- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pea Aphid Rearing, Bacterial Infection and Hemocyte Phagocytosis Assay
Published: Vol 10, Iss 24, Dec 20, 2020 DOI: 10.21769/BioProtoc.3862 Views: 3431
Reviewed by: Andrea PuharSveta ChakrabartiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Mădălina Mocăniță [...] Vanessa M. D'Costa
Mar 5, 2024 2895 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2087 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1655 Views
Abstract
Insects rely on the simple but effective innate immune system to combat infection. Cellular and humoral responses are interconnected and synergistic in insects’ innate immune system. Phagocytosis is one major cellular response. It is difficult to collect clean hemolymph from the small insect like pea aphid. Here, we provide a practicable method for small insects hemocyte phagocytosis assay by taking pea aphid as an example. Furthermore, we provide the protocols for pea aphid rearing and bacterial infection, which offer referential method for related research.
Keywords: ImmuneBackground
Phagocytosis, defined as the cellular uptake of particles bigger than 0.5 μm through formation of a membrane derived phagosome, is an ancient and evolutionarily conserved mechanism of insects cellular response (Lemaitre and Hoffmann, 2007; Melcarne et al., 2019a and 2019b). Phagocytosis is mediated by phagocytes, the dedicated cells, which can digest both “altered-self” particles and pathogens (Hillyer and Strand, 2014; Melcarne et al., 2019b). The phagocytes can be not only circulating hemocytes in hemocoel but also sessile hemocytes on tissues (Hillyer and Strand, 2014; Hillyer, 2016; Sigle and Hillyer, 2016). When pathogens enter hemocoel of insects, the phagocytes rapidly phagocytose pathogens and phagocytosis generally finishes in hours (Hillyer et al., 2003; King and Hillyer, 2012; Sigle and Hillyer, 2016).
There are mainly four different methods to perform hemocyte phagocytosis assay:
In vivo phagocytosis: (1) The insects are injected with fluorescently labeled latex beads or fluorescein-labeled dead bacteria. After incubation, trypan blue is injected to quench extracellular fluorescence. Then, the fluorescence is detected on a fluorescence microscope and the fluorescence intensity from dorsal vessel-associated hemocytes is quantified. The detailed methods were described in literature (Elrod-Erickson et al., 2000; Gonzalez et al., 2013; Garg and Wu, 2014; Nazario-Toole et al., 2018). For some insects, the fluorescent background in the hemolymph and tissues are strong and this may influence the results. (2) The insects are injected with fluorescently labeled latex beads or fluorescein-labeled bacteria. After incubation, the hemocytes are collected by ripping the larval in PBS solution containing trypan blue to quench extracellular fluorescence. The hemocytes are transferred and attached to a glass slide, and then the cells are fixed with formaldehyde. The phagocytosis is observed under microscope and the fluorescence intensity is quantified. The details were described previously (Kocks et al., 2005; Hao et al., 2018). For some insects, such as pea aphid, when inject bacteria into the body cavity, a lot of bacteria are centralized around the wound. Therefore, in vivo phagocytosis is not suitable for these insects.
Ex vivo phagocytosis: (1) After collected in insect medium in low binding tubes, hemocytes are mixed with fluorescein-labeled bacteria. Samples are incubated to enable phagocytosis, and then placed on ice to stop the process. Phagocytosis is quantified using a flow cytometer after quenching fluorescence of extracellular particles. The detailed method is described as previously (Melcarne et al., 2019b). (2) Drops of hemolymph are collected into insect medium and then mixed well with fluorescein-labeled bacteria. The samples are transferred to tissue culture round coverslips in a cell culture plate. After settled and adhered to the coverslips, the cells are fixed with paraformaldehyde. Then, F-actin of cells is stained with Phalloidin after permeabilized with Triton-100. Phagocytosis is observed under a laser scanning confocal microscope. The details of hemolymph collection and phagocytosis methods were described before (Schmitz et al., 2012; Ma et al., 2020).
