- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Characterization of Hippocampal Adult-borne Granule Cells in a Transient Cerebral Ischemia Model
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3890 Views: 3392
Reviewed by: Qihui WuAnthony FlamierAnonymous reviewer(s)
Abstract
Long-term consequences of stroke significantly impair the quality of life in a growing population of stroke survivors. Hippocampal adult neurogenesis has been hypothesized to play a role in the pathophysiology of cognitive and neuropsychiatric long-term sequelae of stroke. Reliable animal models of stroke are paramount to understanding their biomechanisms and to advancing therapeutic strategies. We present a detailed protocol of a transient cerebral ischemia model which does not cause direct ischemic damage in the hippocampus, allowing investigations into the pathophysiology of long-term neurocognitive deficits of stroke. Furthermore, we describe a protocol for obtaining acute hippocampal slices for the purpose of electrophysiological and morphological characterization of adult-borne granule cells. Particularities relating to performing electrophysiological recordings from small cells, such as immature adult-borne granule cells, are also discussed. The present protocol may be complemented by multi-modal investigations (behavioral, morpho-structural, biochemical), to hopefully facilitate research and advances into the long-term sequelae of stroke and the discovery of new therapeutic opportunities.
Keywords: StrokeBackground
Stroke is a major cause of mortality and morbidity in the developed world causing both acute as well as delayed deficits. While intervention strategies targeting restoration of blood flow in ischemic stroke have become more efficient at reducing acute morbidity and mortality, long-term consequences of stroke such as post-stroke depression and post-stroke cognitive impairment and dementia presently evade clinical therapy (Wang et al., 2010; Loubinoux et al., 2012; Mijajlovic et al., 2017). The pathophysiology of these delayed neurocognitive deficits is insufficiently understood. Adult-borne granule cells have been hypothesized to play a role in cognitive and neuropsychiatric sequelae of stroke, however, research in this domain has trailed investigations of other diseases involving adult neurogenesis, such as epilepsy (Jakubs et al., 2006; Jessberger et al., 2007). Several animal models of brain ischemia exist, ranging from reversible global ischemia models to permanent focal ischemia models or even more localized cortical infarcts induced by photothrombosis (Sicard and Fisher, 2009). However, global ischemia models may directly affect the viability of (adult-borne) hippocampal granule cells, thus inducing confounding factors regarding the pathophysiology of neurocognitive deficits, while focal infarcts may cause less robust secondary changes in the brain. Indeed, previous research found that the infarct size correlates with the degree of morphological changes observed in adult-borne granule cells, with transient middle cerebral artery occlusion (MCAO) models having a more profound influence than cortical infarcts (e.g., by photothrombosis, Niv et al., 2012). We therefore present a flexible MCAO model which can reliably induce an extensive, cortico-subcortical ischemia pattern, but without direct ischemic damage of the hippocampus (Sicard and Fisher, 2009). The advantage of the present method is a scalable volume of infarction as a function of ischemia time (with larger infarct volumes inducing a more widespread perturbation in the process of adult neurogenesis), whereby a high percentage of animals survive to allow behavioral, electrophysiological, morphological and biochemical characterization at delayed time points after stroke. Adult borne granule cells can be fluorescently marked either using transgenic models (Couillard-Despres et al., 2006) or by using retroviral labeling techniques (Niv et al., 2012). Transgenic DCX/DsRed mice (Couillard-Despres et al., 2006) express the red fluorescent protein DsRed under the control of the doublecortin (DCX) promoter. DCX is a migration protein which is expressed in immature neurons at the time of synaptic integration and intrinsic maturation roughly up to the fourth week post-mitosis. Alternatively, retroviral labeling can be used to follow the maturation of dentate granule cells born at the moment of intrahippocampal virus injection (Niv et al., 2012).
Materials and Reagents
Middle cerebral artery occlusion
- Mini Vessel Clip (Aesculap Surgical Instruments, catalog number: FD562R )
- Silicone rubber-coated monofilament (Doccol, catalog number: 702056PK5Re )
- Silk suture size 7-0, 100 yards/spool (Teleflex, catalog number: 103-S )
- Silk suture size 3-0 with needle (Covidien, Sofsilk, catalog number: SS694 )
- Surgical tape (3M Transpore, 3M ID 70200739582 )
- Softasept N (Braun, catalog number: 3887138 )
- Cotton pads and cotton swabs
Acute hippocampal slice electrophysiology
- Petri dish
- 50 ml Falcon tube (e.g., Falcon, catalog number 10788561 )
- 15 ml Falcon tube (e.g., Falcon, catalog number 10773501 )
- Razor blades (e.g., Gillette silver blue)
- Air stone (Robu Glasfilter, catalog number 18105 )
- Filter paper (Sartorius, catalog number: FT-3-303-055 )
- Superglue (e.g., UHU, catalog number: 36016 )
- Syringe filters, 0.22 µm (Kinesis, catalog number: ESF-MC-13-022 )
- Thick walled borosilicate glass capillaries (Science products, catalog number: GB150F-8P )
Reagents
NaCl (Carl Roth, catalog number: 9265.1 )
KCl (Carl Roth, catalog number: 6781.1 )
CaCl2 (Carl Roth, catalog number: A119.1 )
MgCl2 (Carl Roth, catalog number: 2189.1 )
