- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Muscle Cryoinjury and Quantification of Regenerating Myofibers in Mice
Published: Vol 11, Iss 11, Jun 5, 2021 DOI: 10.21769/BioProtoc.4036 Views: 3983
Reviewed by: Giusy TornilloYann Simon GallotChao Wang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Absolute Quantification of mRNA Isoforms in Adult Stem Cells Using Microfluidic Digital PCR
Shubhangi Das Barman [...] Antoine de Morree
Sep 5, 2023 1852 Views

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2500 Views

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views
Abstract
Cryoinjury, or injury due to freezing, is a method of creating reproducible, local injuries in skeletal muscle. This method allows studying the regenerative response following muscle injuries in vivo, thus enabling the evaluation of local and systemic factors that influence the processes of myofiber regeneration. Cryoinjuries are applicable to the study of various modalities of muscle injury, particularly non-traumatic and traumatic injuries, without a loss of substantial volume of muscle mass. Cryoinjury requires only simple instruments and has the advantage over other methods that the extent of the lesion can be easily adjusted and standardized according to the duration of contact with the freezing instrument. The regenerative response can be evaluated histologically by the average maturity of regenerating myofibers as indicated by the cross-sectional areas of myofibers with centrally located nuclei. Accordingly, cryoinjury is regarded as one of the most reliable and easily accessible methods for simulating muscle injuries in studies of muscle regeneration.
Keywords: Muscle injuriesBackground
Muscle injuries are a common type of injury that can result from various causes, from exertion to blast trauma. These injuries lead to a complex cascade of inflammatory and regenerative responses that result in the repair of the injured tissues. To characterize muscle injuries and the following responses and to evaluate the efficacy of potential treatments, various experimental models have been developed to replicate the process of muscle injury. In vitro approaches offer some practical advantages, and the advancement in 3D culture technique has enabled bioengineering of human cell-derived muscle tissue surrogates that may be used in muscle injury studies (Mills et al., 2017). In vivo models, however, remain imperative to understanding muscular injuries, as the complex processes involving the local and systemic responses that govern the overall regenerative process and the accompanying functional impairments/recovery can only be observed in a whole system of organisms. Rodent models of muscle injury are well-established and the most used systems for studying muscle regeneration today.
There are various modalities of injuries and their models, including laceration, crushing, blast injuries, volumetric muscle loss, myotoxins, and freezing. While all the modalities of injury go through common phases of healing (Huard et al., 2002; Jarvinen et al., 2005), some heterogeneity exists in the extent and quality of repair that follows regarding susceptibility/responsiveness of muscle stem cells to injury, tissue remodeling, inflammatory response, re-vascularization, and re-innervation (Caldwell et al., 1990; Lefaucheur and Sebille, 1995). Cryoinjuries, or traumatic injuries due to freezing, create localized injuries accompanied by substantial inflammatory and vascular responses (Warren et al., 2007), the extent of which can be adjusted and standardized according to the duration of the contact with the freezing instrument (Oh et al., 2016; Sinha et al., 2017; Endo et al., 2020). This makes it an easy and reliable method for generating reproducible outcomes, in contrast with the use of various myotoxins that lack comparative information needed for standardization. A step of skin incision is, however, required for the cryoinjury model, which could be considered a disadvantage over the use of injectable myotoxins. The cryoinjury model is applicable to a wide range of muscle injuries and is generally an appropriate choice for studying injuries without a substantial loss of muscle volume. In particular, cryoinjuries can simulate non-traumatic and mild traumatic injuries in striated muscles, including crushing injuries in skeletal muscle and myocardial infarction in cardiac muscles. Regenerative responses following cryoinjury can be identified histologically with clearly demarcated borders between injured and uninjured areas. Here, we describe a method of generating cryoinjuries in mouse skeletal muscle using dry ice and quantifying the regenerative response in myofibers.
