrupts host immune responses (Forsgren and Sjöquist, 1966).
We have previously shown that SpA engages the SecA-mediated secretion pathway for translocation across the cytoplasmic membrane (Yu et al., 2018). To reveal where SpA precursors accumulate in the cytoplasm upon secA depletion, we developed a protocol to detect the localization of intracellular proteins (protocol C, Figure 1) based on methods described earlier by Harry et al., (1995) and Pinho and Errington (2003). In this protocol, staphylococcal cells are fixed with paraformaldehyde and glutaraldehyde, which adhere to the poly-L-lysine-coated glass slide. Cells are digested on the slide with a robust staphylococcal cell wall hydrolase, lysostaphin, to generate protoplasts (Schindler and Schuhardt, 1964). The protoplasts are fixed and permeabilized with methanol and acetone, respectively, and subsequently subjected to immunofluorescence staining. Depending on the genetic background of different strains, protocol C can be used to localize membrane-bound or cytoplasmic-localized surface protein precursors. Furthermore, protocol C is not restricted to surface proteins; it can be used to localize cytoplasmic or membrane proteins in S. aureus in general. The protocols described here can also be adapted for use in other Gram-positive bacteria.
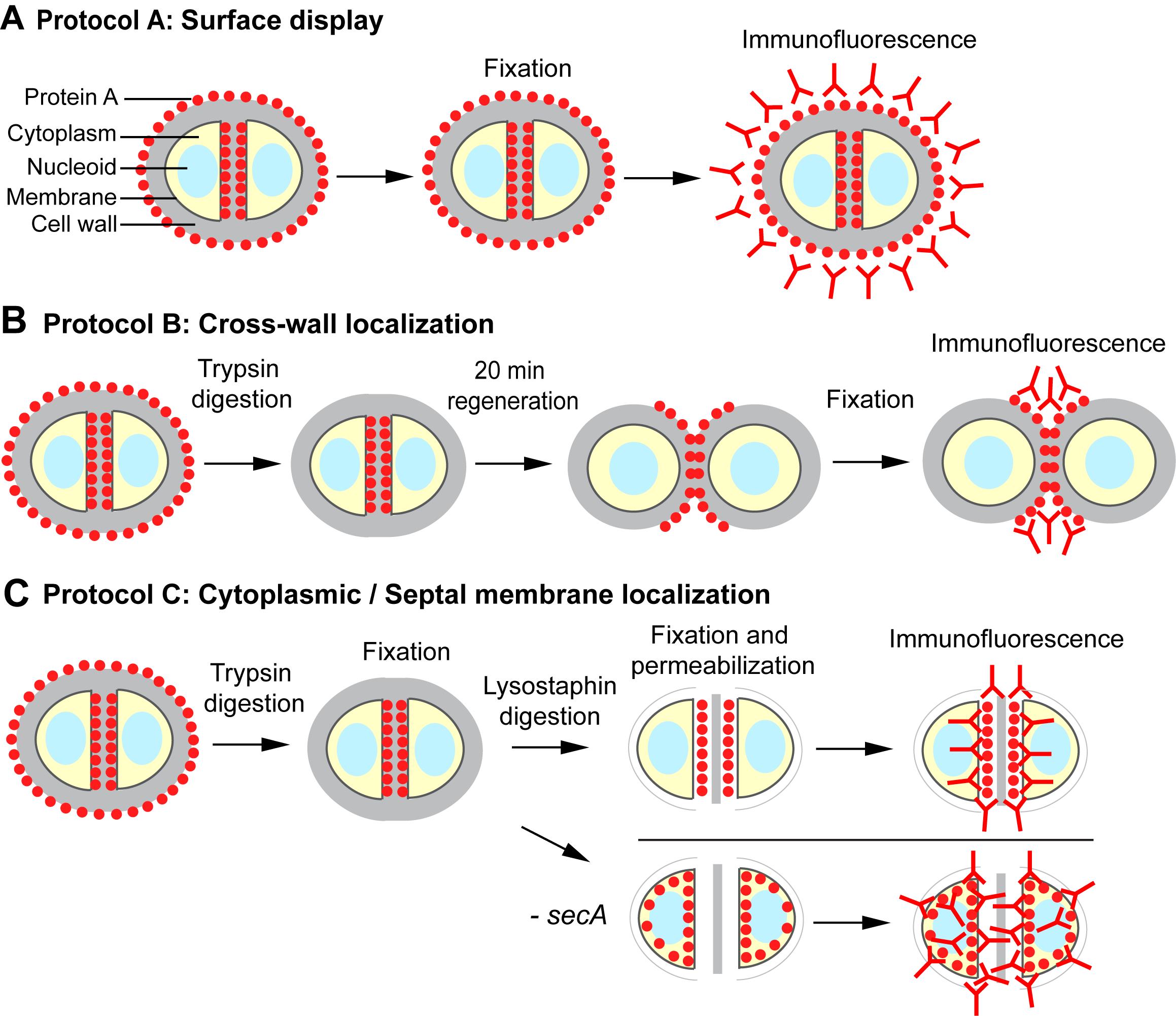
Figure 1. Schematic overview of the protocols described in this work
Materials and Reagents
8-well glass slides (MP Biomedicals/Thermo Fisher Scientific, catalog number: 096040805E)
Disposable borosilicate glass tubes, 16 mm diameter, 125 mm length (Thermo Fisher Scientific, catalog number: 1496130)
1,250 µl XL graduated tips (USA Scientific, catalog number: 1112-1720)
200 µl graduated tips (USA Scientific, catalog number: 1110-1700)
10 µl graduated tips (USA Scientific, catalog number: 1111-3700)
10 ml serological pipets (Thermo Fisher Scientific, catalog number: 1367811E)
25 ml serological pipets (Thermo Fisher Scientific, catalog number: 13-678-11)
1.5 ml microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
2.0 ml microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-138)
Scienceware microcentrifuge tube rack (Thermo Fisher Scientific, catalog number: 10029259)
1.5 ml microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
2.0 ml microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-138)
Nunc 15 ml conical sterile tubes (Thermo Fisher Scientific, catalog number: 12565269)