The second method of ex vivo phagocytosis overcomes the difficulty due to small size of some insects for clean hemolymph collection. An accurate assay was described as previously: the fluorescence intensity in phagocytosing hemocytes is calculated and phagocytic index is represented as the capacity of hemocytes (Melcarne et al., 2019b). Combining these advantages, we provide a practicable method for pea aphid hemocyte phagocytosis assay in this study. Meanwhile, we provide protocols for pea aphid rearing and bacterial infection, which offer referential methods for related research.
Materials and Reagents
Test tubes (Kangsheng Medical Glass Factory, Kangsheng, catalog number: GT-16125 ), diameter x length: 16 mm x 125 mm; Volume: 18 ml
Conical flasks (Sichuan Glass Factory, Fuhai, catalog number: ZXP-250 ), volume: 250 ml
Soil matrix (Shandong Luhao Agricultural Science and Technology co., Itd, Luhao), volume: 50 L
Seedling pots, diameter: 13 cm (purchased from a local supermarket)
Capillaries (Drummond, catalog number: 3-000-203-G/X )
Centrifuge tubes 50 ml (KIRGEN, catalog number: KG2821 ); 1.5 ml (Axygen, catalog number: MCT-150-C )
Sterile Petri dishes (Biofound, catalog number: FM-90-G )
48-well cell culture plates (Corning, Costar, catalog number: 3548 )
Tissue culture-treated round coverslips (8 mm diameter) (Solarbio, catalog number: YA0353-100 )
Broad bean (Vicia faba) seeds (purchased from local farmers market)
A. pisum strain (originally collected from Yunnan, China and maintained in the lab of Prof. Zhiqiang Lu at Northwest A&F University)
Gram-negative bacteria Pseudomonas aeruginosa (PAO1, from Dr. Xihui Shen at Northwest A&F University); Gram-positive bacteria Micrococcus luteus (Kept in the lab of Prof. Zhiqiang Lu at Northwest A&F University)
NaCl (Guangdong Guanghua Sci-tech Co., Ltd, Huada, catalog number: 1.01307.040 )
Yeast extract (Oxoid, catalog number: LP0021 )
Tryptone (Oxoid, catalog number: LP0042 )
Phosphate-Buffered Saline (PBS) (Thermo Fisher, catalog number: 10010023 )
Agar power (Solarbio, catalog number: 9002-18-0 )
Grace’s medium (Sigma-Aldrich, catalog number: S0146 )
Phenylthiourea (Sigma-Aldrich, catalog number: P7629 )
Heat-inactivated fetal bovine serum (Biological Industries, catalog number: 04-011-1A )
Escherichia coli (K-12) and Staphylococcus aureus Alexa Fluor 594 BioParticles (Invitrogen, catalog numbers: E23370 and S23372 )
4% paraformaldehyde (Solarbio, catalog number: P1110 )
SF488 Phalloidin (Solarbio, catalog number: CA1646 )
Anti-fading reagent (Solarbio, catalog number: S2100 )
Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
Luria-Bertani liquid medium (see Recipes)
Luria-Bertani agar medium (see Recipes)
0.85% NaCl solution (see Recipes)
Hemolymph collection medium (see Recipes)
Equipment
Dissecting forceps (ideal-tek, catalog number: 5.SA)
Growth chamber (Ningbo Jiangnan Instrument Factory, model: GXZ-500B)
Ultra-cold storage freezer (Whirlpool Corporation, Sanyo, model: 09S338)
Ice machine (Scotsman ice systems Co., Ltd., Scotsman, model: MF36)
Laminar flow cabinet (Shiheng Instrument Equipment Co., LTD., Dinco, model: SW-CJ-2F)
Incubator (Taicang Experimental Equipment Factory, Peiying, model: HZQ-F100)
Eppendorf Biophotometer (Eppendorf, catalog number: 6133000044)
Laser scanning confocal microscope (Olympus, model: FV3000)
Software
ImageJ (NIH, Bethesda, MD, USA)
GraphPad Prism 5.0 (GraphPad, Inc., La Jolla, CA, USA)
Procedure
Pea aphid rearing (Figure 1)
Put the broad bean seeds into water for imbibing absorption of water adequately for 2 days.
