- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Synaptoneurosomes to Study the Synapse in the Murine Cerebral Cortex
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3896 Views: 4455
Reviewed by: Oneil Girish BhalalaNarayan SubramanianHong Lian

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Single-Particle Tracking of AMPA Receptor-Containing Vesicles
Victor C. Wong [...] Erin K. O’Shea
Jun 5, 2025 2260 Views

Local Iontophoretic Application for Pharmacological Induction of Long-Term Synaptic Depression
Borys Olifirov [...] Pavel Belan
Jun 5, 2025 1897 Views

Ultrafast Isolation of Synaptic Terminals From Rat Brain for Cryo-Electron Tomography Analysis
Rong Sun and Qiangjun Zhou
Sep 5, 2025 3556 Views
Abstract
The synapse is a complex structure where the transmission of information takes place. Synaptic dysfunction is one of the earliest pathophysiological events in several diseases, such as traumatic brain injury, cerebral ischemia, and neurodegenerative diseases. Thus, a methodology to study synaptic structure and function is crucial for the development of potential strategies for the treatment of many neurological diseases. Synaptoneurosomes (SNs) are structures assembled by the sealed presynaptic bouton and the attached post-synaptic density. Despite the fact that for a long time it has been recognized that SNs are a powerful tool to study synaptic function, composition, and structure, its use has been limited by the requirement of relatively large amounts of material to successfully isolate them. Here we describe a three-step centrifugation procedure performed under hypotonic conditions to isolate SNs from small volumes of the cerebral cortex.
Graphic abstract
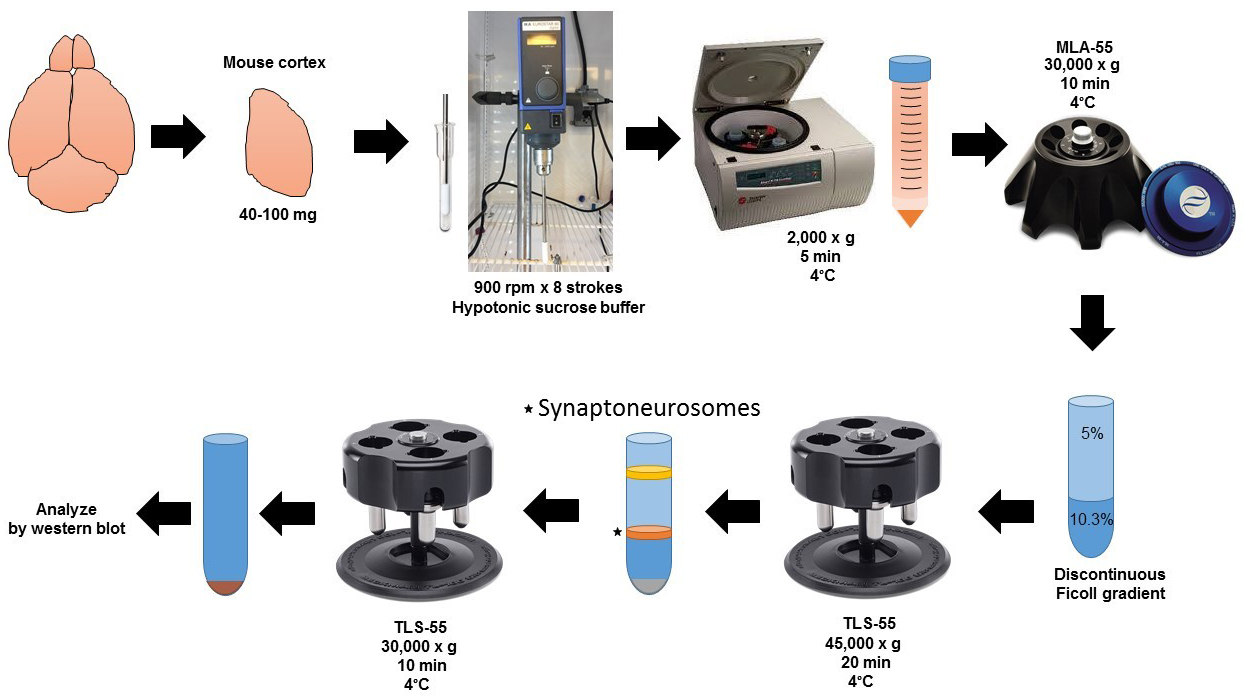
Schematic flowchart for the preparation of synaptoneurosomes.