Materials and Reagents
3.15% Chlorhexidine Gluconate and 70% Isopropyl Alcohol swabs (PDI, catalog number: S40750)
8-week-old C57BL/B male mice
Isoflurane, USP (Patterson Veterinary, catalog number: 14043070406)
Dry ice (or liquid nitrogen)
Buprenorphine (Patterson Veterinary, catalog number: 07-892-5235)
3-0 PROLENE® polyprorylene suture (Ethicon, catalog number: 8665G)
10% formalin solution (Sigma, catalog number: HT501128)
HistoPrepTM 70% Ethyl Alcohol (Fisher Scientific, catalog number: HC1500)
Equipment
Stainless-steel metal rods, 5 mm diameter (Uxcell, catalog number: UX657206)
Note: The metal rod should be chosen so its diameter to matches the muscle size. Inspect and measure the approximate width of the muscle and ensure that the diameter of the rod is smaller than the width of the muscle of all the animals undergoing cryoinjury.
Surgical scissors and forceps (Roboz Surgical Instrument Co, catalog number: RS-5910; Symmetry Surgical, catalog number: 30-1185)
Stainless steel scalpel, #15 (Healthcare Supply Pros, catalog number: MDS15215)
Animal Clipper (Kent Scientific Corporation, catalog number: CL7300)
Glass shell vials (Fisher Scientific, catalog number: 03-339-26A)
Light microscope (Olympus, catalog number: UCMAD3)
Shandom Microtome (Thermo Scientific, catalog number: A78100001K)
Software
ImageJ (National Institute of Health, http://imagej.nih.gov/ij)
GraphPad Prism version 9 (GraphPad Software, https://www.graphpad.com)
Procedure
Cryoinjury
Sterilize the surgical instruments and metal rod prior to the surgeries.
Anaesthetize mice using isoflurane.
Remove hair from the whole leg using a clipper and sterilize the skin anterior to the muscle of interest with chlorhexidine and alcohol swabs (Figure 1 Step 3).
Using aseptic techniques, make an incision in the skin and muscle fascia and expose the underlying muscle using surgical scissors or a scalpel (Figure 1 Step 4a and 4b).
Note: The size of the skin incision should be adjusted according to the size of the muscle and metal rod used (see Figure 1 Step 4a); it must allow the skin to be retracted wide enough so that the edges of the incised skin do not touch the metal rod. This usually requires an incision that spans over 80% of the muscle in the longitudinal direction, with the center of the incision aligning with that of the underlying muscle.
Cool a metal rod in dry ice for 5 min, wipe the rod with the chlorhexidine and alcohol swabs, and apply the flat end of the metal rod to the center of the muscle for a given amount of time (see Figure 1 Step 5).
Note: Standardize the size of the injury by maintaining the duration of the contact with the rod constant. Adjust the duration to achieve the desired extent of injury. A duration of 5 s is recommended for histological evaluation of injuries up to 10 d post-injury; 10 s are recommended for evaluation 14-21d postinjury. A pilot study may be required to establish the optimal duration of the cold injury for your animal model and study design.
Suture the skin and muscle fascia to close the incision using 3-0 PROLENE® polyprorylene suture (Figure 1 Steps 6a and 6b).
Provide the appropriate post-operative analgesia to the animal, according to the relevant guideline, and carefully and regularly observe them for at least 48 h after surgery.
Example: 0.05 mg/kg buprenorphine subcutaneous injection every 12 h for 48 h.