Remove the seeds peels gently.
Plant one peeled seed about 1 cm depth in a seedling pot filled with soil matrix.
The planted seeds grow in the growth chamber at 21 ± 1 °C and 70 ± 5% relative humidity under a 16-h light (L): 8-h dark (D) photoperiod.
Seedlings with more than six leaves can be used.
Place ten adult aphids on each seedling through clamping the tentacles with tweezers and allow to produce offspring in the growth chamber.
Remove the adult aphids from the seedlings two days later.
Rear the nymphs on the seedlings for nine days until they reach wingless adults.
Use these newly emerged aphid adults to do the following experiments.
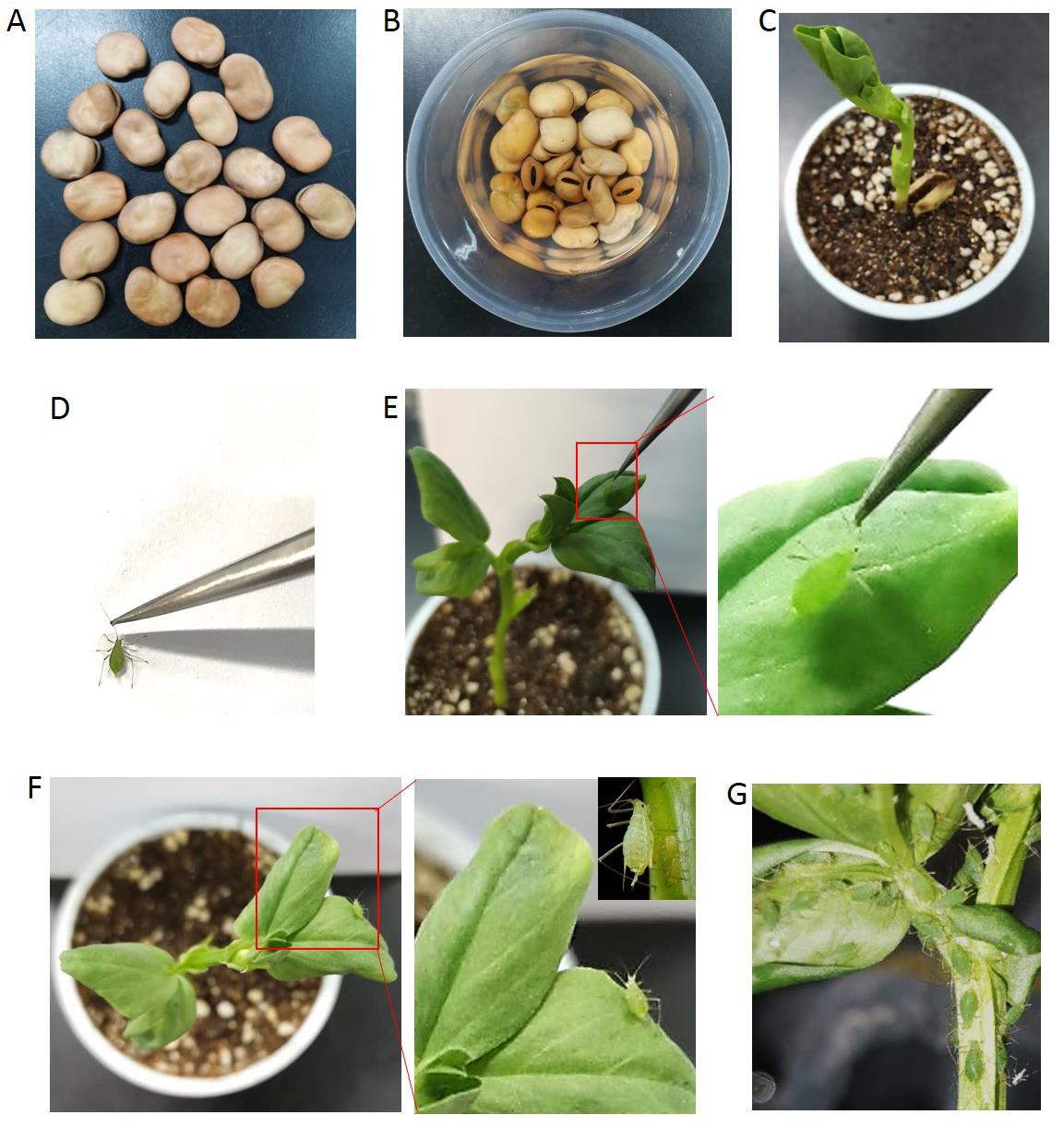
Figure 1. Pea aphid rearing. Put the broad bean seeds into water for imbibing absorption of water adequately for 2 days (A and B). Plant one peeled seed about 1 cm depth into a seedling pot filled with soil matrix and then the seed sprouts (C). Place adult aphids on each seedling through clamping the tentacles with tweezers (D and E) and allow them to produce offsprings (F). Remove the adult aphids from the seedlings two days later and the nymphs grow into wingless adults nine days later (G).
Bacterial infection
Prepare Luria-Bertani liquid medium, Luria-Bertani agar medium, 0.85% NaCl and sterilize these reagents in an autoclave.
Streak culture the P. aeruginosa and M. luteus (keep in a ultra-cold storage freezer at -80 °C) on Luria-Bertani agar plates respectively in an incubator at 37 °C overnight.
