- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Antibacterial Activity of Film Coatings against Four Clinically Relevant Bacterial Strains
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3887 Views: 4732
Reviewed by: Juan Facundo Rodriguez AyalaChao JiangFilipa Vaz

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Functional Assay for Measuring Bacterial Degradation of Gemcitabine Chemotherapy
Serkan Sayin and Amir Mitchell
Sep 5, 2023 1741 Views

Identification of Mycobacterium tuberculosis and its Drug Resistance by Targeted Nanopore Sequencing Technology
Chen Tang [...] Guangxin Xiang
Feb 5, 2025 1986 Views

A Highly Sensitive and Selective DAMP Assay for the Detection of Bacterial Pathogens Associated With Brain Inflammation
Liubov A. Shkodenko [...] Elena I. Koshel
Mar 20, 2025 1641 Views
Abstract
Antibacterial coatings have currently gained great importance in biomedical technology investigations. Because of the spatial arrangement of the film coatings, evaluation of antibacterial activity presents a new challenge regarding traditional bacterial counting methods. In this protocol, four clinically relevant pathogens, Salmonella typhimurium, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus were incubated on titania mesostructured thin film coatings for 24 h. Then, cell viability was studied considering three methods: counting of the number of colony forming units (CFU), live/dead staining, and quantification of extracellular DNA in suspension. Firstly, bacterial count was determined by the standard plate-count technique. Secondly, bacteria membrane integrity was evaluated by utilization of two fluorescent dyes, which allow distinction between live (membrane intact) and dead (membrane disrupted) bacteria. Lastly, extracellular DNA was quantified by spectrophotometry. In this manner, the three aforementioned techniques enabled the study of bacterial viability by qualitative and quantitative analyses.
Keywords: Antibacterial activityBackground
Functional coatings are being widely investigated owing to the valuable advantages they offer to the area of materials engineering. They are capable of introducing new surface properties, with no changes in the material bulk characteristics. Among their functionalities, antibacterial coatings have demonstrated great importance for biomedical applications (Mateescu et al., 2015). In this sense, nanotechnology provides substantial tools for the fabrication of antibacterial coatings. Our group has achieved the synthesis of nanostructured coatings with broad spectrum bactericidal activity, without utilizing antibiotics or bacteria repelling substances (Scilletta et al., 2019). Here, only the superstructure is responsible for the bactericidal activity. To find the specific superstructure capable of bacteria killing many assays with distinct coatings configurations have been performed. All the coatings were composed of poloxamer block copolymers (also named as Pluronic®) within a titania matrix. Thus, film coatings were prepared using two copolymers which differ in the length of the hydrophilic and hydrophobic portions (Pluronic F127 and Pluronic P123). The quantities of the copolymers varied systematically, therefore modifying the titania: pluronic mol ratios. These modifications modulate the mesophase, which we proved is directly related to the bactericidal capacity. The mesostructured titania thin film coatings were synthesized by the sol-gel method in combination with evaporation-induced self-assembly of the pluronic micelles and deposited by spin-coating on glass substrates (Scilletta et al., 2019).
In order to find the best bactericidal performance, the aforementioned distinct coating configurations were compared based on their antibacterial efficacy. We utilized three methods to determine the most effective antibacterial coating: counting of the number of colony forming units (CFU), live/dead staining, and quantification of extracellular DNA in suspension. To perform the counting of CFU after bacterial incubation on the films we modified a previous protocol by Merritt et al. (2006). Afterwards, coatings presenting the best bactericidal performance were further studied to clarify the mechanism of bacteria killing. In this regard, fluorescent staining with SYTO 9 and propidium iodide (PI) was utilized to determine the integrity of the bacteria membrane. After incubating the bacterial inoculum on the films, the recovered suspension was dyed with this mixture. PI only diffuses into damaged membrane cells and binds to DNA with high affinity, labeling those cells in red. SYTO 9 only stains live cells with intact membranes, labeling them in green. This protocol is a modification of the one published by Smith and Hunter (2008). Lastly, the presence of extracellular DNA was studied as an indicator of cell membrane disruption. To perform this determination we modified the previous protocols from Chen and Cooper (2002) and from Yuan et al. (2019).
As a result of the three considered aspects, this protocol describes an integral approach for the determination of antibacterial activity of film coatings. The protocol can be utilized to evaluate the effects of any coatings on bacterial viability, which includes bactericidal superstructures like the one studied by us, and drug-loaded coatings, either with traditional antibiotics or novel antibacterial agents.