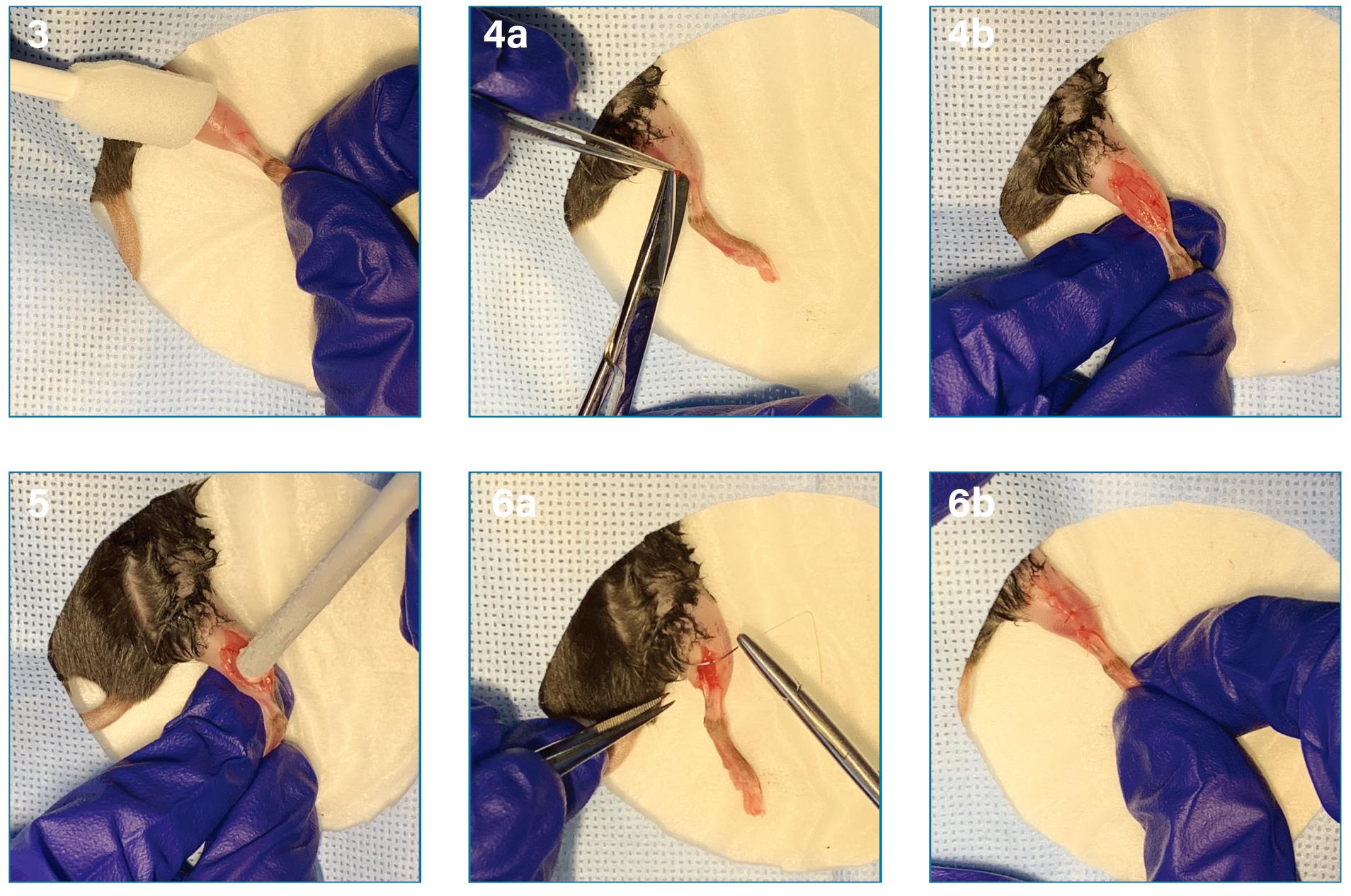
Figure 1. Induction of cryoinjury using a cooled stainless-steel metal rod. After the induction with sufficient anesthesia in the animal, cryoinjury can be inflicted in the following steps: removal of the hair and sterilization of the skin over the whole leg (3), exposure of the muscle (4a and 4b), application of the cooled metal rod over the muscle (5), and closure of the skin incision (6a and 6b).
Muscle harvesting
Euthanize mice according to institutional guidelines.
Note: Cervical dislocation is a preferred method of euthanizing adult mice to minimize the time between the completion of euthanasia and muscle harvesting.
Open the previous incision on the skin and remove the whole length of the injured muscle from the tendon attachments.
Place the muscle in a 10% formalin solution in a glass shell vial.
Replace formalin with 70% ethyl alcohol after 48 h for future sectioning and store the sample at room temperature (RT), avoiding direct sun and heat exposure.
Create five 6-8 μm thick paraffinized transverse sections of the injury site on glass slides using a microtome.