Nunc 50 ml conical sterile tubes (Thermo Fisher Scientific, catalog number: 12565271)
Corning PES syringe filters (Thermo Fisher Scientific, catalog number: 09-754-29)
20 ml filter syringes (Thermo Fisher Scientific, catalog number: 14-955-460)
Round Petri dishes (100 × 15 mm) (Thermo Fisher Scientific, catalog number: FB0875712)
Transfer pipettes (Thermo Fisher Scientific, catalog number: 13-711-7M)
Kimberly-Clark ProfessionalTM KimwipesTM Delicate Task Wipers (Thermo Fisher Scientific, catalog number: 06-666A)
BD BactoTM Tryptic Soy Broth (TSB) (Thermo Fisher Scientific, catalog number: DF0370-07-5)
BD Tryptic Soy Agar (TSA) (Thermo Fisher Scientific, catalog number: DF0369-07-8)
Sodium chloride (NaCl) (Thermo Fisher Scientific, catalog number: S271-1)
Hydrochloric acid (HCl) (Thermo Fisher Chemical, catalog number: 187066)
Potassium chloride (KCl) (Thermo Fisher Scientific, catalog number: AM9640G)
Potassium phosphate dibasic (K2HPO4) (Thermo Fisher Scientific, catalog number: BP363-500)
Sodium phosphate dibasic, anhydrous (Na2HPO4) (Thermo Fisher Scientific, catalog number: BP332-1)
Ethylenediamine tetraacetic acid, EDTA (Thermo Fisher Scientific, catalog number: BP118-500)
Tris base (Thermo Fisher Scientific, catalog number: BP152-5)
D-(+)-glucose (Sigma-Aldrich, catalog number: G8270-1KG)
Acetone (Thermo Fisher Scientific, catalog number: A929-4)
Methanol (Thermo Fisher Scientific, catalog number: A454-4)
Ethanol (Thermo Fisher Scientific, catalog number: A405P-4)
Bovine Serum Albumin (BSA) (Thermo Fisher Scientific Bioreagents, catalog number: BP1600-100)
0.1% poly-L-lysine solution (Sigma-Aldrich, catalog number: P8920-100ML)
Paraformaldehyde (PFA) 4% in PBS (Thermo Fisher Scientific, catalog number: AAJ19943K2)
Glutaraldehyde 50% in H2O (Sigma-Aldrich, catalog number: 340855-25ML)
Primary antibody: SpAKKAA antiserum (Kim et al., 2010). Store at 4°C
Secondary antibodies:
Goat anti-rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Thermo Fisher Scientific, catalog number: A-11034). Store at 4°C
Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (Thermo Fisher Scientific, catalog number: A-21244). Store at 4°C
Molecular ProbesTM SlowFadeTM Diamond Antifade Mountant (Invitrogen, catalog number: S36963)
Nile Red (Sigma-Aldrich, catalog number: 19123-10MG)
Hoechst 33342 DNA dye, 10 mg/ml (Thermo Fisher Scientific, catalog number: H3570), store at 4°C.