MgSO4 (Carl Roth, catalog number: 0261.1)
NaHCO3 (Carl Roth, catalog number: HN01.2 )
NaH2PO4 (Carl Roth, catalog number: T879.2 )
Glucose (Carl Roth, catalog number: HN06.3 )
Sucrose (Carl Roth, catalog number: 9286.2 )
Na-Ascorbate (Sigma-Aldrich, catalog number: A4034 )
K-gluconate (Carl Roth, catalog number: 2621.2 )
MgATP (Sigma-Aldrich, catalog number: A9187 )
NaGTP (Sigma-Aldrich, catalog number: G8877 )
EGTA (Sigma-Aldrich, catalog number: 03777)
HEPES (Carl Roth, catalog number: HN77.2 )
KOH (Carl Roth, catalog number: K017.1 )
Biocytin (Tocris, catalog number: 3349 )
Streptavidin-Cy3 (Sigma-Aldrich, catalog number: S6402-1ML )
TBS (Thermo Fisher, catalog number: 28358 )
Normal Donkey Serum (abcam, catalog number: ab7475 )
Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
4% PFA (Sigma-Aldrich, catalog number: 1004965000 )
Fluoromount (Sigma-Aldrich, catalog number: F4680 )
Slicing solution (final) (see Recipes)
Artificial cerebrospinal fluid (final) (see Recipes)
Slicing solution 10x stock (see Recipes)
aCSF 10x Stock, 500 ml (see Recipes)
2 M Glucose stock solution (see Recipes)
1 M CaCl2 stock solution (see Recipes)
1 M MgCl2 stock solution (see Recipes)
Slicing solution (see Recipes)
aCSF (see Recipes)
Intracellular solution (see Recipes)
TBS Plus (see Recipes)
Equipment
Middle cerebral artery occlusion
Needle holder (Fine Science Tools, catalog number: 12010-14 )
Blunt surgical scissors (Fine Science Tools, catalog number: 14003-14 )
Standard forceps (Fine Science Tools, catalog number: 11000-13 )
Curved standard forceps (Fine Science Tools, catalog number: 11001-12 )
Hartman hemostat (Fine Science Tools, catalog number: 13002-10 )
Delicate suture tying forceps (Fine Science Tools, catalog number: 11063-07 )
Spring scissors (Fine Science Tools, catalog number: 15018-10 )
Temperature controller (FHC, catalog number: 40-90-8D )
Heating pad (FHC, catalog number: 40-90-2-02 )
Mini Rectal Thermistor Probe (FHC, catalog number: 40-90-5D-02 )
Stereomicroscope with cold-light source (Zeiss, Stemi 508, catalog number: 495009-0016-000 )
200 ml beaker
Laboratory timer
Curved blunt surgical scissors (Fine Science Tools, catalog number: 14003-14 )
Small scissors (Fine Science Tools, catalog number: 14184-09 )
Adson forceps (Fine Science Tools, catalog number: 11006-12 )
Small curved forceps (Fine Science Tools, catalog number: 11152-10 )
Spatula (Fine Science Tools, catalog number: 10099-15 )
Microloader microcapillaries (Eppendorf, catalog number: 5242956003 )
Vibrating blade microtome (Leica VT1200 S, catalog number: 1491200S001 )
Water bath (GFL Water Bath, catalog number: 1108 )
Osmometer (Gonotec Osmomat 010)
Peristaltic pump (Ismatec ISM830, catalog number: GZ-78016-02 )
Upright electrophysiology microscope ( Zeiss Axio Examiner.Z1 )
Patch clamp amplifier (Molecular Devices, catalog number: Axopatch 200B-2 )
Digitizer (Molecular Devices, catalog number: Digidata 1550B4 )
Micropipette puller (Sutter Instruments, P-97 Flaming/Brown, catalog number: P-97 )
Constant current stimulation unit (Digitimer Ltd., DS3)
(Optional) Commercial slice chamber (Science Products, Brain Slice Keeper1, catalog number: BSK1 )
Software
ZEN 2.3 (blue edition) imaging software (ZEISS Germany)
pCLAMP 10.7 (Molecular Devices, Axon Instruments)
Procedure
Transient middle cerebral artery occlusion
Prepare the necessary instrumentation before starting the experiments (Figure 1).

Figure 1. Necessary instrumentation for the MCAO procedureWeigh the mouse and apply antalgic treatment with one drop of meloxicam (0.5 mg/ml, ca. 25 µg for a 25 g mouse) orally 30-60 min before starting the operation.
Place the mouse in an anesthesia box and turn on a gas mixture of 2.5% Isoflurane in 3:1 N2O:O2 and wait for the mouse to fall asleep. Test pain reaction by pinching the tail or the paw.
Place the mouse supine on a heating pad. Secure the paws with surgical tape, introduce the mini rectal thermistor probe, and keep the body temperature at 37 °C on the operating table under a continuous flux of isoflurane/oxygen/nitrous oxide.
Note: A lower body temperature in the mouse MCAO model will result in a smaller infarct size, and, more generally, variations in body temperature during the MCAO protocol will result in a greater variability in infarct size and severity of neurological deficit. The age of the mouse is also relevant for the outcome (Sieber et al., 2010).
Disinfect the ventral cervical region with Softasept N and make a midline incision. Use the standard forceps and the curved standard forceps to expose the common carotid artery by gently pushing to the side fat tissue, muscle, and the thyroid. Use the fine tip tweezers under the stereomicroscope to dissect the common carotid artery, the bifurcation, and the internal and external carotid arteries.
Place a silk suture around the proximal external carotid artery after the bifurcation and another suture around the common carotid artery close to the bifurcation. Using the mini vessel clip, clamp the internal carotid artery proximally to cause a slight vessel engorgement in the common carotid artery, helping in the next step.
Make a small incision in the common carotid artery proximal to the suture, open the mini vessel clip and insert the monofilament to occlude the middle cerebral artery until no further advancement to light pressure is possible (insertion length of about 10 mm). Fix the monofilament in place with a suture around the internal carotid artery which should be fixed with only one node. Close the wound.
Note: Write down the insertion depth of the monofilament both to control that it did not move during the ischemic period and as a control across mice.