Perform hematoxylin-eosin (HE) staining according to standard procedures.
Histological evaluation
Under a light microscope, identify regenerating myofibers recognizable by their centrally located nuclei. Avoid counting multi-nucleated myofibers (green arrows) and inflammatory cells (red arrow) (Figure 2).
Take high-power field images (the use of 20× or 40× objectives is recommended) spanning the entire injury site.
Using ImageJ or an equivalent software, measure the cross-sectional area (CSA) of a total of at least 1,000 distinct regenerating fibers per sample.
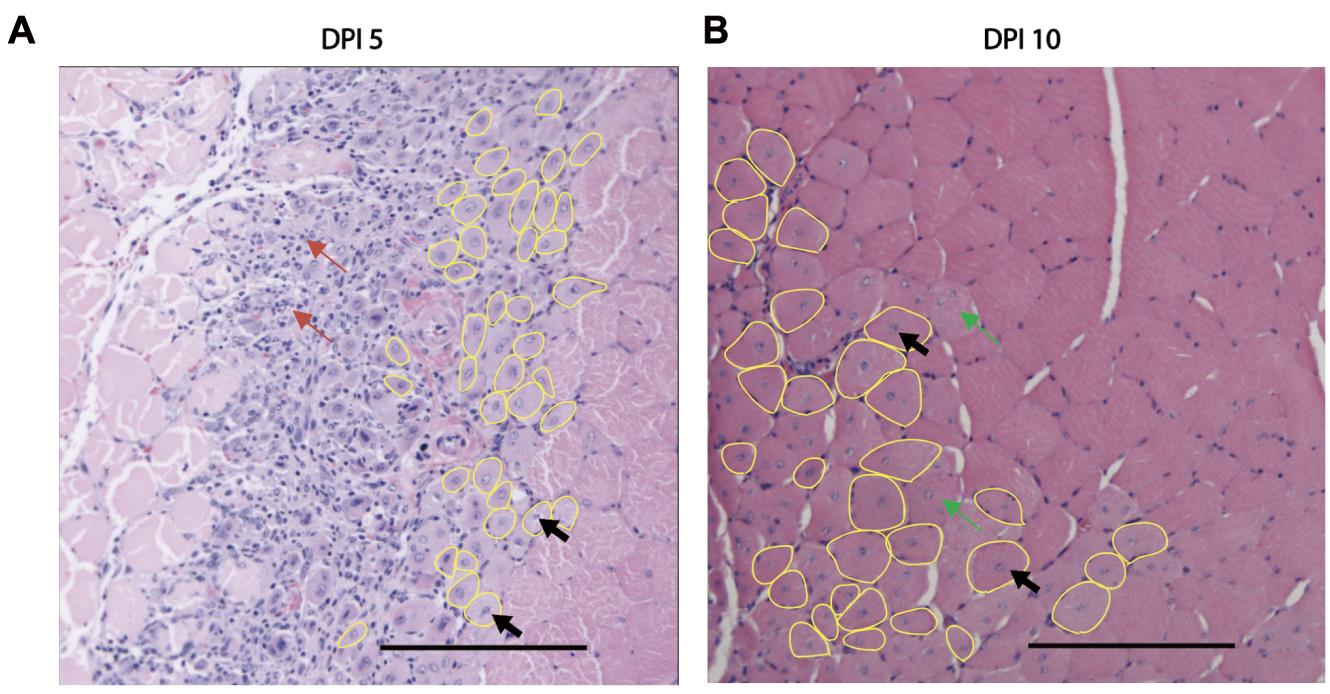
Figure 2. Identification of regenerating myofibers in HE-stained transverse muscle sections. A. Regenerating myofibers (yellow circles) are distinguishable from the surrounding mature myofibers by their centrally located nuclei (black arrows). On day 5 post-injury (DPI 5), regenerating myofibers with centrally located nuclei are in the inflammatory foci (red arrows) (A). B. By day 10 post-injury (DPI 10), regenerating myofibers have formed an organized structure with substantially less inflammatory infiltration. Both images are of tibialis anterior muscle of 8-week-old C57BL/B male mice, with cryoinjury inflicted for 5 s. Scale bars = 100 μm.