Pick a P. aeruginosa bacterial colony into a test tube containing 5 ml Luria-Bertani liquid medium and pick a M. luteus bacterial colony into a conical flask containing 100 ml Luria-Bertani liquid medium in a laminar flow cabinet.
Culture the bacteria in a bed temperature incubator at 37 °C with the rotate speed at 220 r/min.
Measure the absorbance of the culture at 600 nm on an Eppendorf biophotometer until the optical density reaches approximately 1 (Figure 2A).
Harvest the cells by centrifugation at 8,000 x g for 10 min and resuspend the pellets in sterilized 0.85% NaCl solution and wash the bacteria three times with 0.85% NaCl solution through centrifugation and resuspension.
Bring the P. aruginosa suspension to 2 x 109 colony formation units (CFU)/ml (Figure 2B) and M. luteus cell suspension to 2 x 1010 CFU/ml.
Anesthetize the adult aphids on ice for ten minutes and prick through the abdominal wall into the hemocoel of the aphids about 0.5 mm deep with a capillary dipped in bacteria suspensions or sterilized 0.85% NaCl solution (Figures 2C and 2D).
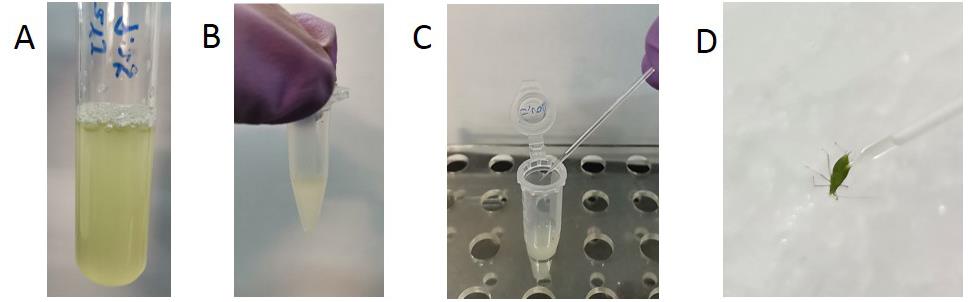
Figure 2. Bacterial infection. Culture P. aeruginosa in a test tube containing 5 ml Luria-Bertani liquid medium until the optical density at 600 nm reach approximately 1 (A). Harvest the P. aeruginosa cells by centrifugation and resuspend the cells to 2 x 109 (CFU)/ml (B). Prick through the abdominal wall into the hemocoel of the aphids about 0.5 mm deep with a capillary dipped in the P. aeruginosa suspension (C and D).