Materials and Reagents
50 ml sterile Erlenmeyer flasks (DWK Life Science, Duran®, catalog number: 2121617 )
5 ml sterile Borosilicate tubes (IVA®, catalog number: VID-01548 )
1.5 ml sterile Eppendorf centrifuge tubes (Eppendorf, catalog number: 022364111 )
2-20 µl, 20-100 µl, 100-1,000 µl Kartell pluripet micropipettes (Kartell LABWARE, catalog numbers: 13000 , 13210 , 13220 ) and 1-10 ml Acura® manual micropipette (Socorex Swiss, catalog number: 825/835 )
Sterile pipette tips 1-200 µl, 100-1,000 µl (Corning®, catalog numbers: S4860 , S9032)
Sterile 50, 100 and 1,000 ml borosilicate measuring cylinders (VILABO, catalog numbers: 3501114 , 3501115 , 3501118 )
Sterile Petri dish glass plates (80 mm diameter) (Brand®, catalog number: BR455732 )
Bacterial strains Salmonella typhimurium (LT2), Escherichia coli K12, Pseudomonas aeruginosa (PAO1) and Staphylococcus aureus (ATCC 29213 )
Neutral detergent (Extran®, Merck Millipore, catalog number 140000 )
Milli-Q Water
Acetone (Merck, catalog number 67-64-1 )
Isopropanol (Merck, catalog number 3979-51-9 )
Titanium tetrachloride (Merck, catalog number , 7550-45-0 )
Pluronic® F-127 (Sigma-Aldrich, catalog number 9003-11-6 )
Pluronic® P-123 (Carbo synth, catalog number 9003-11-6 )
Glass slides (YEGREN® , catalog number: 7101 )
Cover glasses (Merck, catalog number C9056-1CS )
Cell culture plate (Sigma®, catalog number SIAL0516-50EA )
Plastic box (15 cm x 20 cm x 5 cm)
Paper towel (WypAll* X 60 Jumbo Roll, KCWW, Kimberly-Clark, catalog number: 30218593 )
Inoculating loop (DeconTM, catalog number: MP 19025 )
Glycerol for molecular biology, ≥ 99.0% (Sigma-Aldrich, catalog number: G5516-500ML )
Distilled water (G-BIOSCIENCES, catalog number: 786-1713 )
Immersion oil (Leica, standar and type “F”, CAS Number: 195371-10-9 )
Tryptone (OXOID, catalog number: LP0042 )
Yeast extract (Merck, catalog number: 103753 )
Granulated agar (Difco, catalog number 214530 )
Sodium Chloride (NaCl) (Biopack, catalog number: 1646.08 )
SYTOTM 9 Green Fluorescent Nucleic Acid Stain (Invitrogen, catalog number S34854 )
Propidium Iodide (Invitrogen, Catalog number P1304MP )
LB medium (see Recipes)
LB agar solid medium (see Recipes)
4 M NaCl solution (see Recipes)
Saline solution (see Recipes)
Staining SYTO 9/PI solution (see Recipes)
Titania mesostructured thin film coatings (see Recipes)
Equipment
50 ml Erlenmeyer flasks
Inoculation loop
Conventional incubator shaker (New Brunswick Scientific Co., INC, model: G25 )
UV-Vis Spectrophotometer (Biotraza, model: 752 )
Autoclave (Hirayama HICLAVETM, model: HVE-50 )
Hot air oven sterilizer (Dalvo Instrumentos, model: OHR/T )
Vortex (Velp, model: ZV3, 201251076 )
Epifluorescence microscope (Olympus, model: BX51 )
Incubation stove (Precision, Scientific Group, model 4, catalog number: 31483 )
Bunsen burner (EiscoTM, catalog number CH0091B )
Nanodrop 2000 (Thermo Scientific, NanoDrop®, catalog number: ND-2000 )
Refrigerated centrifuge (Hanil Scientific, model: Combi 514R )
Spin coater (Laurell Technologies Corporation, model: WS-650-23B )
Procedure
A schematic diagram depicting the whole experimental procedure is shown in Figure 1.