BODIPYTM Vancomycin-FL (Thermo Fisher Scientific, catalog number: V34850)
CorningTM Rectangular Cover Glasses No.1 (22 × 50 mm) (Thermo Fisher Scientific, catalog number: 12-553-461) (see Note 1)
Clear nail polish (cheapest one in any grocery store)
Trypsin, from bovine pancreas (Sigma-Aldrich, catalog number: T1426)
Trypsin inhibitor, from glycine max soybean (Sigma-Aldrich, catalog number: T9128)
Lysostaphin (AMBI, catalog number: LSPN-50)
Phosphate-buffered saline (PBS) (see Recipes)
Fixation solution (see Recipes)
GTE solution (see Recipes)
Trypsin stock solution (see Recipes)
Trypsin inhibitor stock solution (see Recipes)
BSA blocking solution (see Recipes)
Lysostaphin stock solution (see Recipes)
Nile Red stock solution (see Recipes)
BODIPYTM Vancomycin-FL stock solution (see Recipes)
Equipment
Eppendorf pipettes 100–1,000 µl, 20–200 µl, 2–20 µl, 0.1–2.5 µl (Eppendorf, catalog number: 2231000714)
Eppendorf Easypet®3 (Eppendorf, catalog number: 4430000018)
FisherbrandTM TraceableTM Multi-Colored Timer (Thermo Fisher Scientific, catalog number: 02-261-840)
Forceps (MilliporeSigmaTM Filter Forceps/Thermo Fisher Scientific, catalog number: XX6200006P)
In-house vacuum
Shaker (Eppendorf New BrunswickTM Innova® 42 shaker, catalog number: EPM1335-0010)
Table centrifuge (Eppendorf, model: Centrifuge 5425, catalog number EP5405000441)
Spectrophotometer (Thermo Scientific Genesys GENESYSTM 30 Visible Light Spectrophotometer, catalog number: 14-380-442)
Mini-tube rotator (FisherbrandTM Mini Tube Rotator, catalog number: 88-861-051)
Incubator microbiological (Fisher Scientific, catalog number: 51030513)
-20°C freezer
4°C refrigerator
LP Vortex Mixer (Thermo Fisher Scientific, catalog number: 88880017)
Leica SP5 2-photon Laser Scanning Confocal microscope (Leica Microsystems, product name: Leica TCS SP5 Confocal)
Software
Image J (Rasband W.S./U. S. NIH, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/)
Leica microscope software LAS_AF Leica (Leica microscopes)
Prism GraphPad Software for statistical analysis (https://www.graphpad.com/scientific-software/prism/)
Adobe Illustrator to assemble figures
Procedure
Slide preparation
Add 50 µl 0.1% poly-L-lysine to each well of an 8-well glass slide and allow to sit for 5 min at room temperature (see Note 2).
Briefly rinse the wells with ddH2O, remove the excess liquid using a vacuum, and allow the slide to air dry completely (see Notes 3 and 4).
Bacterial cultures
Prepare an overnight culture: inoculate one single colony from a streaked agar plate to 3 ml TSB in a glass test tube; add the appropriate antibiotics, if needed.
Grow the overnight culture at 37°C with rigorous shaking at 220 rpm.
The next morning, inoculate 30 µl overnight culture to 3 ml fresh TSB (1:100 dilution).
Grow the cultures at 37°C with rigorous shaking at 220 rpm.
Measure the optical density OD600 of the culture every hour in a spectrophotometer.
Place the cultures on ice when OD600 reaches 0.8–1.0 (see Note 5).
Continue the sample preparation in Part C below according to the different protocols (A, B, or C).
Sample preparation
Protocol A – Surface display:
Transfer 2 ml bacterial culture into a 2 ml microcentrifugation tube.
Spin at 18,000 x g for 3 min in a tabletop centrifuge to pellet the bacterial cells.
Remove the supernatant without disturbing the pellet.
Resuspend the pellet in 1 ml PBS and vortex well.
Spin at 18,000 × g for 3 min and remove the supernatant (steps 4–5 are “wash with PBS” steps).
Resuspend the pellet in 1 ml PBS, vortex thoroughly; mix 250 µl bacterial suspension with 250 µl fixation solution (see Recipes) in a clean 1.5 ml microcentrifuge tube, briefly vortex to mix, and incubate for 20 min at room temperature (fixation step).
Wash twice with PBS, as described in steps 4–5 (see Note 6).
Resuspend the pellet in 150 µl PBS, vortex thoroughly (see Note 7).
Add 50 µl bacterial suspension to poly-L-lysine-coated glass slides and allow to sit for 5 min.
Remove the excess liquid (non-adherent cells) using a vacuum.
Add one drop of PBS to each well using a plastic disposable transfer pipette and remove using a vacuum (this is the “drop and remove on-slide wash” step).
Repeat the drop and remove on-slide wash step.
Continue with immunofluorescence in Part D.
Transfer 2 ml bacterial culture into a 2 ml microcentrifugation tube.
Spin at 18,000 × g for 3 min in a tabletop centrifuge to pellet the bacterial cells.
Remove the supernatant without disturbing the pellet.
Wash once with PBS.
Resuspend the pellet in 900 µl PBS, vortex thoroughly; add 100 µl 5 mg/ml trypsin stock solution (see Recipes) and briefly vortex (trypsin final concentration: 0.5 mg/ml).
Incubate the tubes in a Mini-tube rotator at 37°C for 1 h at a rotation speed of 16 (medium speed).
Wash twice with PBS.
Resuspend the pellet in 900 µl fresh TSB, vortex thoroughly; add 100 µl 10 mg/ml soybean trypsin inhibitor stock solution (see Recipes) and briefly vortex to mix (final concentration of soybean trypsin inhibitor: 1 mg/ml).
Incubate the tubes in a Mini-tube rotator at 37°C for exactly 20 min at a rotation speed of 16 (see Note 8).
Add 250 µl fixation solution to a clean 1.5 ml microcentrifuge tube during the 20 min incubation.
At the 20-min timepoint, quickly transfer 250 µl bacterial sample to the microcentrifuge tubes prepared in the previous step.
Vortex to mix and allow the sample to sit at room temperature for 20 min.
Wash twice with PBS.
Resuspend the pellet in 150 µl PBS and vortex thoroughly (adjust the volume depending on the pellet size).
Add 50 µl bacterial suspension to a glass slide coated with poly-L-lysine and allow to sit for 5 min.
Remove the liquid (non-adherent cells) using a vacuum.
Perform the drop and remove on-slide wash twice for each well as described above.
Continue with immunofluorescence in Part D.