Allow the animal to recover from anesthesia under a heat lamp. Ischemia time is usually 45 min but can vary from 30 to 45 min depending on the intended size of the infarct. With longer time intervals there is also cortex involvement of the infarct.
Note: There is a trade-off in the time of ischemia: shorter durations may lead to a number of animals not becoming a sufficiently large enough infarct, while longer periods of ischemia are associated with increased mortality and hence a need for larger experimental groups. Qualified and well-practiced personnel is essential for reducing variability.
Re-anesthetize the animal 5 min before the end of the ischemia time. Loosen the suture over the monofilament and remove the monofilament. Suture the common carotid artery, check for bleeding and close the wound. Observe the animal during recovery from anesthesia and place it back in its cage.
For mice from the control group Step A7 is skipped.
Control and score the mice according to a pre-established scoring table immediately after the operation, at three hours postoperatively and daily afterwards.
Note: Body weight should be checked once or twice daily and early interventions such as providing wet food and a beaker of water on the cage floor for mice with reduced mobility can help reduce mortality. Scoring would also allow correlation of clinical stroke severity with single-cell electrophysiological properties.
Acute hippocampal slices
Before starting:
Assemble a dissecting tray by gluing a smaller, plastic Petri dish into a larger, glass one, as in Figure 2 (lower row, middle). Optionally, the bottom of the small Petri dish can be covered in a couple of mm of silicone rubber to provide an anti-slip surface during dissection.
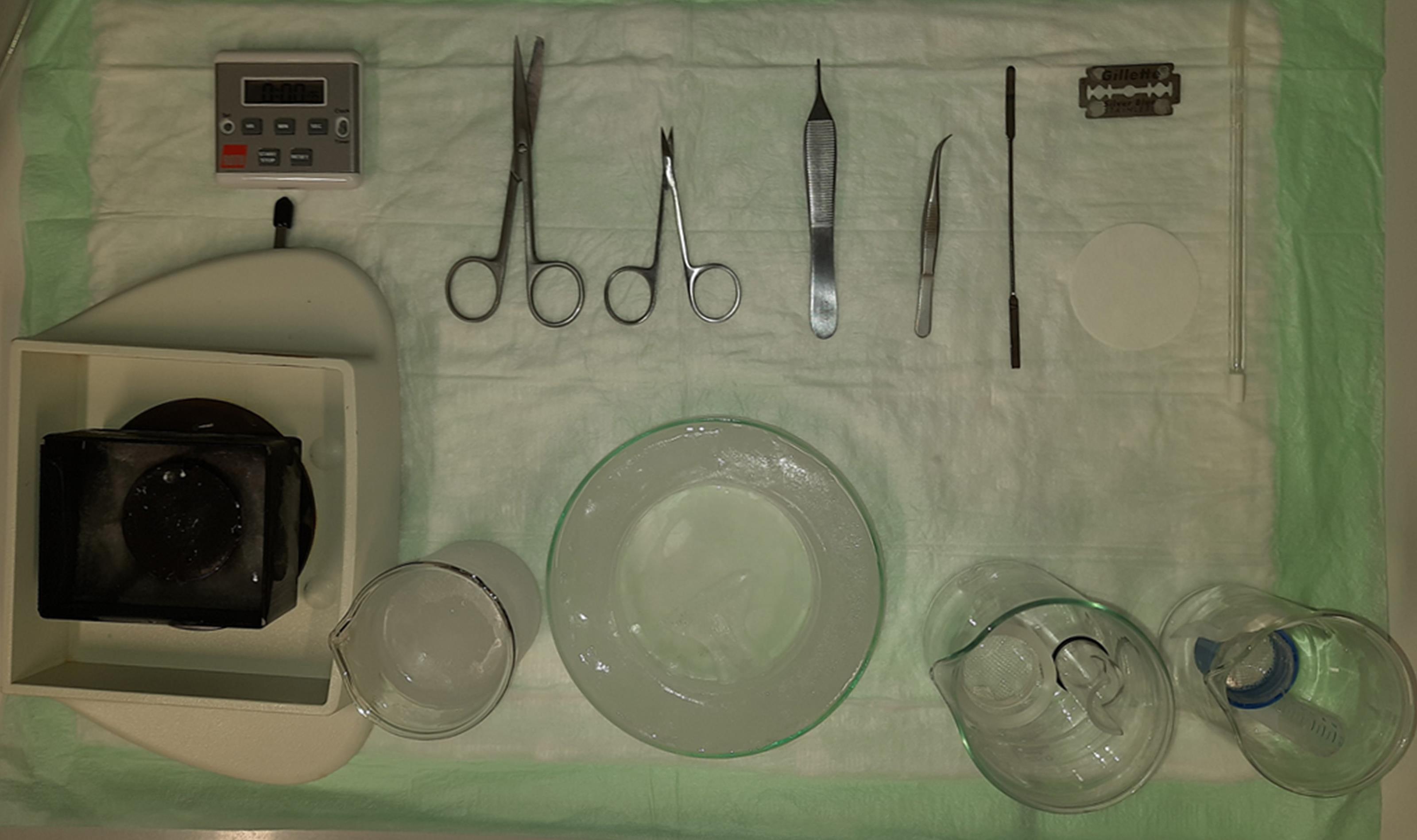
Figure 2. Necessary instrumentation for the preparation of acute hippocampal slicesAssemble a brain slice chamber: Cut the top part of a 50 ml Falcon tube and cut out the lid of the tube so that only the outer cylinder remains. Place a cotton or nylon net between the cut-out lid and the top part of the Falcon tube and screw it in place. Use a 15-ml Falcon tube with the bottom cut out and a small plastic weigh dish to secure all elements in place as in Figure 2 (lower row, rightmost). Alternatively, and if an associated research workshop is available, a more stable construction made from acrylic glass can pe prepared or can be bought commercially.
