- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Extracellular Vesicles Derived from Mesenchymal Stromal Cells by Ultracentrifugation
(*contributed equally to this work) Published: Vol 10, Iss 24, Dec 20, 2020 DOI: 10.21769/BioProtoc.3860 Views: 6460
Reviewed by: Xiaoyi ZhengMei Po ChanAlexandros C KokotosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1536 Views

A Protocol to Induce Brown and Beige Adipocyte Differentiation From Murine and Human Adipose-Derived SVF
Rohit Raj Yadav [...] Narendra Verma
Dec 5, 2025 1642 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views
Abstract
Extracellular vesicles (EVs) are a heterogeneous group of membranous vesicles that differ on their biogenesis and release pathways, such as exosomes, microvesicles and apoptotic bodies. They are involved in cell-to-cell communication delivering signal molecules (proteins, nucleic acids, lipids, etc.) that can regulate different physiological processes, as well as the development and progression of several diseases. There are different methods and commercial kits to isolate EVs and depending on the methodology one could obtain EVs with different degrees of efficiency, purity and it can be more or less time-consuming. Then, the choice has to be according to the different advantages and disadvantages, and their use for downstream applications. Here, we describe the EVs isolation method from mesenchymal stromal cells by ultracentrifugation. This EVs isolation can be performed using common media and buffers, and only with the requirement of an analytical ultracentrifuge. Moreover, this method can be used to obtain large quantity of EVs with a good reproducibility for developing in vitro and in vivo experiments and studying their biological actions.
Keywords: Mesenchymal stromal cellsBackground
Mesenchymal stem cells (MSCs) have a protective effect on the progression of different diseases contributing to the immunomodulation and the inflammatory state (Bartholomew et al., 2002; Togel et al., 2005; Azmi et al., 2013; Ebrahimi et al., 2013; Ben-Ami et al., 2014). Their protective action is not only due to their transdifferentiation, but also their paracrine mechanisms such as release of extracellular vesicles (EVs) showing an immunomodulatory, anti-inflammatory, anti-apoptotic and proangiogenic function (Bruno et al., 2009; Camussi et al., 2010a; Grange et al., 2020). Moreover, the application of EVs from MSCs in clinical practice has the advantage of being a safe therapy without the disadvantages related to other MSCs therapies, including the possibility to be rejected, gene stability, poor long-term differentiation and probability of viral transfer (Kunter et al., 2007; Wang et al., 2012).
In the last years, there is an increasing interest in the application of EVs in the transplant area, as an immunomodulatory and anti-inflammatory therapy on transplant recipients, and their addition on perfusion solution to pre-condition the graft before transplantation (Koch et al., 2015; Gregorini et al., 2017; Stone et al., 2017; Rigo et al., 2018; Ramírez-Bajo et al., 2020b). In particular, EVs therapy in the context of a hypothermic or normothermic perfusion machine could increase the long-term survival and increase the number of available organs. This improvement could be related to a decrease of inflammation, the preservation of energy metabolism and microvascular permeability avoiding the formation of edema. These properties show the great therapeutic potential of EVs in the transplantation process.
Cell-to-cell communication by EVs is a mechanism that has been preserved throughout the evolution in both prokaryotic and eukaryotic cells (Ratajczak et al., 2006). Since its discovery 30 years ago, it has been demonstrated that EVs are produced by an enormous variety of cell types such as blood cells, dendritic, endothelial and epithelial cells, nervous system cells, adult and embryonic stem cells and even tumor cells (Harding et al., 1984). EVs are a heterogeneous group of membranous vesicles that differ on their biogenesis and release pathways such as exosomes, microvesicles, microparticles, ectosomes, oncosomes and apoptotic bodies (Thery et al., 2002 and 2018). EVs act as paracrine/endocrine effectors that transport bioactive molecules among the cells (cytosolic proteins, lipids, mRNA, miRNA, DNA, mitochondrial DNA and genomic DNA) of the surrounding microenvironment, or being carried remotely in biological fluids (Fevrier and Raposo, 2004; Camussi et al., 2010b; Mittelbrunn et al., 2011). These regulatory biological signals can regulate various physiological processes, as well as the development and progression of disorders (Valadi et al., 2007; Guescini et al., 2010; Balaj et al., 2011).
A large number of studies are focused on EV’s field and their isolation from different biofluids, developing different methods of isolation such as ultracentrifugation, density gradient, size exclusion chromatography, filtration, microfluidics, precipitation kits and magnetic beads technologies. The choice of isolation methods should depend on the type of starting material, volume, available equipment, scientific inquiry and subsequent analysis or use (Momen-
Heravi et al., 2013). There are described different protocols associated with difference in purity and time of isolation. Moreover, EVs composition is dependent on the isolation protocol used, obtaining different subpopulations with different soluble proteins and nucleic acids. For these reasons, it is important to follow a series of criteria based on current best practice that represents the minimal experimental requirements for definition of EVs that allow the interpretation and their replication by other researchers (Lotvall et al., 2014).
The most commonly used protocol for isolation is ultracentrifugation which has advantages such as the applicability for EVs isolation from large volumes as urine or cell culture conditioned media, the use of common media and buffers, easy to handle, and absence of impact on EVs except gravitational force (Konoshenko et al., 2018). This method can be used to obtain large quantity of EVs with a good reproducibility for developing studies about their biological actions. However, it needs expensive equipment and the isolated EVs could present co-isolating contaminants such as soluble proteins and nucleic acids. In our previous publications, we showed a comparison of the therapeutic effect of MSC and their EVs in an in vivo model of chronic kidney allograft rejection (Ramírez-Bajo et al., 2020a) and chronic cyclosporine nephrotoxicity (Ramírez-Bajo et al., 2020b). The ultracentrifugation protocol allows to produce a great quantity of EVs from high volumes of MSC-conditioned medium required for a periodic administration of therapies to different animal groups. We observed a good reproducibility between EVs batches by this methodology.