Protocol C – Cytoplasmic/septal membrane localization:
Place two 50 ml tubes containing approximately 25 ml methanol and 25 ml acetone, respectively, into a -20°C freezer.
Normalize all the bacterial cultures to OD600 = 1.
Transfer 2 ml normalized bacterial culture to a 2 ml microcentrifuge tube (this step is to have same cell numbers for the following enzymatic digestion step).
Spin at 18,000 × g for 3 min in a tabletop centrifuge to pellet the bacterial cells.
Remove the supernatant without disturbing the pellet.
Wash once with PBS.
Resuspend the pellet in 900 µl PBS and vortex thoroughly; add 100 µl 5 mg/ml trypsin stock solution and briefly vortex (see Note 9).
Incubate in a Mini-tube rotator at 37°C for 1 h at a rotation speed of 16.
Wash twice with PBS.
Resuspend the pellet in 500 µl PBS, vortex thoroughly; add 500 µl fixation solution and vortex to mix.
Incubate the sample for 15 min at room temperature and then on ice for 15 min to fix.
Wash three times with PBS.
Resuspend the pellet in 1 ml freshly made GTE buffer (see Recipes) and vortex thoroughly (see Note 10).
Add 50 µl cell suspension to poly-L-lysine-coated glass slides (this is the control without lysostaphin digestion) (see Note 11).
Prepare a timer, add 10 µl lysostaphin working solution (see Recipes) to the rest of the cell suspension; quickly vortex and immediately add 50 µl to the glass slides (see Note 12).
Incubate for 2 min on the slide (see Note 13).
Remove the liquid using a vacuum until completely dry.
Immediately place the slide into prechilled methanol at -20°C for 5 min.
Take out the slide using forceps and place into prechilled acetone at -20°C for 30 s (see Note 14).
Take out the slide using forceps and allow to air-dry completely.
Once the slide is dry, apply 50 µl PBS to the sample well to rehydrate.
Perform the drop and remove on-slide wash twice for each well.
Continue with immunofluorescence in Part D.
Immunofluorescence
Remove PBS, add BSA blocking solution (see Recipes), and incubate for 30 min at room temperature.
Remove the blocking solution, add 50 µl primary antibody solution (rabbit serum SpAKKAA 1:4,000 dilution in BSA blocking solution), and incubate overnight at 4°C or at room temperature for 1 h (see Note 15).
Remove the unbound primary antibody solution and wash 8 times with PBS with the last wash step for 5 min.
Remove the washing solution, add 50 µl secondary antibody diluted in BSA blocking solution (e.g., Alexa Fluor 647-IgG or Alexa Fluor 488-IgG, 1:500 dilution), and incubate in the dark for 1 hour at room temperature (see Note 16).
Perform the on-slide wash 10 times with PBS.
Take a clean 1.5 ml microcentrifuge tube, add 1 ml PBS, 5 µl Hoechest stock solution (1:200 dilution), 2 µl Van-FL stock solution (1:500 dilution), or 5 µl Nile Red stock solution (1:200 dilution) (see Recipes) and mix well; add 50 µl staining solution to each well.
Incubate in the dark for 10 min at room temperature.
Perform the on-slide wash three times with PBS.
After the last wash, remove all the excess liquid from the well.
Add a 5-μl drop of Slow Fade Diamond Antifade Mountant at 3 different places between the sample wells (see Note 17).
Brush a thin layer of nail polish around the edges of the slide and seal with a cover slip; gently press the cover slip and use a Kim wipes to remove the excess antifade solution around the edges (see Note 18).
Image the samples using a microscope with the appropriate fluorescent channels (Part E).
The prepared slides can be stored at 4°C for a few days and at -20°C for a longer period; however, immediate imaging is recommended.
Imaging
The samples prepared above are suitable to be imaged by different imaging systems, including epi-fluorescence microscopy, confocal microscopy, or deconvolution microscopy. However, to reveal bacterial cellular features and define protein localization in tiny bacterial cells, a microscope with high resolution is recommended. A 60× or 100× objective lens with a higher numerical aperture is needed. We used a Leica DM 2000 coupled with a sensitive CCD camera, a Leica SP5 2-photon Laser Scanning Confocal microscope, and a Leica SP8 3X STED Laser Confocal Microscope. All showed good imaging results.
Representative images of protocols A, B, and C are displayed in Figure 2. The images were captured using a Leica SP5 2-photon Laser Scanning Confocal microscope.