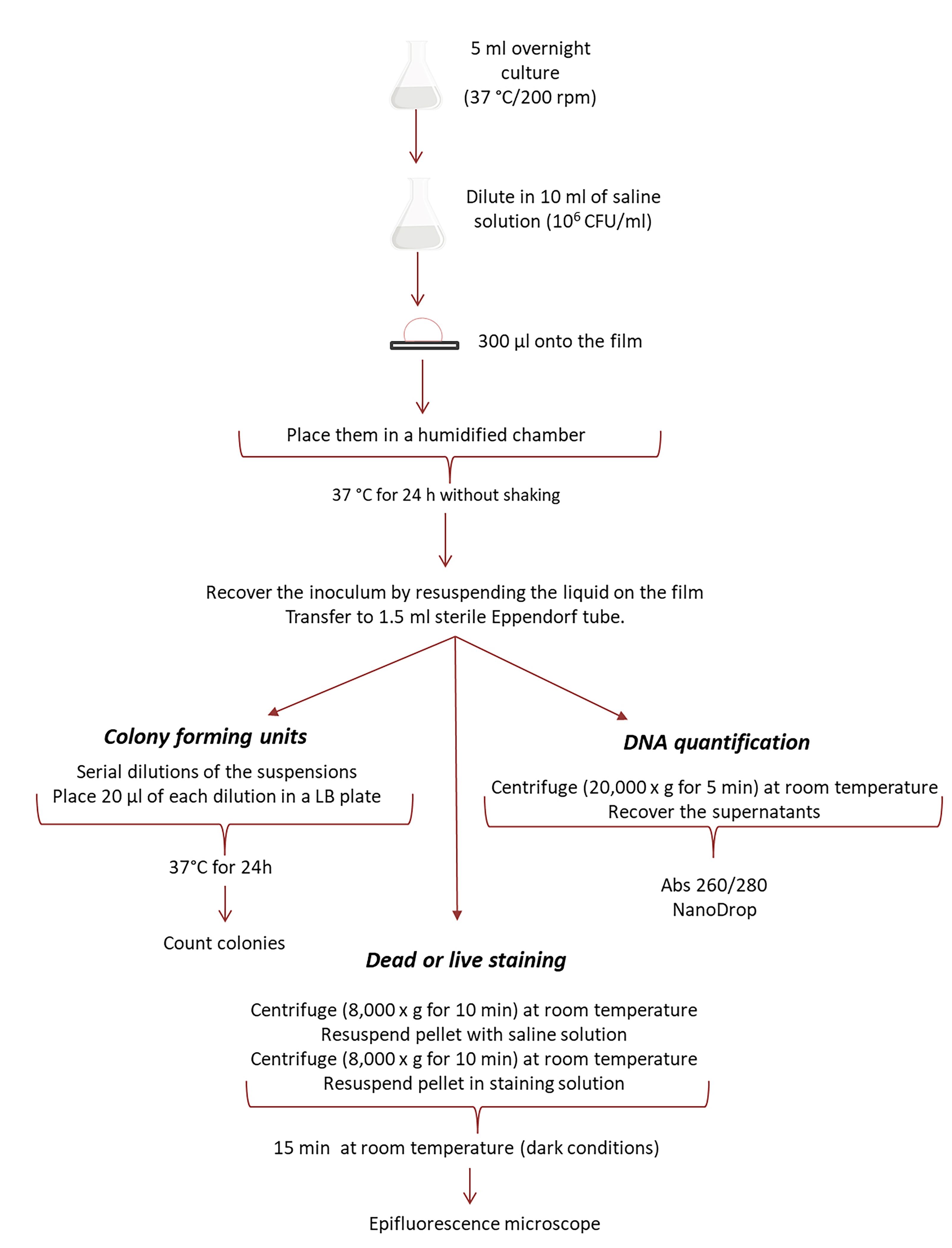
Figure 1. Schematic diagram of the experimental procedure
Bacterial incubation on film coating
Salmonella typhimurium (LT2), Escherichia coli K12, Pseudomonas aeruginosa (PAO1) and Staphylococcus aureus (ATCC 29213 ) strains are maintained in glycerol stocks in liquid nitrogen. Prepare stocks by mixing by inversion or vortexing 130 µl of sterile glycerol and 770 µl of 5 ml overnight bacterial cultures grown in LB medium at 37 °C in sterile 50 ml Erlenmeyer flasks.
Prepare fresh LB agar plates by pouring 25 ml of LB agar medium in the Petri dishes. After solidification, allow them to dry by leaving them opened and upside down in a stove for at least 30 min at 55 °C.
Plate bacteria with a sterile inoculating loop from glycerol stocks onto solid LB plates and grow them overnight at 37 °C in an incubation stove.