Prepare 500 ml sucrose-based slicing solution and fill the half of a 200 ml beaker, the bottom of the buffer tray of the vibratome (not covering the stage) and the outer ring of the dissecting tray, and place these in the freezer at -20 °C overnight.
On day of the experiment, prepare 500 ml fresh sucrose-based slicing solution and 1,000 ml aCSF. Fill the brain slice chamber with aCSF and put it in the water bath at 33 °C while bubbling Carbogen (5% CO2/95% O2). Fill up the 200 ml beaker and the bottom of the inner ring of the dissecting tray with slicing solution. Place the rest of the slicing solution on ice with constant Carbogen bubbling.
Note: Surgical instruments can also be cooled by placing them on ice before starting the experiments.
Prepare the vibratome according to the instruction manual: place a razor blade in its holder and align the blade using the Vibrocheck.
Anesthetize the mouse with isoflurane, start the timer and decapitate the anaesthetized mouse with the surgical scissors. Place the head immediately in the ice-cold slicing solution in the 200 ml beaker.
Note: Removal of the brain is a critical step that must be completed in under one minute. Keep visual track of time during these critical steps and fill it in the lab book afterwards to account for possible variations in slice or measurement quality. Inadvertent stress on the day of recording should be avoided, as activation of β-adrenergic receptors may lead to GluA3 AMPA receptor potentiation and variability in the data (Renner et al., 2017).
Use the small scissors to cut the occipital skull from the foramen magnum towards the auditory meatus on each side, then cut medially from the foramen magnum towards lambda. Keep the cranium in ice-cold slicing solution between steps.
Use the Adson forceps to remove the occipital bone by flapping it medially to laterally, then remove the parietal bone similarly along the sagittal suture, exposing the brain. Keep the cranium in ice-cold slicing solution between steps.
Insert the spatula along the clivus, severing the cranial nerves, and remove the brain into the dissecting tray.
Use a razor blade make a coronal cut at the level of the superior colliculus, and separate and remove the cerebellum. Separate the two hemispheres with a median sagittal cut and place the hemisphere ipsilateral to the stroke (right) on its medial side. Next, make a longitudinal cut along the parietal cortex (Figure 3A) and perpendicular to the sagittal plane (Figure 3B) and separate the frontal lobes by sectioning in the coronal plane (Figure 3C).
Note: This is a critical step as the plane of section will determine perhaps to the greatest extent cellular viability and hence slice quality. The goal is to achieve a transverse sectioning plane, parallel to the plane of the apical dendritic tree and perpendicular to the long axis of the hippocampus. However, due to the complex, three-dimensional configuration of the hippocampus (similar to a seahorse or a banana), no one plane will be optimal for the entire length of the hippocampus. The above coordinates will give viable slices from the middle to ventral hippocampus (Figure 3a, 3b [Lein et al., 2007]). Depending on slice thickness, about three good quality slices can be expected from one hippocampus. For other subregions of the hippocampus, different slicing angles are chosen (Bischofberger et al., 2006). We recommend allowing a generous amount of time and (animal) resources at the beginning of the project for finding and perfecting the slicing angles which will result in the optimal slice viability. The removed frontal lobes may be used for estimating the size of the ischemia (e.g., by Map2 staining), since affected areas usually include the frontoparietal cortex and lateral caudatoputamen.