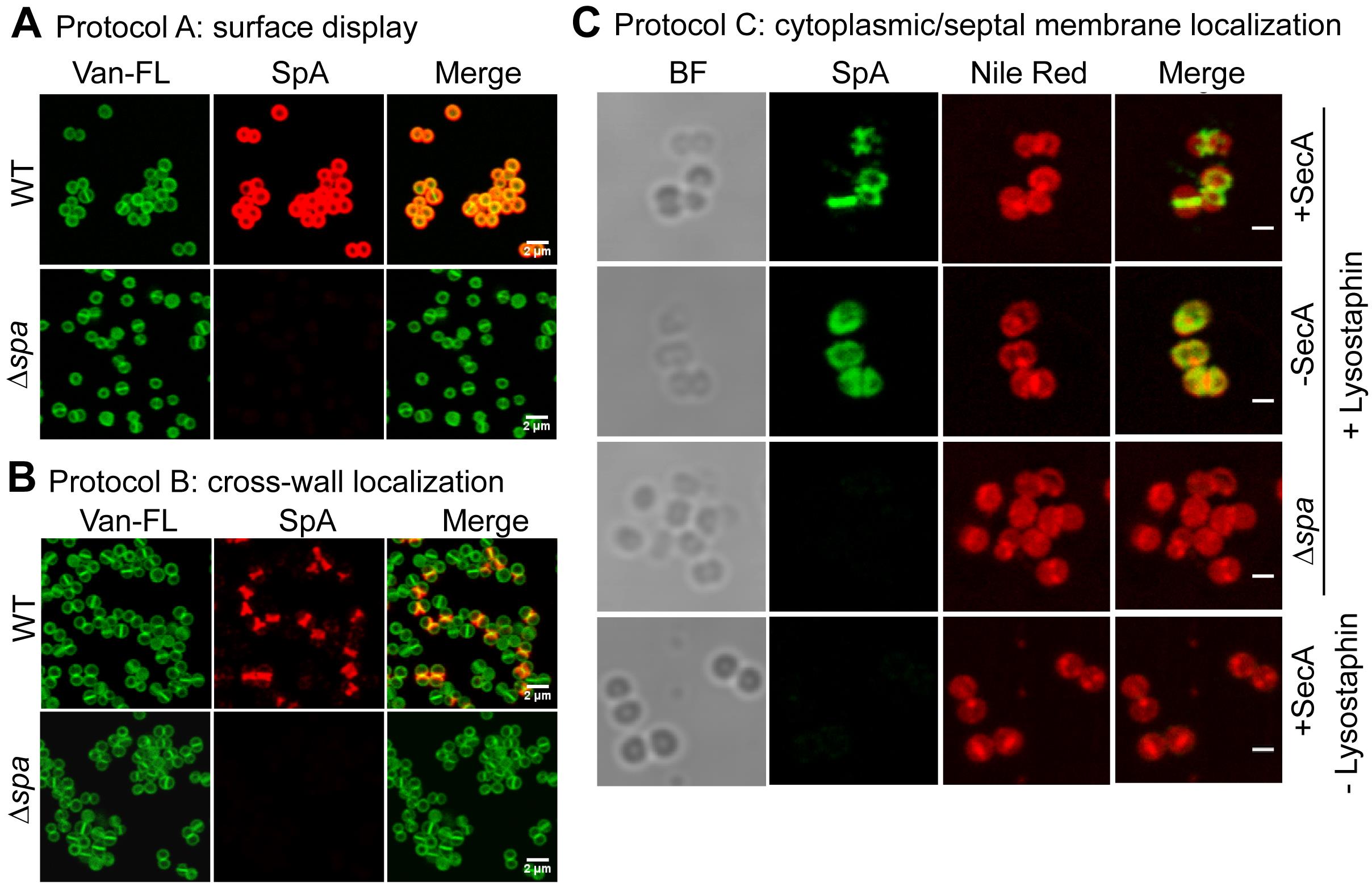
Figure 2. Representative images from (A) protocol A, showing surface display of SpA; (B) protocol B, showing cross-wall localization of SpA; and (C) protocol C, showing septal localization of SpA in the presence of SecA and cytoplasmic localization of SpA precursors in the absence of SecA. Van-FL: BODIPYTM Vancomycin-FL cell wall staining; BF: brightfield images; Nile Red: membrane staining; scale bar: 2 µm in panels A and B and 1 µm in panel C. Images are adapted from Yu et al. (2018).
Data analysis
Take at least three images with random views for each sample in each experiment. One has to take more random images, especially when there are only a few cells on the slides or when there are different phenotypes on one slide.
Perform the experiment independently at least three times.
To quantitate the percentage of SpA cross-wall localization, open images from protocol B in ImageJ, split the channels, and enlarge the images to allow better visualization.
Open the “cell counter” tool in ImageJ.
Select cell type 1, manually count pairs of diplococci in Van-FL-stained images, and record the number. Count at least 50 pairs of diplococci per image. Diplococci are defined as two connecting daughter cells that have just been split but not yet separated (see sample images in Figure 3) (see Note 19).
Select cell type 2, manually count cross-wall localized SpA signals in the merged images, and record the number. Cross-wall localized SpA signals are defined as clear lines at the cross-wall. To be rigorous, dots are not counted.
Calculate the average of three images per experiment.
Input the average values of three independent experiments to GraphPad Prism.
In GraphPad Prism, use a t-test to statistically analyze significant differences between two groups; use one-way ANOVA for multiple group comparisons; and use Tukey’s multiple comparison test to analyze differences among multiple groups.