Pipet 5 ml of LB in sterile 50 ml Erlenmeyer flasks. Then, by using an inoculation loop, pick colonies from the LB media plate and shake the loop in the liquid LB medium. Grow overnight at 37 °C with shaking (200 rpm) in an incubator shaker. To work in sterile conditions, keep the Bunsen burner on during the entire procedure.
Check OD650 by using a spectrophotometer (1 OD650 = 108 CFU/ml).
Take an aliquot of the overnight culture and dilute in 10 ml of sterile saline solution in a new sterile 50 ml Erlenmeyer flasks to reach 1 x 106 CFU/ml. To work in sterile conditions, keep the Bunsen burner on during the entire procedure.
Place the film-coated glass substrates on a cell culture plate and add 300 µl of the bacterial suspension on the surface of the films, avoiding leaking out of the films. The inoculum should stay on the surface of the coatings for the whole incubation time.
Place the cell culture plate in a plastic box with wet paper to create a humidified chamber.
Incubate at 37 °C in an incubation stove for 24 h without shaking.
Recover the inoculum from incubation by resuspending the liquid on the film surface to capture bacteria potentially attached to the surface. Place in a 1.5 ml sterile Eppendorf tube.
Viability evaluation
Counting for colony forming units (CFU)
Vortex the bacterial suspensions recovered from the incubation on film surfaces (2,000 rpm) for a few seconds.
Make serial dilutions of the suspensions (10-1, 10-2, 10-3, 10-4). For this, add 50 µl of the initial suspension to a sterile borosilicate glass tube carrying 450 µl of saline solution and vortex to mix it. Then take 50 µl of the first dilution 10-1 and pass to a new tube with 450 µl of saline solution, and so on until 10-4 dilutions. Vortex briefly before successive dilutions.
Take 20 µl from each dilution and pour them as a drop onto sterile dried LB solid medium plates. Wait for the drops to be absorbed into the agar and then flip the plates.
Incubate the plates at 37 °C for 24 h in an incubation stove. This incubation time could be modified in order to evaluate bacterial survival at other time points.
Count the colonies formed onto the LB agar plate of each dilution. Only plates containing about 3-30 colonies per drop should be considered.
The total CFU/ml is calculated as follows: the number of colonies corresponding to each drop x dilution factor x total volume (1,000 µl) /volume plated (20 µl). At least three independent films and three independent experiments are needed for statistical analysis.
Centrifuge the bacterial suspensions recovered from the incubation on film surfaces at 8,000 x g for 10 min at room temperature. Separate the pellets from the supernatants.
Resuspend the pellet with 500 µl of saline solution and centrifuge at 8,000 x g for 10 min. Discard the supernatant.
Resuspend the pellet in 200 µl of a staining solution of SYTO 9 and PI.
Incubate in the dark for 15 min at room temperature.
Place 50 µl of the staining suspension in a glass slide and cover it with a cover glass.
Visualize under a fluorescence microscope with 100x objective lens and immersion oil.
Take at least 6 representative photographs per sample.
Centrifuge the bacterial suspensions recovered from the incubation on film surfaces at 20,000 x g for 5 min at room temperature.
Recover the supernatants and place them in a new 1.5 ml sterile Eppendorf tube.
Place 1 µl of the supernatants in the NanoDrop to measure the DNA concentration. Read at 260/280 nm. At least three independent films and three independent experiments are needed for statistical analysis.
Data analysis
As an example of the described methodologies, results of the antibacterial activity of mesostructured titania thin film coatings on Salmonella typhimurium are shown (Scilletta et al., 2019). For this purpose, hybrid titania-Pluronic mesostructured thin films were synthesized by the sol-gel method in combination with evaporation-induced self-assembly of the pluronic micelles, according to protocols described in the literature (Crepaldi et al., 2003; Soler-Illia et al., 2011). Briefly, films were deposited by spin-coating on glass substrates, starting from water/alcohol acidic solutions with the inorganic precursor (TiCl4) and the Pluronic templates. The use of Pluronic F127 and Pluronic P123, and the systematic variation of the titania:Pluronic mol ratios allowed to modulate the mesophase structure. Pluronic P123 indeed presents practically half the molecular weight of Pluronic F127.
Note: Data presented in Figures 2, 3 and 4 were reported in Scilletta et al. (2019).