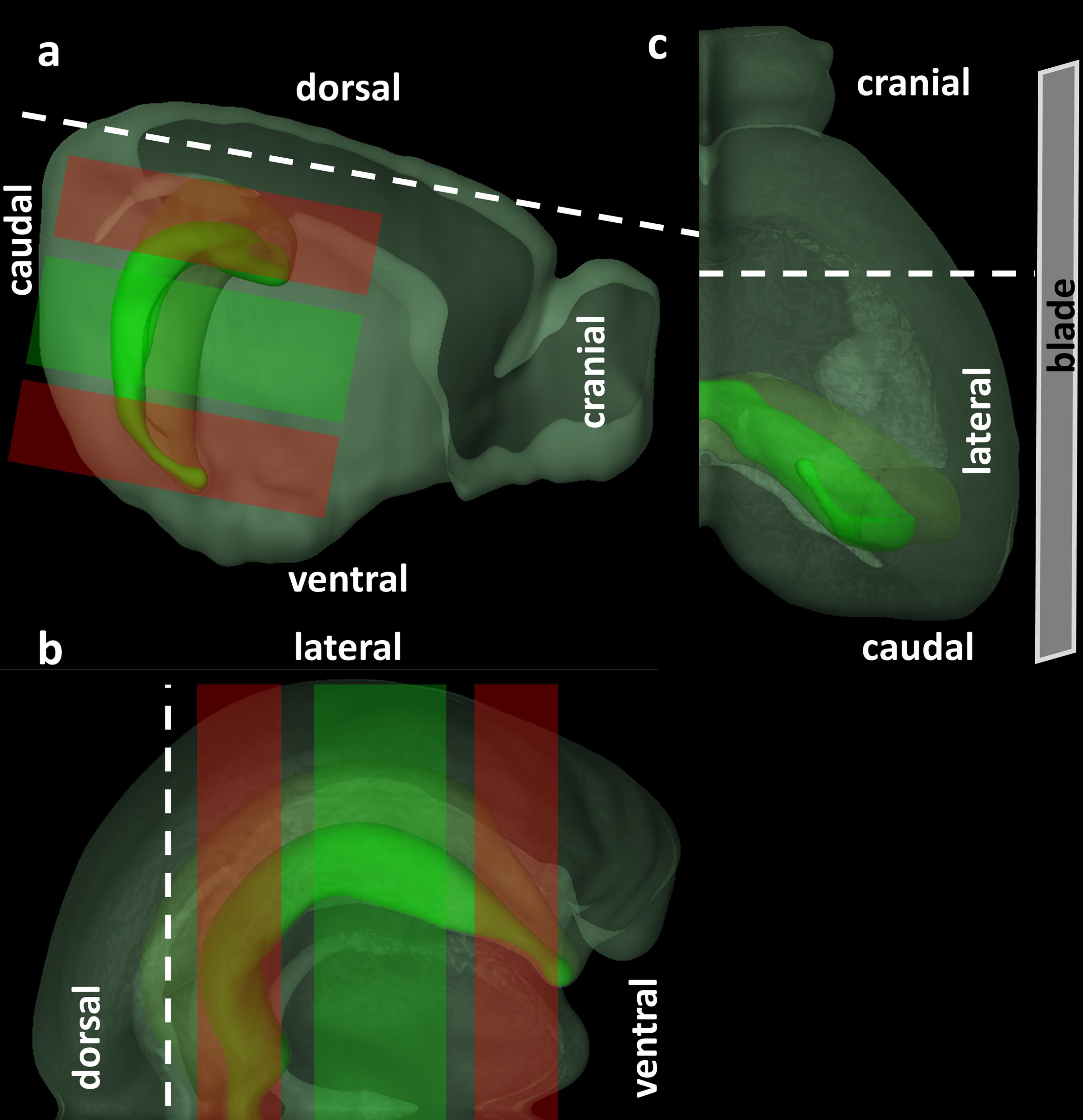
Figure 3. Orientation and cut surfaces of the brain for preparing acute hippocampal slices. A. Lateral view. B. Cranial view. C. Dorsal view. Red shaded areas represent regions of the hippocampus where slice quality in the present orientation will presumably be suboptimal, whereas green shaded areas represent slices with optimal cellular viability. © 2015 Allen Institute for Brain Science. Allen Brain Atlas API. Available from: brain-map.org/api/index.html.Place a couple of drops of cyanoacrylate glue on the dry stage of the buffer tray. With the help of a small curved forceps place the hippocampus dorsal side down on the spatula, use the filter paper to absorb any excess fluid, and carefully place the hippocampus dorsal side down in cyanoacrylate glue on the stage. The brain should be glued with its lateral side facing the blade (Figure 3C). Pour ice-cold slicing solution to completely cover the hippocampus in the buffer tray.
Make 300 µm slices using a speed of 0.05 mm/s and a horizontal oscillation amplitude of 1.2 mm (Geiger et al., 2002). Transfer slices into the brain slice chamber in the water bath at 33 °C and incubate under constant Carbogen bubbling for 45 min.
Remove the brain slice chamber from the water bath and let slices recover for another 60 min at room temperature.
Note: Temperature and time allowed to recover influence the metabolic and electrophysiologic properties of the brain slices and should be carefully monitored and kept constant (zur Nedden et al., 2011).
Electrophysiological considerations for whole cell recordings from adult borne granule cells and biocytin staining
A detailed description of the instrumentation and steps required to obtain the whole-cell configuration is beyond the scope of this paragraph and there are already excellent resources available (Walz, 2007; Sakmann, 2009; Okada, 2012; Graziane and Dong, 2015). Below are presented technical particularities relevant for performing whole cells analysis from adult-borne granule cells.
For acute hippocampal slices from older mice (over 3-6 months), modified formulations of the slicing solution can be adopted for improved slice quality and cellular viability (Ting et al., 2011 and 2014).
The very high input resistance (1-10 GΩ) and low capacitance (5-10 pF) due to a very small size pose technical difficulties for the electrophysiologist. Patch pipettes should be made of thick-walled glass, and the pipette capacitance should be carefully compensated. The seal resistance should be at least 3 times (preferably > 5 times) the input resistance.
An understanding of the membrane dynamics and stability during formation of the seal can help select cells which have formed stable seals and discard cells with a gigaseal but a bad seal structure, ultimately saving time (Suchyna et al., 2009). Variations of promising seals as well as seals with a low probability of successful, qualitative recording, are presented in Figure 4A and Figure 4B respectively.