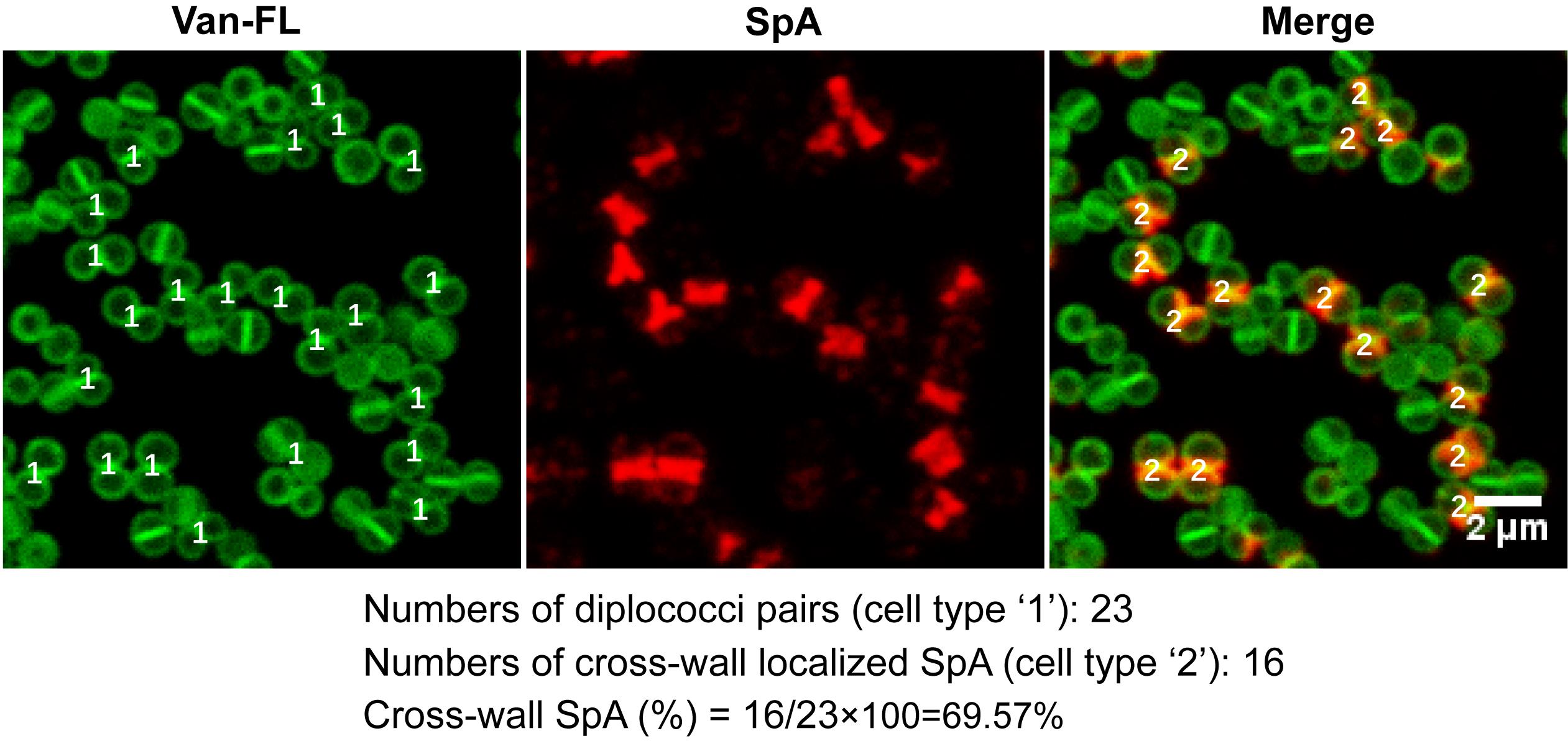
Figure 3. Sample images with the cell counting window, demonstrating how to quantitate the percentage of SpA cross-wall localization. Images are adapted from Yu et al. (2018).
Notes
The choice of thickness of the cover slip depends on the imaging system.
A 1:10 dilution of poly-L-lysine to 0.01% also works.
It takes about 15 min to air-dry the poly-L-lysine-coated slides. One can also use a vacuum to dry the slides.
To assemble the vacuum system, connect an in-house vacuum to a tube, cut the extremity off a 200 µl pipette tip, and insert the tip into the tube. It is important not to touch the samples on the glass slides during drying.
It usually takes about 2–3 h for S. aureus to reach an OD600 of 0.8–1.0 under standard lab culture conditions.
Cells tend to clump after fixation; a longer vortex may be needed.
The volume can be adjusted according to the pellet size; if unsure, one can add less PBS and make dilutions. The key point here is to have a proper number of cells on the slide so that most of the cells are well separated. Too many cells will lead to bacterial clumping and cause artifacts in immunostaining; too few cells will not provide reliable results.
The time of re-generation was determined experimentally in our protocol. It should be tested and optimized depending on different growth conditions and antigens.
This step is not critical for protocol C, as lysostaphin digestion will remove most of the cell wall as well as the existing SpA. We include this step in our protocol to minimize any potential background caused by existing SpA.
GTE buffer is an osmotic stabilizing buffer. Lysostaphin is a zinc-dependent endopeptidase (Sabala et al., 2014). Although EDTA in the GTE buffer can chelate zinc, it does not have any obvious negative effects in our experiments. We have tried other osmotic stabilizing buffers without EDTA, such as TSM [50 mM Tris-HCl (pH 7.5), 0.5 M sucrose, 10 mM MgCl2], which also works.
It is important to have this control. Staphylococcal cells after lysostaphin digestion will become more translucent in brightfield images, whereas undigested cells have a dark cell contour. Moreover, the two closely attached daughter cells will separate after lysostaphin digestion (see Figure 2C).