Bacterial viability was assessed after 24 h of incubation on the surfaces (Figure 2). The results showed that hybrid coatings possess bactericidal activity, while the titania control films (without Pluronic) presented no antibacterial activity. Indeed, the data evidenced that to one tuned amount of template, the antibacterial activity increases for both Pluronic F127 and P123. Besides, at the extremes of Ti:Pluronic ratio the surfaces become slightly active.
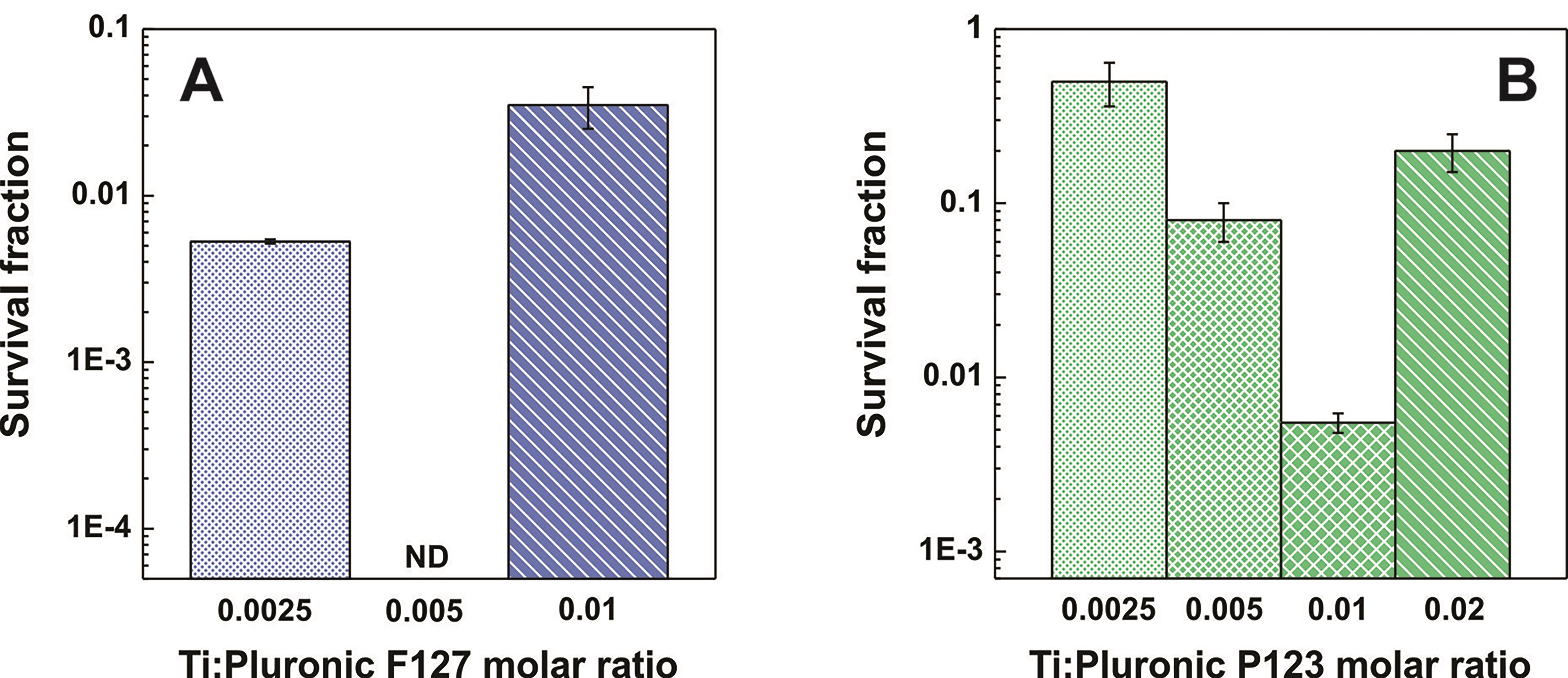
Figure 2. Bactericidal activity of the mesostructured surfaces: Pluronic F127 (A) and Pluronic P123 (B) loaded films with variable concentrations of surfactant were challenged with 106 CFU ml−1 of Salmonella typhimurium. Survival fraction of bacteria after 24 h was calculated by dividing the CFU at 24 h respect to initial time. ND: Non-detectable. [Reproduced from Scilletta et al. (2019) by permission from Elsevier].