Hemocyte phagocytosis assays
Anesthetize the adult aphids in a sterile Petri dish on ice for 10 min.
Remove one aphid legs gently with dissecting forceps and mixed the drops of hemolymph with a 5 μl Grace’s medium drop containing 1 μM phenylthiourea and 10% (vol/vol) heat-inactivated fetal bovine serum (FBS).
Collect the hemolymph from 20 aphids per test group as above and mix the collected hemolymph well with 2 μl of 1mg/ml E. coli or S. aureus Alexa Fluor 594 BioParticles.
Perform the operations of 5-14 steps in the dark.
Transfer the prepared samples to tissue culture-treated round coverslips (8 mm diameter) in a 48-well cell culture plate.
Allow the hemocytes to settle and adhere through incubating at room temperature for 1 h.
Wash the coverslips twice with 200 μl hemolymph collection medium.
Fix the washed hemocytes for 10 min with 200 μl 4% paraformaldehyde in PBS.
Wash the coverslips three times (10 min each) with 200 μl PBS.
Permeabilize the hemocytes with 200 μl 0.1% Triton X-100 in PBS for 10 min and wash twice with 200 μl PBS (10 min each).
Incubate the permeabilized hemocytes with 200 μl diluted SF488 Phalloidin by 1:200 in PBS for 1 h.
Wash the coverslips with 200 μl PBS three times (10 min each).
Mount the coverslips on slides using anti-fading reagent (3 μl per coverslip).
Observe and take photos under a laser scanning confocal microscope (Figure 3). The excitation and acquisition wavelengths of the bacteria were respectively 590 nm and 617 nm. The excitation and acquistion wavelengths of Phalloidin were respectively 495 nm and 519 nm.