Background
The synapse is a structure assembled by a presynaptic bouton attached to a postsynaptic terminal and ensheathed by an astrocytic elongation (Halassa et al., 2007). The last decade has witnessed the development of a large number of highly sophisticated techniques to study its structure and function. However, SNs are still used by a large number of investigators because they are relatively easy to isolate and yield valuable information on synaptic structure and function. The preparation of SNs is a modified cell-fractioning procedure that is performed under specific hypotonic conditions, and allows the isolation of the presynaptic boutons and attached postsynaptic elements. In the initial phases of their identification, they were called cell-free responsive preparations because their response to hormones (Chasin et al., 1974; Horn and Phillipson, 1976). This was followed years later by a detailed description of a procedure to isolate a suspension of snowman-shaped structures (synaptoneurosomes) assembled by a sealed presynaptic terminal (synaptosomes) attached to a postsynaptic sac (neurosomes; Hollingsworth et al., 1985). Since then several modifications have been introduced to their preparation, including the use of different hypotonic solutions and rotors (Rao and Steward, 1991; Villasana et al., 2006). However, despite their importance, the use of SNs has been limited by the requirement of relatively large amounts of tissue for their preparation. To address this limitation, here we describe a modified procedure to isolate SNs from small amounts of fresh and frozen brain tissue, and cultured neurons (Diaz et al., 2017 and 2020). This protocol has been used to successfully study the tripartite synapse in neuronal-astrocytic co-cultures (Diaz et al., 2019).
Materials and Reagents
15 ml Conical Centrifuge tubes (any brand)
10 ml, Open-Top Thickwall Polycarbonate tube, 16 x 76 mm (Beckman Coulter, catalog number: 355630 )
2.2 ml, Open-Top Thinwall Ultra-Clear tube, 11 x 34 mm (Beckman Coulter, catalog number: 347356 )
Pipette tips
60 mm cell culture dish (EMD Millipore, catalog number: CLS430589 )
Nitrocellulose Membrane (Bio-Rad, catalog number: 1620112 )
One 8- to 12-week-old mouse
Isoflurane, USP (Piramidal Critical Care, catalog number: 66794-013-25 )
Restore PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific, catalog number: 46430 )
Protease inhibitors, complete tablets EDTA-free EASYpack (Roche, catalog number: 04693132001 )
Phosphatase inhibitors, PhosSTOP EASYpack (Roche, catalog number: 04906837001 )
Rabbit anti-Syntaxin 1 antibody (1:1,000; EMD Millipore, catalog number: AB5820-50UL )
Mouse anti-PSD-95 antibody 6G6-1C9 (1:1,000; Novus Biologicals, catalog number: NB300-556 )
Mouse anti-β-Actin antibody (1:100,000; Sigma-Aldrich, catalog number: A1978 )
Mouse anti-Synaptophysin antibody (1:1,000; EMD Millipore, catalog number: MAB5258 )
Rabbit anti-Histone H3 antibody (1:1,000; Cell signaling, catalog number: 4499 )
IRDye 800CW Donkey anti-Rabbit IgG secondary antibody (1:10,000; LI-COR, catalog number: 926-32213 )
IRDye 680RD Donkey anti-Mouse IgG secondary antibody (1:10,000; LI-COR, catalog number: 926-68072 )
Intercept (TBS) Blocking Buffer (LI-COR, catalog number: 927-60001 )
Mini-PROTEAN TGX Stain-Free Gels (Bio-Rad, catalog number: 4568123 )
Mouse Surgical Kit (Kent Scientific, INSMOUSEKIT)
Sucrose (Fisher Chemical, catalog number: S612 )
Trizma base (Sigma-Aldrich, catalog number: T6066-5KG )
RIPA buffer (TEKnova, catalog number: R3792 )
Phosphate Buffered Saline 10x (Growcells, catalog number: RGF-6235 )
Regular ice
Ficoll (GE Healthcare, catalog number: 17-0300-10 )
100 mM EGTA (see Recipes)
10 mM Tris pH 8.1 (see Recipes)
Homogenization buffer (HB) (see Recipes)
Ficoll solutions (see Recipes)
Discontinuous Ficoll gradient (see Recipes)
Tris-buffer Saline (TBS) 20x (see Recipes)
TBS-Tween (TBS-T) (see Recipes)
Equipment
Micropipettes set
-80 °C freezer
Swinging-bucket clinical centrifuge (Beckman Coulter) or any centrifuge with temperature control compatible with 15 ml tubes
Optima Max-XP ultracentrifuge (Beckman Coulter)
TLS-55 Swinging-Bucket Rotor (Beckman Coulter)
MLS-50 Swinging-Bucket Rotor (Beckman Coulter)
Universal laboratory stirrer IKA EuroStar 60 Digital (Sigma-Aldrich, catalog number: Z766976 )
Potter-Elvehjem Tissue Homogenizer 5 ml (Omni International, catalog number: 07-358034 )
Mini-PROTEAN Tetra Cell for Ready Gel Precast Gels (Bio-Rad, catalog number: 1658005EDU )
Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, catalog number: 1703940 )
Odyssey Fc Imaging System (Li-COR)
Software
ImageStudio v5.2 (Li-COR)
Procedure
SN from brain tissue (work on ice all the time)
Induce anesthesia in the mouse using the isoflurane.