Other cell wall hydrolases can substitute lysostaphin if this protocol is to be adapted for another bacterium. Lysozyme, for example, has been used in Bacillus subtilis (Harry et al., 1995). Most S. aureus strains are lysozyme-resistant, which limits its use in S. aureus. The digestion time and buffer will have to be adjusted experimentally for a different enzyme or bacterium.
It is critical to perform on-slide digestion to stabilize the protoplasts.
This step stabilizes the protoplast after lysostaphin digestion and permeabilizes the cytoplasmic membrane. Depending on the bacterial strain and abundance of antigens, Triton X-100 can be used to further permeabilize the cytoplasmic membrane.
For any new antigen, serial dilution of primary antibody is necessary to determine the optimal concentration. A negative control that does not express the antigen is an essential control. If there is no mutant available, one should include at least a control without primary antibody. Minimal background signals should be seen in the negative control.
Depending on the microscope system, one can choose different fluorescent-labeled secondary antibodies. We consistently use the secondary antibody at a 1:500 dilution.
Different kinds of antifade solution are commercially available. One should choose the antifade solution compatible with the imaging system.
Make sure that the antifade solution covers every well as a very thin layer without bubbles, and that the cover slip is leveled on the slide.
The reason for counting diplococci is because cross-wall localized SpA signals can only be detected at the cross-wall of these diplococci under our experimental conditions; however, as defining diplococci may be subjective, it can introduce bias. Thus, one can count total cell numbers instead of diplococci.
Recipes
Phosphate-buffered saline (PBS)
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM K2HPO4
pH 7.4
Dissolve 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.24 g K2HPO4 in 1 L ddH2O
Adjust the pH to 7.4 with HCl and autoclave at 121°C for 20 min
Fixation solution
2.4% paraformaldehyde and 0.01% glutaraldehyde in PBS
Mix 30 ml 4% PFA and 10 µl 50% glutaraldehyde and add PBS to a 50-ml total volume. Store at 4°C (stable for at least two weeks).
GTE solution
50 mM glucose
10 mM EDTA
20 mM Tris-HCl pH 7.5
Note: Make fresh and filter-sterilize before use.
Make stock solutions of 0.5 M EDTA (pH 8) and 1 M Tris-HCl (pH 7.5)
Add 0.9 g D-glucose, 2 ml 0.5 M EDTA, and 2 ml 1 M Tris-HCl to a final volume of 80 ml ddH2O
Adjust the pH to 7.5 with HCl, add ddH2O to 100 ml, filter-sterilize, and store at 4°C
Trypsin stock solution
5 mg/ml trypsin in PBS, filter-sterilize, and store at -20°C
Trypsin inhibitor stock solution
10 mg/ml trypsin inhibitor in ddH2O, filter-sterilize, store at -20°C
BSA blocking solution: 3% BSA in PBS
Dissolve 0.3 g BSA powder in 10 ml PBS; make fresh and filter-sterilize before use, store at 4°C
Lysostaphin stock solution
Make a stock solution of 10 mg/ml in 20 mM sodium acetate (pH 4.5), store at -20°C
Dilute with 200 mM Tris-HCl (pH 8) to 2 mg/ml as a working solution, store at 4°C
Nile Red stock solution
Dissolve in 100% ethanol to make a 0.5 mg/ml stock solution, store at -20°C
Add 5 µl Nile Red stock solution to 1 ml PBS (1:200 dilution) to stain the samples
BODIPYTM Vancomycin-FL stock solution
Dissolve 100 µg in 100 µl DMSO to make a 1 µg/µl stock solution, store at -20°C
Add 2 µl Van-FL stock solution to 1 ml PBS (1:500 dilution) to stain the samples
Acknowledgments
This work was supported by the start-up funds to W.Y. from the University of South Florida. We thank Olaf Schneewind and Dominique Missiakas for their mentorship during the initial development of the protocols. We thank Vytas Bindokas, Robert Hill, and Byeong Cha for their assistance with microscope facilities. We thank lab members for suggestions regarding the manuscript. This work reports the fluorescence microscopy methods used in our previous paper (Yu et al., 2018). Images from Yu et al. (2018) have been adapted in this report to demonstrate the methods.
Competing interests
The authors declare that there are no conflicts of interest or competing interests.
References
- Carlsson, F., Stalhammar-Carlemalm, M., Flardh, K., Sandin, C., Carlemalm, E. and Lindahl, G. (2006). Signal sequence directs localized secretion of bacterial surface proteins. Nature 442(7105): 943-946.
- Cole, R. M. and Hahn, J. J. (1962). Cell wall replication in Streptococcus pyogenes. Science 135(3505): 722-724.
- DeDent, A., Bae, T., Missiakas, D. M. and Schneewind, O. (2008). Signal peptides direct surface proteins to two distinct envelope locations of Staphylococcus aureus. EMBO J 27(20): 2656-2668.
- Forsgren, A. and Sjöquist, J. (1966). "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol 97(6): 822-827.
- Foster, T. J., Geoghegan, J. A., Ganesh, V. K. and Hook, M. (2014). Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12(1): 49-62.
- Harry, E. J., Pogliano, K. and Losick, R. (1995). Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol 177(12): 3386-3393.