The analysis of coating effects on the bacterial membrane evidenced that bacteria membrane integrity was considerably disrupted, so bacteria died after the incubation on the hybrid Pluronic F127-loaded coatings. Nevertheless, they remained mainly intact when they were incubated on the Pluronic-free titania films (Figure 3).
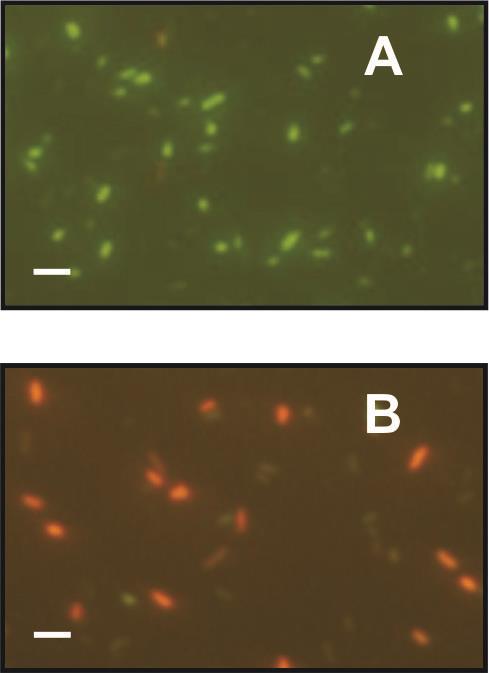
Figure 3. Cell membrane integrity: Fluorescence microscopy images of the bacteria suspension of Salmonella typhimurium after 24 h inoculating F127-free films (A) and F127-loaded films (B). Bacteria that have damaged membranes are labelled in red and cell with intact membranes in green. Scale bars represent 10 μm. [Reproduced from Scilletta et al. (2019) by permission from Elsevier].
DNA present in the bacteria suspension after incubation on the Pluronic-loaded films supports the results observed in the survival fraction and microscopy assays on cell membrane disruption occasioned by these mesostructured surfaces (Figure 4).
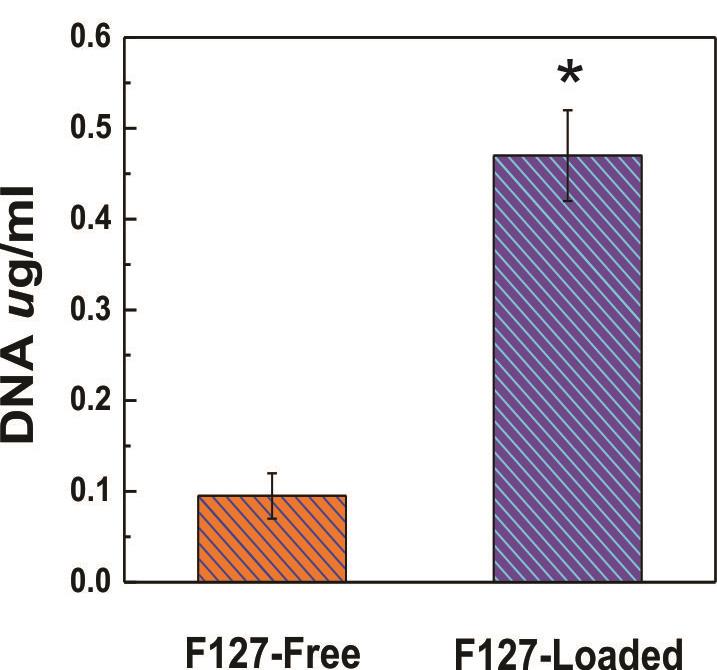
Figure 4. The amount of DNA present in the bacteria suspension of Salmonella typhimurium after incubation on the surfaces. t-test, P < 0.005, * significantly different from F127-free coatings. [Reproduced from Scilletta et al. (2019) by permission from Elsevier].