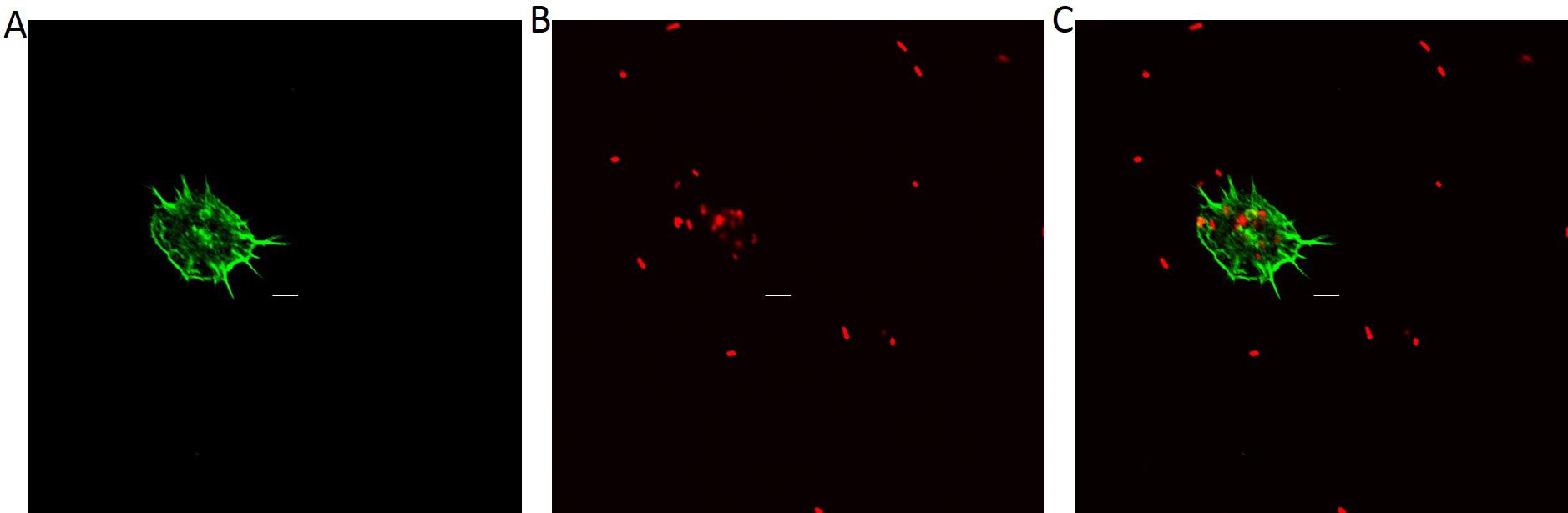
Figure 3. The confocal images of hemocytes with F-actin stained by SF-488 Phalloidin (A), E. coli AlexaFluo 594 BioParticles (B) and the overlay of hemocytes and E. coli. Scale bars: 5 μm.Calculate the bacterial fluorescence intensities in phagocytosing hemocytes with ImageJ software (The calculation processes were shown as Figure 4).
Take the phagocytic index (PI) as the phagocytic capacity of hemocytes according to a previous description ( Melcarne et al., 2019b).
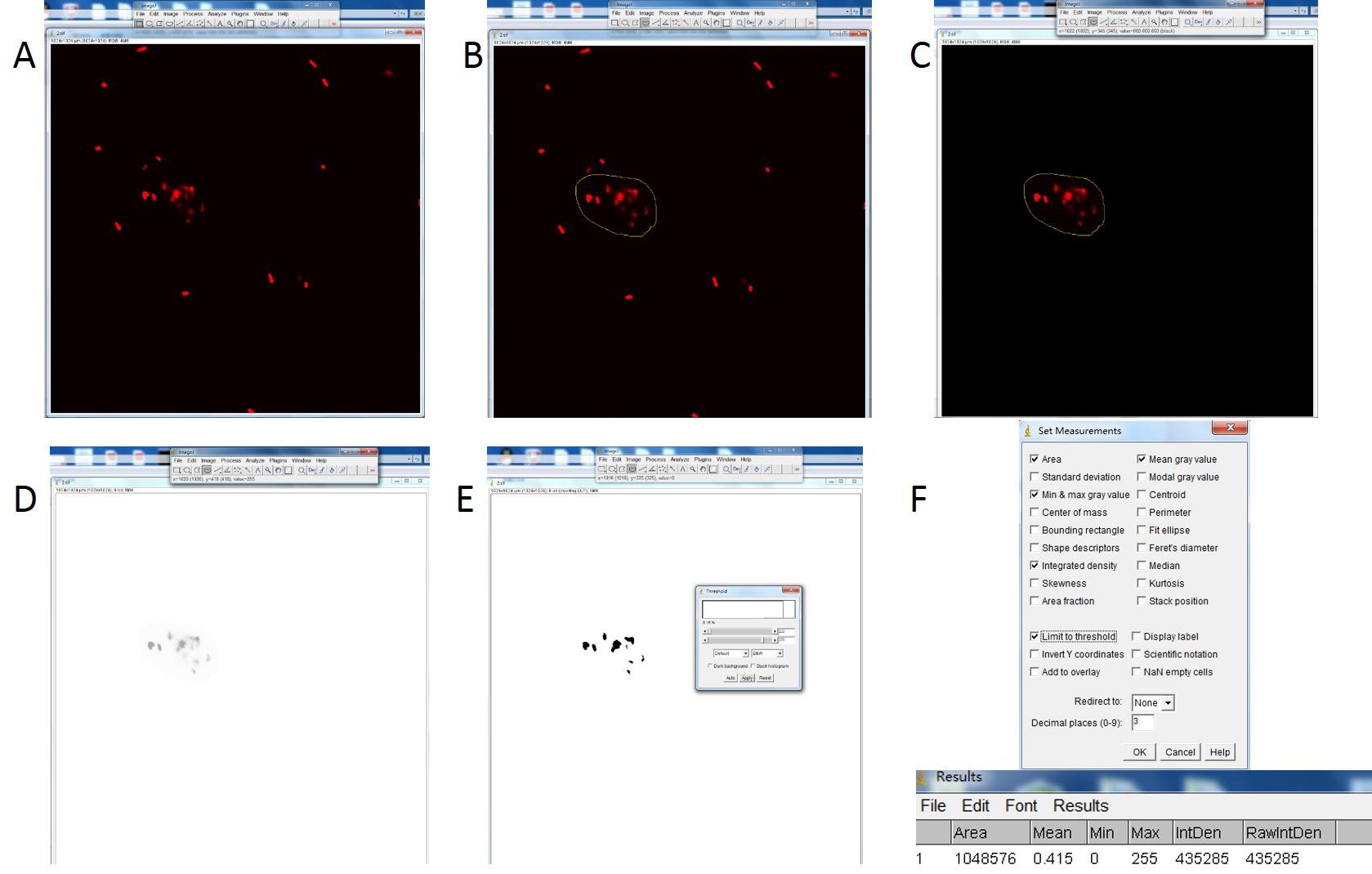
Figure 4. The calculation processes of the bacterial fluorescence intensities in phagocytosing hemocytes with ImageJ software. The image (the image is from Figure 3B) was loaded into ImageJ software (A). The phagocytizing bacteria were circled (B) and the bacteria outside of the hemocyte were cleared out (C). The image type was set as 8 bit and inverted (D). Adjusted the threshold and chose the “Default” options as “B&W” (black and white) (E). Got the analyzed result after set the measurements and the IntDen (integrated density) value was represented the fluorescence intensity (F).
Data analysis
The data analysis of hemocyte phagocytosis assay is referred to our recent article ( Ma et al., 2020). The intensity of excitation light and the magnification times were the same during all observation processes. The photos taken from about twenty different views per coverslip were used to calculate the bacterial fluorescence intensities in phagocytosing hemocytes (the calculation processes were shown as Figure 4).