- Kajimura, J., Fujiwara, T., Yamada, S., Suzawa, Y., Nishida, T., Oyamada, Y., Hayashi, I., Yamagishi, J., Komatsuzawa, H. and Sugai, M. (2005). Identification and molecular characterization of an N-acetylmuramyl-L-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol Microbiol 58(4): 1087-1101.
- Kim, H. K., Cheng, A. G., Kim, H. Y., Missiakas, D. M. and Schneewind, O. (2010). Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med 207(9): 1863-1870.
- Mazmanian, S. K., Liu, G., Ton-That, H. and Schneewind, O. (1999). Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285(5428): 760-763.
- Monteiro, J. M., Fernandes, P. B., Vaz, F., Pereira, A. R., Tavares, A. C., Ferreira, M. T., Pereira, P. M., Veiga, H., Kuru, E., VanNieuwenhze, M. S., Brun, Y. V., Filipe, S. R. and Pinho, M. G. (2015). Cell shape dynamics during the staphylococcal cell cycle. Nat Commun 6: 8055.
- Oshida, T., Sugai, M., Komatsuzawa, H., Hong, Y. M., Suginaka, H. and Tomasz, A. (1995). A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A 92(1): 285-289.
- Pinho, M. G. and Errington, J. (2003). Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol Microbiol 50(3): 871-881.
- Raz, A., Talay, S. R. and Fischetti, V. A. (2012). Cellular aspects of the distinct M protein and SfbI anchoring pathways in Streptococcus pyogenes. Mol Microbiol 84(4): 631-647.
- Rosenstein, R. and Götz, F. (2000). Staphylococcal lipases: biochemical and molecular characterization. Biochimie 82(11): 1005-1014.
- Sabala, I., Jagielska, E., Bardelang, P. T., Czapinska, H., Dahms, S. O., Sharpe, J. A., James, R., Than, M. E., Thomas, N. R. and Bochtler, M. (2014). Crystal structure of the antimicrobial peptidase lysostaphin from Staphylococcus simulans. FEBS J 281(18): 4112-4122.
- Schindler, C. A. and Schuhardt, V. T. (1964). Lysostaphin: A New Bacteriolytic Agent for the Staphylococcus. Proc Natl Acad Sci U S A 51: 414-421.
- Schneewind, O. and Missiakas, D. (2019). Sortases, Surface Proteins, and Their Roles in Staphylococcus aureus Disease and Vaccine Development. Microbiol Spectr 7(1).
- Sugai, M., Komatsuzawa, H., Akiyama, T., Hong, Y. M., Oshida, T., Miyake, Y., Yamaguchi, T. and Suginaka, H. (1995). Identification of endo-beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol 177(6): 1491-1496.
- Swanson, J., Hsu, K. C. and Gotschlich, E. C. (1969). Electron microscopic studies on streptococci. I. M antigen. J Exp Med 130(5): 1063-1091.
- Tettelin, H., Nelson, K. E., Paulsen, I. T., Eisen, J. A., Read, T. D., Peterson, S., Heidelberg, J., DeBoy, R. T., Haft, D. H., Dodson, R. J., Durkin, A. S., Gwinn, M., Kolonay, J. F., Nelson, W. C., Peterson, J. D., Umayam, L. A., White, O., Salzberg, S. L., Lewis, M. R., Radune, D., Holtzapple, E., Khouri, H., Wolf, A. M., Utterback, T. R., Hansen, C. L., McDonald, L. A., Feldblyum, T. V., Angiuoli, S., Dickinson, T., Hickey, E. K., Holt, I. E., Loftus, B. J., Yang, F., Smith, H. O., Venter, J. C., Dougherty, B. A., Morrison, D. A., Hollingshead, S. K. and Fraser, C. M. (2001). Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293(5529): 498-506.
- Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L. and Fowler, V. G., Jr. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management.Clin Microbiol Rev 28(3): 603-661.
- von Eiff, C., Becker, K., Machka, K., Stammer, H. and Peters, G. (2001). Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 344(1): 11-16.
- Yamada, S., Sugai, M., Komatsuzawa, H., Nakashima, S., Oshida, T., Matsumoto, A. and Suginaka, H. (1996). An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol 178(6): 1565-1571.
- Yu, W., Missiakas, D. and Schneewind, O. (2018). Septal secretion of protein A in Staphylococcus aureus requires SecA and lipoteichoic acid synthesis. Elife 7.
- Zhou, X., Halladin, D. K., Rojas, E. R., Koslover, E. F., Lee, T. K., Huang, K. C. and Theriot, J. A. (2015). Bacterial division. Mechanical crack propagation drives millisecond daughter cell separation in Staphylococcus aureus. Science 348(6234): 574-578.
Article Information
Copyright
Scaffidi et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Scaffidi, S. J., Shebes, M. A. and Yu, W. (2021). Tracking the Subcellular Localization of Surface Proteins in Staphylococcus aureus by Immunofluorescence Microscopy. Bio-protocol 11(10): e4038. DOI: 10.21769/BioProtoc.4038.
- Yu, W., Missiakas, D. and Schneewind, O. (2018). Septal secretion of protein A in Staphylococcus aureus requires SecA and lipoteichoic acid synthesis. Elife 7.
Category
Microbiology > Microbial cell biology > Cell imaging
Microbiology > Microbe-host interactions > Bacterium
Cell Biology > Cell staining > Cell wall
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