Data analysis
Calculate the CSAs of at least 1,000 distinct regenerating fibers per sample. Create a contingency table (Figure 3A) for frequency distribution of various fiber sizes (steps of 100 μm2 are recommended for adequate statistical resolution) of all samples and draw a frequency histogram of the mean frequency or percentage frequency of the group (Figure 2B). Calculate the mean CSAs per sample of a given group (Figure 2C). Perform statistical analysis using unpaired the Student’s t-test or one-way ANOVA to evaluate the presence of statistically significant variation between groups. Data can be expressed as mean ± standard deviation (SD) or standard error of means (SEM), and significance may be set at P-value < 0.05.
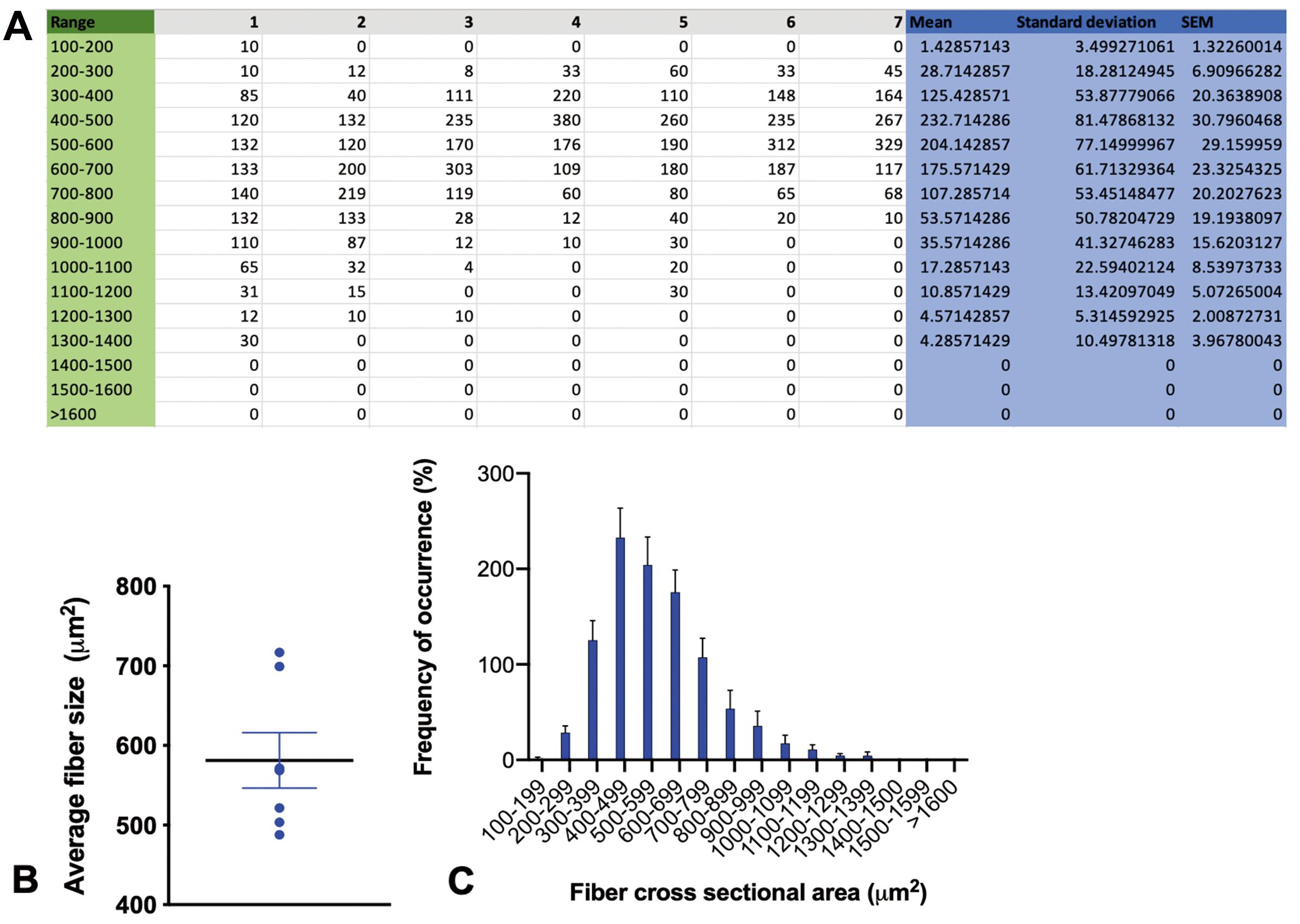
Figure 3. Data analysis and presentation. A. Contingency table containing the number of myofibers per 1,000 distinct regenerating fibers with a cross-sectional area (CSA) within a given range. The range was separated by 100 μm2 steps in this example. B. Histogram showing the mean percentage frequency of different myofiber CSA ranges of seven samples. C. Graph of the mean and standard error of the average myofiber CSAs of seven samples.