Recipes
LB medium
Dissolve:
10 g tryptone
5 g yeast extract
5 g NaCl
Bring the volume up to 1,000 ml in distilled water
Autoclave at 1atm for 20 min
LB solid medium
Dissolve:
10 g tryptone
5 g yeast extract
5 g NaCl
15 g agar
Bring the volume up to 1,000 ml in distilled water
Autoclave at 1atm for 20 min
4 M NaCl
Dissolve 46.7 g NaCl in 200 ml of distilled water
Autoclave at 1atm for 20 min
Saline solution
Mix 7.5 ml sterile 4 M NaCl in 300 ml of sterile distilled water
Staining SYTO 9/PI solution
0.7 μl of 5 mM SYTO 9 (stock solution; stored at -20 °C protected from the light)
26 μl of 0.77 mM PI (stock solution; stored at 4 °C protected from the light)
1,000 ml of distilled water
Prepare fresh for every experiment
The solution can be kept in the dark at room temperature during the experiment
Glass coating with titania mesostructured thin film
Wash microscope slides (26 mm x 20 mm x 1 mm) with neutral detergent, and then rinse sequentially with MilliQ water, acetone, and isopropanol
Starting from a TiCl4/ethanol solution (1:40), add Pluronic F127 or Pluronic P123 and water to achieve a final composition of TiCl4:ethanol:H2O:Pluronic equivalent to 1:40:10:S mol ratios. S varies between 0.0025 and 0.02 mol ratios
Perform the spin-coating at 2,500 rpm, 35 °C and relative humidity of 15%
Place the films in a chamber with 50% relative humidity for 24 h
Perform a consolidation thermal treatment including three consecutive steps of 1 h at 60 °C, 130 °C and 180 °C
Acknowledgments
N.A.S. is grateful for her doctoral fellowship granted by CONICET. M.P. is member of CNEA and CONICET. M.F.D., G.J.A.A.S-I, M.G.B., and P.N.C. are members of CONICET. This work was supported by CONICET (PIP 561) and ANPCYT (PICT-2016-0647 and PICT- 2016-1781), Argentina.
The counting of colony forming units procedure is based on a previous protocol by Merritt, Kadouri and O'Toole (Merritt et al., 2006). The live/dead staining method for membrane integrity evaluation is a modification of a protocol published by Smith and Hunter (2008). The quantification of extracellular DNA was based on a previous protocol by Chen and Cooper (2002).
Competing interests
The authors declare no conflict of interest.
References
- Chen, C. Z. and Cooper, S. L. (2002). Interactions between dendrimer biocides and bacterial membranes. Biomaterials 23 (16): 3359-3368.
- Crepaldi, E. L., Soler-Illia, G. J., Grosso, D., Cagnol, F., Ribot, F. and Sanchez, C. (2003). Controlled formation of highly organized mesoporous titania thin films: from mesostructured hybrids to mesoporous nanoanatase TiO2. J Am Chem Soc 125(32): 9770-9786.
- Mateescu, M., Baixe, S., Garnier, T., Jierry, L., Ball, V., Haikel, Y., Metz-Boutigue, M. H., Nardin, M., Schaaf, P., Etienne, O. and Lavalle, P. (2015). Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS One 10(12): e0145143.
- Merritt, J. H., Kadouri, D. E. and O'Toole, G. A. (2005). Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1: Unit 1B 1.
- Scilletta, N. A., Pezzoni, M., Desimone, M. F., Soler-Illia, G. J., Catalano, P. N. and Bellino, M. G. (2019). Transforming an inert nanopolymer into broad-spectrum bactericidal by superstructure tuning. Colloid Surface B 178: 214-221.
- Smith, K. and Hunter, I. S. (2008). Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol (8): 966-973.
- Soler-Illia, G. J., Angelomé, P. C., Fuertes, M. C., Calvo, A., Wolosiuk, A., Zelcer, A., Bellino, M. G. and Martínez, E. D. (2011). Mesoporous hybrid and nanocomposite thin films. A sol–gel toolbox to create nanoconfined systems with localized chemical properties. J Sol-gel Sci Techn 57(3): 299-312.
- Yuan, Z., Ouyang, P., Gu, K., Rehman, T., Zhang, T., Yin, Z., Fu, H., Lin, J., He, C., Shu, G., Liang, X., Yuan, Z., Song, X., Li, L., Zou, Y. and Yin, L. (2019). The antibacterial mechanism of oridonin against methicillin-resistant Staphylococcus aureus (MRSA). Pharm Biol 57(1): 710-716.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Scilletta, N. A., Pezzoni, M., Desimone, M. F., Soler-Illia, G. J. D. A. A., Bellino, M. G. and Catalano, P. N. (2021). Determination of Antibacterial Activity of Film Coatings against Four Clinically Relevant Bacterial Strains. Bio-protocol 11(2): e3887. DOI: 10.21769/BioProtoc.3887.
Category
Microbiology > Antimicrobial assay > Antibacterial assay
Cell Biology > Cell-based analysis > Colony formation
Molecular Biology > DNA > DNA quantification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