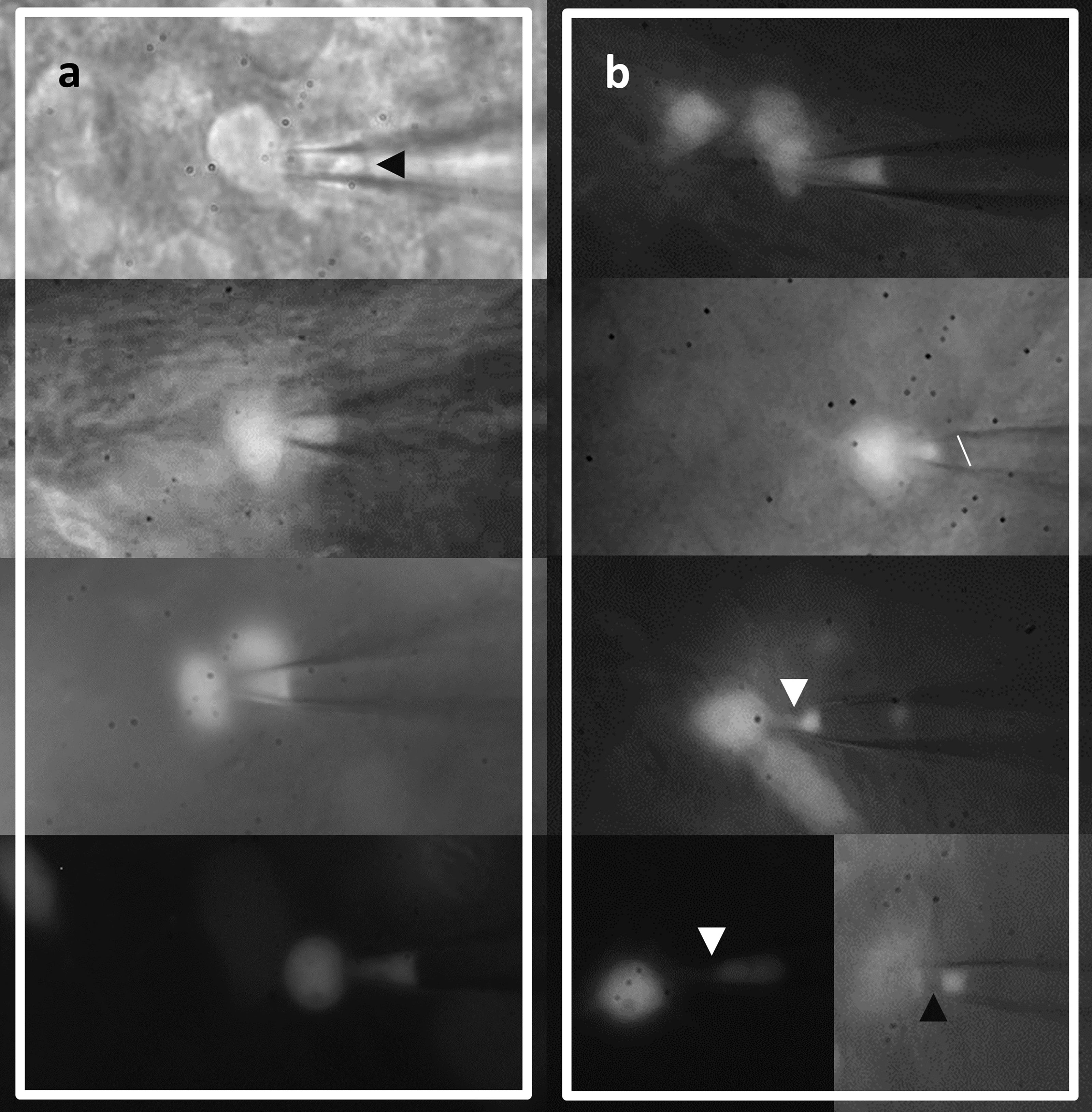
Figure 4. Aspect of cell membrane during gigaseal formation can inform on the probability of a good recording. A. Promising aspects include a convex, straight, or slightly concave seal membrane with a relatively homogenous fluorescent signal (slightly dimmer at the pipette tip). B. Seals unlikely of giving stable recordings showing a more concave patch membrane with inhomogeneous fluorescent signal, a sloped patch membrane (line added for reference), and more inhomogeneous membrane patches indicative of membrane blebbing (arrowheads).Electrophysiological properties of a group of neurons (e.g., dentate granule cells) display heterogeneity in multiple space dimensions (Cembrowski and Spruston, 2019), therefore settling on a particular hippocampal region (e.g., ventral or dorsal hippocampus) may help reduce variability.
The liquid junction potential should be measured and be compensated for before experiments (Barry and Lynch, 1991).
Due to the very high input resistance of immature, adult-borne granule cells, relatively small current injections may be enough to destabilize the membrane and damage the cell. Incrementing steps of 5-10 pA should be initially implemented, since the median rheobase is around 30 pA in immature granule cells (Ceanga et al., 2019). The resting potential at the start of the protocol is of critical importance, since at depolarized potentials Na+ channels are in the inactive state. Some neurons which only produce spikelets/immature action potentials when depolarized starting from the resting membrane potential (Ihold = 0 pA), may produce taller action potentials when depolarized starting from a more negative potential (Ihold = -20 pA).
Biocytin staining of recorded cells is complicated by the precarious anchoring of immature adult-borne granule cells in the tissue due the small soma size and underdeveloped dendritic tree (Figure 5), which often lead to the removal of the cell with the pipette (cells remain glues to the pipette tip). Very slow removal of the pipette from the neuron can help increase the percentage of patched cells which remain in the slice. Good quality reconstructions usually require at least 10 min for filling of the neurons.