Acknowledgments
This protocol is derived from “Loss of ARNT in skeletal muscle limits muscle regeneration in aging” (Endo et al., 2020). This work was funded by the National Institute of Aging (K76AG059996 to IS), the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK036836 to AJW), the Glenn Foundation for Medical Research (AJW), and a Research and Education Committee Grant (IS) from the Boston Claude D. Pepper Center, supported by the National Institute of Aging (P30AG031679 to SB).
Competing interests
The authors declare no competing interests.
Ethics
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Brigham and Women’s Hospital and were conducted within the guidelines of the U.S. National Institutes of Health (NIH). IACUC approval ID: 2016N000375, valid between 2020-2023.
References
- Caldwell, C. J., Mattey, D. L. and Weller, R. O. (1990). Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol 16(3): 225-238.
- Endo, Y., Baldino, K., Li, B., Zhang, Y., Sakthivel, D., MacArthur, M., Panayi, A. C., Kip, P., Spencer, D. J., Jasuja, R., Bagchi, D., Bhasin, S., Nuutila, K., Neppl, R. L., Wagers, A. J. and Sinha, I. (2020). Loss of ARNT in skeletal muscle limits muscle regeneration in aging. FASEB J 34(12): 16086-16104.
- Huard, J., Li, Y. and Fu, F. H. (2002). Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84(5): 822-832.
- Jarvinen, T. A., Jarvinen, T. L., Kaariainen, M., Kalimo, H. and Jarvinen, M. (2005). Muscle injuries: biology and treatment. Am J Sports Med 33(5): 745-764.
- Lefaucheur, J. P. and Sebille, A. (1995). Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol 57(1-2): 85-91.
- Mills, R. J., Voges, H. K., Porrello, E. R. and Hudson, J. E. (2017). Cryoinjury Model for Tissue Injury and Repair in Bioengineered Human Striated Muscle. Methods Mol Biol 1668: 209-224.
- Oh, J., Sinha, I., Tan, K. Y., Rosner, B., Dreyfuss, J. M., Gjata, O., Tran, P., Shoelson, S. E. and Wagers, A. J. (2016). Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging (Albany NY) 8(11): 2871-2896.
- Sinha, I., Sakthivel, D., Olenchock, B. A., Kruse, C. R., Williams, J., Varon, D. E., Smith, J. D., Madenci, A. L., Nuutila, K. and Wagers, A. J. (2017). Prolyl Hydroxylase Domain-2 Inhibition Improves Skeletal Muscle Regeneration in a Male Murine Model of Obesity. Front Endocrinol (Lausanne) 8: 153.
- Warren, G. L., Summan, M., Gao, X., Chapman, R., Hulderman, T. and Simeonova, P. P. (2007). Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol 582(Pt 2): 825-841.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Endo, Y., Karvar, M. and Sinha, I. (2021). Muscle Cryoinjury and Quantification of Regenerating Myofibers in Mice. Bio-protocol 11(11): e4036. DOI: 10.21769/BioProtoc.4036.
Category
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Tissue analysis > Injury model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