Perfuse the mouse intracardially with cold PBS 1x.
Open the skull, remove the meninges, and dissect the brain cortex (or the anatomical area of interest) in a 60 mm cell culture dish. 40-100 mg of brain tissue is enough for one experiment.
Save the other hemisphere (see Step A8).
Chop the tissue using small scissors in 1 ml of HB.
Homogenize tissue (homogenate 1, H1) in 4 ml of HB using a Potter-Elvehjem glass/teflon homogenizer.
Stroke 8 times at 800 rpm on ice.
Homogenize the other hemisphere with 1 ml of RIPA buffer containing protease and phosphatase inhibitors, centrifuge at 18,000 x g at 4 °C, and recover supernatant with a pipette and store at -80 °C. This will be used to prepare whole brain extract (WBE) which will constitute an internal control for SNs enrichment (see Data analysis).
Centrifugation
Centrifuge homogenate H1 at 2,000 x g for 5 min at 4 °C in a swinging-bucket clinical centrifuge.
Transfer the supernatant (S1) to a 10 ml, Open-Top Thickwall Polycarbonate tube, 16 x 76 mm, discard pellet (P1), and centrifuge the S1 in an MLA-55 fixed angle rotor at 30,000 x g for 10 min at 4 °C to obtain pellet 2 (P2; this pellet does not disrupt easily because is dense and thick), discard the supernatant 2 (S2) with a pipette (work on ice).
Resuspend P2 in 400 µl of HB (homogenate 2; H2) by pipetting several times (work on ice).
Discontinuous Ficoll gradient
Layer the H2 on top of a 5-10.3% discontinuous Ficoll gradient (Recipe 4) and centrifuge at 45,000 x g for 20 min at 4 °C in a TLS-55 rotor using a Beckman Optima Max-XP tabletop ultracentrifuge.
Collect 400 μl of the white layer in the 5-10.3% Ficoll interface that contains SNs.
(Figure 1; this fraction may also be used for other procedures or analyses, such as isolation of presynaptic terminals, synaptic vesicles and postsynaptic terminals, as well flow cytometry, omics, and electron microscopy).

Figure 1. 2.2 ml, Open-Top Thinwall Ultra-Clear tube at the end of Step C1. Note the white 5%/10.3% interface band containing SNs that will be collected in step C2. The pellet contains mainly mitochondria and cell debris.Complete volume with 1,400 μl of HB.
Centrifuge the SNs layer at 30,000 x g for 10 min at 4 °C in a TLS-55 rotor using a Beckman Optima Max-XP tabletop ultracentrifuge. Then, using a 200 μl tip pipette connected to the vacuum remove the supernatant (the SNs pellet does not disrupt easily because is dense and thick).
Resuspend the SNs pellet using 150 μl of RIPA buffer containing protease and phosphatase inhibitors store at -80 °C.
Data analysis
Run an SDS-PAGE
Quantify proteins using the laboratory routine protocol.
Mount the precast gel and load 10 μg of SNs, WBE (internal control), and a molecular weight marker.
Run gel.
Immunoblot and interpretation
Transfer proteins to a nitrocellulose membrane.
Block membrane with blocking buffer.
Cut membrane between the 55 and 72 kDa molecular weight marker.
Incubate the upper half with anti-PSD-95 antibody.
Incubate the lower half with antibodies against syntaxin 1 and synaptophysin.
Incubate overnight at 4 °C with gentle agitation.
Wash membranes using TBS-T and incubate them with specific LI-COR secondary antibodies mix for 1 h at room temperature.
Wash membranes with TBS-T and detect signal using the Odyssey Fc Imaging System.