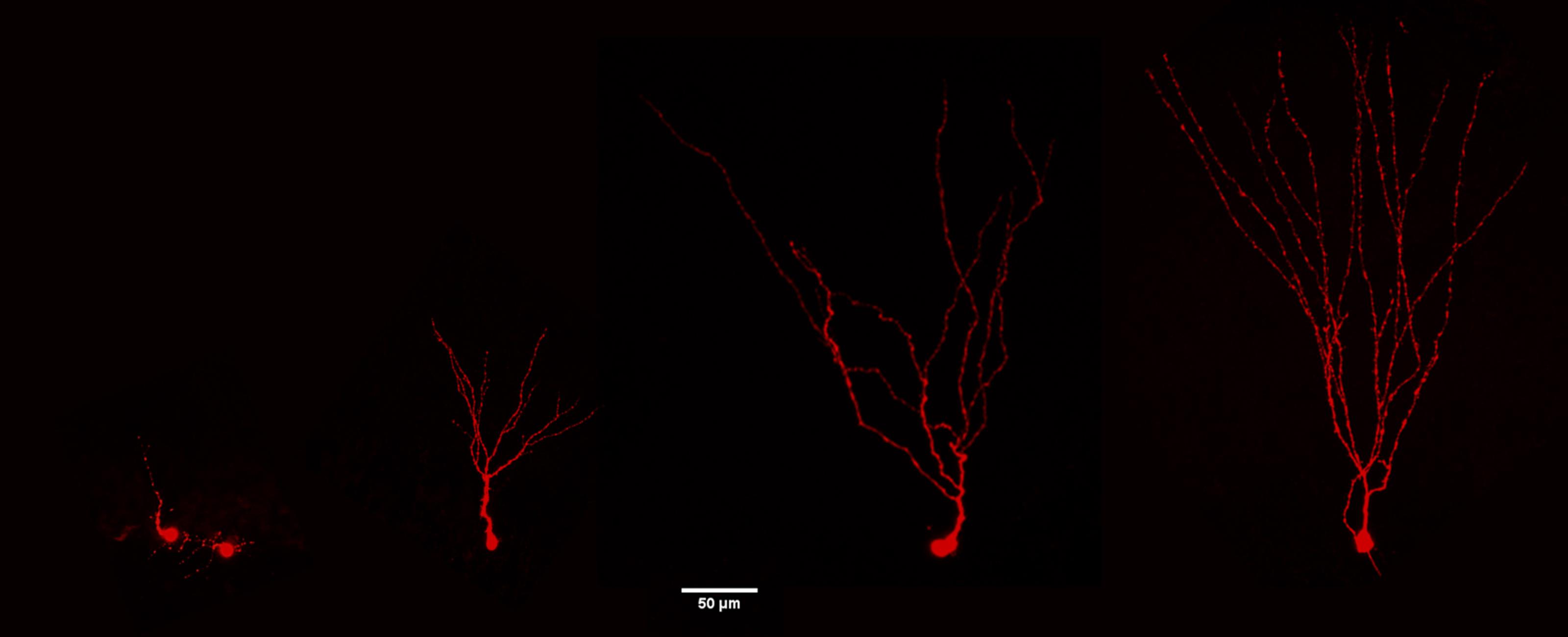
Figure 5. Confocal images of adult-borne granule cells following biocytin filling at different maturation stages. The neurons show a progressive development of the dendritic tree from immature to the left to a mature phenotype to the right (3.5 month-old mice). Bar=50 μm.After recordings, transfer slices into 4% PFA (e.g., in 4-well plates, one slice/well) and store overnight at 4 °C (however slices may also be processed after 4 h in 4% PFA).
Next, gently wash the slices 4 × 15 min in TBS, then incubate the slices for 2 h in TBS Plus. Then add 1:500 Streptavidin-Cy3 and incubate overnight at 4 °C.
Next day wash the slices 4 × 15 min in TBS and embed slices in Fluoromount.
Note: The orientation of the slice must be noted on the cover of the well plates (e.g., tip of the dentate gyrus pointing left or right). Since slices are 300 µm thick, they should be placed with the cut surface closest to the recording cells facing upwards, otherwise scatter and autofluorescence from the tissue will affect image quality when cells are scanned. Recorded cells are often near the surface, around 50 µm deep–slices should be handled and transferred gently. When embedding the slices, press only very gently on the cover glass to avoid morphological disruption of the recorded cells.
Recipes
Slicing solution (final)
Concentrations in mM:
87 NaCl
25 NaHCO3
2.5 KCl
1.25 NaH2PO4
7 MgCl2
25 glucose
75 sucrose
1.3 ascorbate
0.5 CaCl2
Artificial cerebrospinal fluid (final)
Concentrations in mM
126 NaCl
26 NaHCO3
3 KCl
1.25 NaH2PO4
1 MgSO4
2 CaCl2
10 glucose
1.3 ascorbate
Slicing solution 10x stock
870 mM NaCl
25 mM KCl
250 mM NaHCO3
12.5 mM NaH2PO4, prepare 500 ml for a week, store at 4 °C
aCSF 10x Stock, 500 ml
1,260 mM NaCl
30 KCl
260 NaHCO3
12.5 NaH2PO4
Prepare 500 ml for a week, store at 4 °C
2 M Glucose stock solution
72.064 g for 200 ml stock solution in H2O
1 M CaCl2 stock solution
22.198 g for 200 ml stock solution in H2O
1 M MgCl2 stock solution
40.660 g for 200 ml stock solution in H2O
Slicing solution
10x stock slicing solution (100 ml stock for 1 L final)
7 mM MgCl2 from stock (7 ml stock for 1 L final)
25 mM glucose from stock (12.5 ml stock for 1 L)
75 mM sucrose
1.3 mM Na-ascorbate
0.5 mM CaCl2 from stock (250 µl stock for 1 L final). Add slowly at the end to prevent precipitation of CaCO3, equilibrate with Carbogen, and keep at 4 °C. pH should settle at about 7.4, osmolarity 340-350 mOsm.
aCSF
10x stock slicing solution (100 ml stock for 1 L final)
1 mM MgSO4
10 mM glucose from stock (5 ml stock for 1 L)
1.3 mM Na-ascorbate
2 mM CaCl2 from stock (1 ml stock for 1 L final). Add slowly at the end to prevent precipitation of CaCO3 and equilibrate with Carbogen. pH should settle at about 7.4, osmolarity 300-310 mOsm.
Intracellular solution
140 mM K-gluconate
10 mM NaCl
2 mM MgATP
0.3 mM NaGTP
0.6 mM EGTA
10 mM HEPES, pH 7.2 with KOH, osmolarity 285 mosm/kg
Prepare 50-100 ml intracellular solution in a measuring cylinder on ice, filter and aliquot in 1 ml tubes and store at -20 °C for up to six months
On the day of the experiment add 4 mg/ml biocytin and centrifuge the tube
An ultrasonic bath can help biocytin dissolve faster
After vigorous vortexing, the intracellular solution can be aspirated in a 1 ml syringe which is filtered with a 0.22 µm filter and a microloader microcapillary. The syringe with the intracellular solution should be kept on ice during recordings
TBS Plus
TBS
5% Donkey Serum
0.3% Triton X-100
Acknowledgments
This work was supported by the Interdisciplinary Center for Clinical Research (IZKF) Jena. These protocols were used for the article (Ceanga et al., 2019).