PI calculation: Fraction of hemocytes phagocytosing (f) = number of hemocytes in fluorescence positive gate/total number of hemocytes. Phagocytic index (PI) = [mean fluorescence intensity of hemocytes in fluorescence positive gate] x f. Every experiment was performed three independent biological repetitions. All PI data was plotted using GraphPad Prism 5.0. Student’s t-test was used to determine other statistical values, which were presented as the mean ± SEM.
Notes
Remove the legs of pea aphids gently to prevent linking of other tissues.
Put the coverslips in the center of the cell culture plates. Make sure all the collected hemolymph and bacteria mixed samples bestrew the coverslips, but don’t flow to the lacuna neighbor to the wall of cell culture plate.
Recipes
Luria-Bertani liquid medium
Dissolve 5 g NaCl, 5 g Yeast extract and 10 g Tryptone in 1 L ddH2O and mix well
Sterilize the medium in an autoclave
Luria-Bertani agar medium
Dissolve 5 g NaCl, 5 g Yeast extract, 10 g Tryptone, 15 g Agar power in 1 L ddH2O and mix well
Sterilize the medium in an autoclave
0.85% NaCl solution
Dissolve 0.85 g NaCl in 100 ml ddH2O and mix well and sterilize the solution in an autoclave
Hemolymph collection medium
Grace’s medium containing 1 μM phenylthiourea and 10% (vol/vol) heat-inactivated fetal bovine serum (FBS)
Acknowledgments
This protocol is adapted from the publication of Ma et al. (2020). Funding for the study was provided by the National Natural Science Foundation of China grants (31970467 and 31772530) and the Fundamental Research Funds for the Central Universities (Z1090219001).