Synaptophysin is a marker of pre-synaptic vesicles (Kwon and Chapman, 2011); Syntaxin 1 detects the presynaptic membrane (Bennett et al., 1992), and PSD-95 is a scaffold protein of the postsynaptic compartment (Hunt et al., 1996). A successful procedure should enrich the pre- and post-synaptic markers in the SNs fraction as compared to WBE (Figure 2).
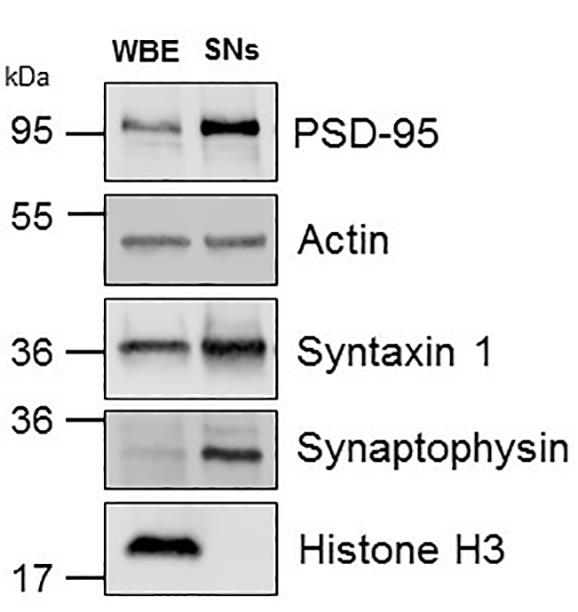
Figure 2. Representative Western blot analysis. Whole brain extracts (WBE) and synaptoneurosomes (SNs) prepared from the cerebral cortex of a C57 mouse and immunoblotted with antibodies against syntaxin 1, synaptophysin, PSD-95, and histone H3.Remove antibodies signal from the membranes using Restore PLUS Western Blot Stripping Buffer 20 min at room temperature.
Wash membrane with TBS-T.
Incubate overnight the lower half of the membrane at 4 °C and gentle agitation with antibodies against H3 and β-Actin.
Wash membranes using TBS-T and incubate them with specific LI-COR secondary antibodies mix for 1 hour at room temperature.
Detect signal using the Odyssey Fc Imaging System.
H3 is a nuclear marker; β-Actin is a loading control. SNs should be immunoreactive to β-Actin but not H3 (Figure 2).
Notes
This protocol requires basic knowledge of laboratory and animal surgical procedures.
We recommend to work on ice and/or 4 °C for each step.
For this protocol, we used an 8-week old C57bl/6J male mouse. However, we have also used it to isolate synaptoneurosomes from 3- days/ and 12 months/old male and female mice. To our knowledge, the strain and genetic background are not limitations for this procedure.
40-100 mg of tissue is enough for one experimental point, however, this amount could be further reduced by using smaller tubes and/or changing the volumes and/or concentrations of the Ficoll gradient.
In our experience, WBE and SNs can be stored for at least 1 year at -80 °C. We recommend to use small aliquots to avoid freeze-thaw cycles.
In our experience, following their preparation the solutions described below can be stored for at least 1 year.
Recipes
100 mM EGTA
Dissolve 3.8 g of EGTA in 100ml of 10 mM Tris pH 8.1
Store solution at room temperature
10 mM Tris, pH 8.1
Dissolve 2.42 g of Trizma base in 1.8 L of MilliQ grade H2O
Adjust pH 8.1 with HCl/NaOH as needed
Add water to obtain 2 L final volume
Store at room temperature
HB (0.25 M Sucrose/0.5 mM EGTA buffer in 10 mM Tris, pH 8.1)
For 1 L: 85.57 g Sucrose, 5 ml of 100 mM EGTA in 10 mM Tris pH 8.1, store at 4 °C
Add protease and phosphatase inhibitors to 50 ml of HB
Ficoll solutions
5% Ficoll
Dissolve 5 g of Ficoll in 100 ml of HB
Filter using 0.22 μm pore, store at 4 °C
14.5% Ficoll
Dissolve 14.5 g of Ficoll in 100 ml of HB
Filter using 0.22 μm pore, store at 4 °C
10.3% Ficoll
Mix 865 μl of Ficoll 14.5% and 345 μl of HB (for two samples), prepare the same day
Discontinuous Ficoll gradient
Note: Prepare the gradient before start the experiment or during Step B2.