Competing interests
The authors declare no competing financial interests.
Ethics
All experimental procedures were in accordance with the EU Directive 2010/63/EU and were approved by the German Animal Care and Use Committee.
References
- Barry, P. H. and Lynch, J. W. (1991). Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol 121(2): 101-117.
- Bischofberger, J., Engel, D., Li, L., Geiger, J. R. and Jonas, P. (2006). Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc 1(4): 2075-2081.
- Ceanga, M., Keiner, S., Grünewald, B., Haselmann, H., Frahm, C., Couillard-Després, S., Witte, O. W., Redecker, C., Geis, C. and Kunze, A. (2019). Stroke accelerates and uncouples intrinsic and synaptic excitability maturation of mouse Hippocampal DCX+ adult-born granule cells. J Neurosci 39(9): 1755-1766.
- Cembrowski, M. S. and Spruston, N. (2019). Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat Rev Neurosci 20(4): 193-204.
- Couillard-Despres, S., Winner, B., Karl, C., Lindemann, G., Schmid, P., Aigner, R., Laemke, J., Bogdahn, U., Winkler, J., Bischofberger, J. and Aigner, L. (2006). Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci 24(6): 1535-1545.
- Graziane, N. and Dong, Y. (2015). Electrophysiological analysis of synaptic transmission. New York: Humana Press..
- Geiger, J. R., Bischofberger, J., Vida, I., Frobe, U., Pfitzinger, S., Weber, H. J., Haverkampf, K. and Jonas, P. (2002). Patch-clamp recording in brain slices with improved slicer technology. Pflugers Arch 443(3): 491-501.
- Jakubs, K., Nanobashvili, A., Bonde, S., Ekdahl, C. T., Kokaia, Z., Kokaia, M. and Lindvall, O. (2006). Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52(6): 1047-1059.
- Jessberger, S., Zhao, C., Toni, N., Clemenson, G. D., Li, Y. and Gage, F. H. (2007). Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27(35): 9400-9407.
- Lein, E. S., Hawrylycz, M. J., Ao, N., Ayres, M., Bensinger, A., Bernard, A., Boe, A. F., Boguski, M. S., Brockway, K. S. and Byrnes, E. J. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124): 168-176.
- Loubinoux, I., Kronenberg, G., Endres, M., Schumann-Bard, P., Freret, T., Filipkowski, R. K., Kaczmarek, L. and Popa-Wagner, A. (2012). Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med 16(9): 1961-1969.
- Mijajlovic, M. D., Pavlovic, A., Brainin, M., Heiss, W. D., Quinn, T. J., Ihle-Hansen, H. B., Hermann, D. M., Assayag, E. B., Richard, E., Thiel, A. et al. (2017). Post-stroke dementia- a comprehensive review. BMC Med 15(1): 11.
- Niv, F., Keiner, S., Krishna, Witte, O. W., Lie, D. C. and Redecker, C. (2012). Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke 43(9): 2468-2475.
- Okada, Y. (2012). Patch Clamp Techniques: From Beginning to Advanced Protocols. Tokyo: Springer Japan.
- Renner, M. C., Albers, E. H., Gutierrez-Castellanos, N., Reinders, N. R., van Huijstee, A. N., Xiong, H., Lodder, T. R. and Kessels, H. W. (2017). Synaptic plasticity through activation of GluA3-containing AMPA-receptors. Elife 6: e25462.
- Sakmann, B. (2009). Single-channel recording. New York: Springer.
- Sicard, K. M. and Fisher, M. (2009). Animal models of focal brain ischemia. Exp Transl Stroke Med 17.
- Sieber, M. W., Guenther, M., Kohl, M., Witte, O. W., Claus, R. A. and Frahm, C. (2010). Inter-age variability of bona fide unvaried transcripts Normalization of quantitative PCR data in ischemic stroke. Neurobiol Aging 31(4): 654-664.
- Suchyna, T. M., Markin, V. S. and Sachs, F. (2009). Biophysics and structure of the patch and the gigaseal. Biophys J 97(3): 738-747.
- Ting, J. T., Daigle, T. L., Chen, Q. and Feng, G. (2014). Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 1183: 221-242.
- Ting, J., Chen, Q. and Feng, G. (2011). Improved methods for acute brain slice preparation from adult and aging animals. Soc Neurosci Abstr 37.
- Walz, W. (2007). Patch-Clamp Analysis: Advanced Techniques. Totowa, N.J.: Humana Press.
- Wang, S. H., Zhang, Z. J., Guo, Y. J., Sui, Y. X. and Sun, Y. (2010). Involvement of serotonin neurotransmission in hippocampal neurogenesis and behavioral responses in a rat model of post-stroke depression. Pharmacol Biochem Behav 95(1): 129-137.
- zur Nedden, S., Hawley, S., Pentland, N., Hardie, D. G., Doney, A. S. and Frenguelli, B. G. (2011). Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors. J Neurosci 31(16): 6221-6234.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ceanga, M., Guenther, M., Ingrisch, I. and Kunze, A. (2021). Characterization of Hippocampal Adult-borne Granule Cells in a Transient Cerebral Ischemia Model. Bio-protocol 11(2): e3890. DOI: 10.21769/BioProtoc.3890.
- Ceanga, M., Keiner, S., Grünewald, B., Haselmann, H., Frahm, C., Couillard-Després, S., Witte, O. W., Redecker, C., Geis, C. and Kunze, A. (2019). Stroke accelerates and uncouples intrinsic and synaptic excitability maturation of mouse Hippocampal DCX+ adult-born granule cells. J Neurosci 39(9): 1755-1766.
Category
Neuroscience > Nervous system disorders
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