Competing interests
The authors declare no conflicts of interest.
References
- Elrod-Erickson, M., Mishra, S. and Schneider, D. (2000). Interactions between the cellular and humoral immune responses in Drosophila. Current Biology 10(13): 781-784.
- Garg, A. and Wu, L. P. (2014). Drosophila Rab14 mediates phagocytosis in the immune response to Staphylococcus aureus. Cell Microbiol 16(2): 296-310.
- Gonzalez, E. A., Garg, A., Tang, J., Nazario-Toole, A. E. and Wu, L. P. (2013). A glutamate-dependent redox system in blood cells is integral for phagocytosis in Drosophila melanogaster. Curr Biol 23(22): 2319-2324.
- Hao, Y., Yu, S., Luo, F. and Jin, L. H. (2018). Jumu is required for circulating hemocyte differentiation and phagocytosis in Drosophila. Cell Commun Signal 16(1): 95.
- Hillyer, J. F., (2016). Insect immunology and hematopoiesis. Dev Comp Immunol 58: 102-118.
- Hillyer, J. F., Schmidt, S. L. and Christensen, B. M. (2003). Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J Parasitol 89(1): 62-69.
- Hillyer, J. F. and Strand, M. R. (2014). Mosquito hemocyte-mediated immune responses. Curr Opin Insect Sci 3: 14-21.
- King, J. G. and Hillyer, J.F. (2012). Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog 8(11): e1003058.
- Kocks, C., Cho, J. H., Nehme, N., Ulvila, J., Pearson, A. M., Meister, M., Strom, C., Conto, S. L., Hetru, C., Stuart, L. M., Stehle, T., Hoffmann, J. A., Reichhart, J. M., Ferrandon, D., Ramet, M. and Ezekowitz, R. A. (2005). Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123(2): 335-346.
- Lemaitre, B. and Hoffmann, J. (2007). The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697-743.
- Ma, L., Liu, L., Zhao, Y., Yang, L., Chen, C., Li, Z. and Lu, Z. (2020). JNK pathway plays a key role in the immune system of the pea aphid and is regulated by microRNA. PLoS Pathog 16(6): e1008627.
- Melcarne, C., Lemaitre, B. and Kurant, E. (2019). Phagocytosis in Drosophila: From molecules and cellular machinery to physiology. Insect Biochem Mol Biol 109: 1-12.
- Melcarne, C., Ramond, E., Dudzic, J., Bretscher, A. J., Kurucz, E., Ando, I. and Lemaitre, B. (2019). Two Nimrod receptors, NimC1 and Eater, synergistically contribute to bacterial phagocytosis in Drosophila melanogaster. FEBS J 286(14): 2670-2691.
- Nazario-Toole, A. E., Robalino, J., Okrah, K., Corrada-Bravo, H., Mount, S. M. and Wu, L. P. (2018). The Splicing Factor RNA-Binding Fox Protein 1 Mediates the Cellular Immune Response in Drosophila melanogaster. J Immunol 201(4): 1154-1164.
- Schmitz, A., Anselme, C., Ravallec, M., Rebuf, C., Simon, J. C., Gatti, J. L. and Poirie, M. (2012). The cellular immune response of the pea aphid to foreigen intrusion and symbiotic challenge. PLoS One 7(7): e42114.
- Sigle, L. T. and Hillyer, J. F. (2016). Mosquito hemocytes preferentially aggregate and phagocytose pathogens in the periostial regions of the heart that experience the most hemolymph flow. Dev Comp Immunol 55: 90-101.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ma, L., Liu, L. and Lu, Z. (2020). Pea Aphid Rearing, Bacterial Infection and Hemocyte Phagocytosis Assay. Bio-protocol 10(24): e3862. DOI: 10.21769/BioProtoc.3862.
Category
Immunology > Animal model > Other
Microbiology > Microbe-host interactions > Bacterium
Cell Biology > Model organism culture > Maintenance
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link