Add 500 μl of 10.3% Ficoll solution into an Open-Top Thinwall Ultra-Clear tubes (11 x 34 mm)
Using the smoothest 1,000 μl pipette available or a 200 μl pipette, add slowly 1,300 μl of 5% Ficoll solution (a 5%/10.3% interface should be visible, if not, discard the gradient and start again using a clean tube)
TBS 20x for 2 L
Dissolve 96 g of Trizma base and 352 g of NaCl in 1 L of Ficoll 5%: dissolve 5 g of MilliQ grade H2O
Adjust pH 7.6 with HCl/NaOH as needed
Add water to obtain 2 L final volume
Tris-buffer Saline Tween 1x
Dilute TBS 20x to 1x with MilliQ grade H2O
Add tween 20 to obtain 0.1% final concentration
Acknowledgments
This work was supported in part by National Institutes of Health Grant NS-NS091201 (to M.Y.), Veterans Administration Merit Award IO1BX003441 (to M.Y.), and American Heart Association Post-Doctoral Fellowship Grant 19POST34380009 (to A.D.).
Competing interests
The authors declare no competing financial interests.
Ethics
Experiments were approved by the Institutional Animal Care and Use Committee (Protocol ID PROTO201700559 from 1/28/2020 to 1/27/2023) of Emory University, Atlanta, GA, USA.
References
- Bennett, M. K., Calakos, N. and Scheller, R. H. (1992). Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257(5067): 255-259.
- Chasin, M., Mamrak, F. and Samaniego, S. G. (1974). Preparation and properties of a cell-free, hormonally responsive adenylate cyclase from guinea pig brain. J Neurochem 22(6): 1031-1038.
- Diaz, A., Merino, P., Manrique, L. G., Ospina, J. P., Cheng, L., Wu, F., Jeanneret, V. and Yepes, M. (2017). A Cross Talk between Neuronal Urokinase-type Plasminogen Activator(uPA) and Astrocytic uPA Receptor(uPAR) Promotes Astrocytic Activation and Synaptic Recovery in the Ischemic Brain. J Neurosci 37(43): 10310-10322.
- Diaz, A., Merino, P., Manrique, L. G., Cheng, L. and Yepes, M. (2019). Urokinase-type plasminogen activator(uPA) protects the tripartite synapse in the ischemic brain via ezrin-mediated formation of peripheral astrocytic processes. J Cereb Blood Flow Metab 39(11): 2157-2171.
- Diaz, A., Merino, P., Guo, J. D., Yepes, M. A., McCann, P., Katta, T., Tong, E. M., Torre, E., Rangaraju, S. and Yepes, M. (2020). Urokinase-type plasminogen activator protects cerebral cortical neurons from soluble Aβ-induced synaptic damage. J Neurosci 40(21): 4251-4263.
- Halassa, M. M., Fellin, T. and Haydon, P. G. (2007). The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13(2): 54-63.
- Hollingsworth, E. B., McNeal, E. T., Burton, J. L., Williams, R. J., Daly, J. W. and Creveling, C. R. (1985). Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3':5'-monophosphate-generating systems, receptors, and enzymes. J Neurosci 5(8): 2240-2253.
- Horn, A. S. and Phillipson, O. T. (1976). A noradren aline sensitive adenylate cyclase in the rat limbic forebrain: preparation, properties and the effects of agonists, adrenolytics and neuroleptic drugs. Eur J Pharmacol 37(1): 1-11.
- Hunt, C. A., Schenker, L. J. and Kennedy, M. B. (1996). PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci 16(4): 1380-1388.
- Kwon, S. E. and Chapman, E. R. (2011). Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70(5): 847-854.
- Rao, A. and Steward, O. (1991). Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptosomes. J Neurosci 11(9): 2881-2895.
- Villasana, L. E., Klann, E. and Tejada-Simon, M. V. (2006). Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J Neurosci Methods 158(1): 30-36.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Diaz, A. E., Torre, E. and Yepes, M. (2021). Preparation of Synaptoneurosomes to Study the Synapse in the Murine Cerebral Cortex. Bio-protocol 11(2): e3896. DOI: 10.21769/BioProtoc.3896.
- Diaz, A., Merino, P., Guo, J. D., Yepes, M. A., McCann, P., Katta, T., Tong, E. M., Torre, E., Rangaraju, S. and Yepes, M. (2020). Urokinase-type plasminogen activator protects cerebral cortical neurons from soluble Aβ-induced synaptic damage. J Neurosci 40(21): 4251-4263.
Category
Neuroscience > Basic technology
Neuroscience > Cellular mechanisms > Synaptic physiology
Cell Biology > Organelle isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link