Materials and Reagents
22 G needle
15 ml conical tubes (Sarstedt, catalog number: 62.554.502 )
50 ml conical tubes (Sarstedt, catalog number: 62.548.004 )
Serological pipettes 10 ml (Sarstedt, catalog number: 86.1254.001 )
Serological pipettes 25 ml (Sarstedt, catalog number: 86.1685.001 )
Tissue Culture Flask, 150 cm2 filter (TPP, catalog number: 90151 )
Tissue Culture Flask, 75 cm2 filter (TPP, catalog number: 90076 )
0.22 μm sterile syringe filters (Sarstedt, catalog number: 831.826.001 )
0.1 μm sterile syringe filters (Pall Life Sciences, catalog number: 4668 )
20 ml Sterile syringes (BD Plastipak, catalog number: 10569215 )
Glass Pasteur pipettes (Deltalab, catalog number: 702 )
Countess cell counting chamber slides (Thermo Fisher Scientific, Invitrogen, catalog number: C10228 )
Open-Top Thinwall Ultra-Clear Tube, 38.5 ml capacity (Beckman Coulter, catalog number: 344058 )
Lewis and Fisher rats (200 g)
Trypan blue (Thermo Fisher Scientific, Invitrogen, catalog number: C10228 )
Alpha MEM (Lonza Bioscience, catalog number: BE02-002F )
RPMI 1640 Medium with L-Glutamine (Lonza Bioscience, catalog number: BE12- 702 F)
Fetal bovine serum (FBS) (EuroClone, catalog number: ECS0180L )
Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher Scientific, Invitrogen, catalog number: 15140122 )
Trypsin-EDTA (0.25%), phenol red (Thermo Fisher Scientific, Invitrogen, catalog number: 25200072 )
Dulbecco’s phosphate buffered saline (PBS) 10x, Without Ca++ and Mg++ (Cultek, catalog number: BE17-517Q )
Sterile water for irrigation, Serrasol solution (Serra Pamies, catalog number: 374561 )
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855 )
L-glutamine solution (Sigma-Aldrich, catalog number: G7513 )
Complete alpha-MEM medium (see Recipes)
Freezing medium (see Recipes)
Equipment
-80 °C freezer
Water bath set to 37 °C (Thermo Fisher Scientific, model: JP SelectaTM Precisterm 6000141 )
Refrigerated benchtop centrifuge (Thermo Fisher Scientific, model: HeraeusTM MultifugeTM X1)
Countess automated cell counter (Thermo Fisher Scientific, Invitrogen, catalog number: C10281 )
DHD Air-Jacketed Automatic CO2 Incubator (NuAire, catalog number: NU-5510E )
Inverted Modulation Contrast Microscope (Leica, model: DMIRB )
C2200 CBC Complete Balance (Cobos, 200-20018 )
Preparative ultracentrifuge (Beckman Coulter, model: Optima L100XP )
SW32 Ti Swinging-Bucket Rotor Package (Beckman Coulter, catalog number: 369694)
NanoDrop Microvolume Spectrophotometers (Thermo Fisher Scientific, model: ND1000)
NanoSight instrument equipped with a 638 nm laser and CCD camera (model F-033) (Malvern, model: LM10)
Cryo-electronmicroscope (Jeol, model: JEM 2011)
EMR Carbon support film on copper 400 square mesh (EMR, catalog number: 22-1MC040-100)
Flow cytometer (Becton Dickinson, Biosciences, model: FACS Canto II)
Software
FlowJo v10 software (BD Bioscience, https://www.flowjo.com/solutions/flowjo/downloads)
Nanosight NTA Software version 3.1 (build 3.1.46) (Malvern Panalytical, https://www.malvernpanalytical.com/en/learn/events-and-training/webinars/W150326NanoSightSoftwareRelease)
Procedure
Mesenchymal stem cell preparation
Bone marrow-MSCs (BM-MSCs) were isolated from femurs of Lewis and Fisher rats (200 g). In brief, the bone shaft was extracted inserting a 22 G needle and flushed out with alpha-MEM supplemented with 10% FBS and 2 mM EDTA and cell clumps desegregated. The cell suspension was centrifuged at 400 x g for 20 min at room temperature. Rat BM-MSCs were seeded and expanded in alpha-MEM supplemented with 10% FBS (Ramírez-Bajo et al., 2020a). Therefore, BM-MSCs were characterized according to standardized criteria defined by International Society for Cellular Therapy (ISCT) (see Note 1; Figure 1).
Culture MSCs in T75 cm2 flask using 7 ml of complete alpha-MEM medium. Trypsinization is performed at approximately 80% confluence.
Wash cell culture with pre-warmed PBS, add 3 ml trypsin and incubate at 37 °C for 4 min.
Stop the reaction with 9 ml of complete medium and spin down at 600 x g for 5 min at room temperature to count cells and check viability of culture.
Count MSC’s number and their viability with an automated cell counter following manufacturer’s instructions (see Note 2).
Plate the cells at a density of 5 x 105 cells in a flasks T150 cm2 with 15 ml of complete alpha-MEM medium.
Incubate at 37 °C in 5% CO2 environment.
Replace with fresh complete alpha-MEM medium (pre-warmed to 37 °C) every two days thereafter.
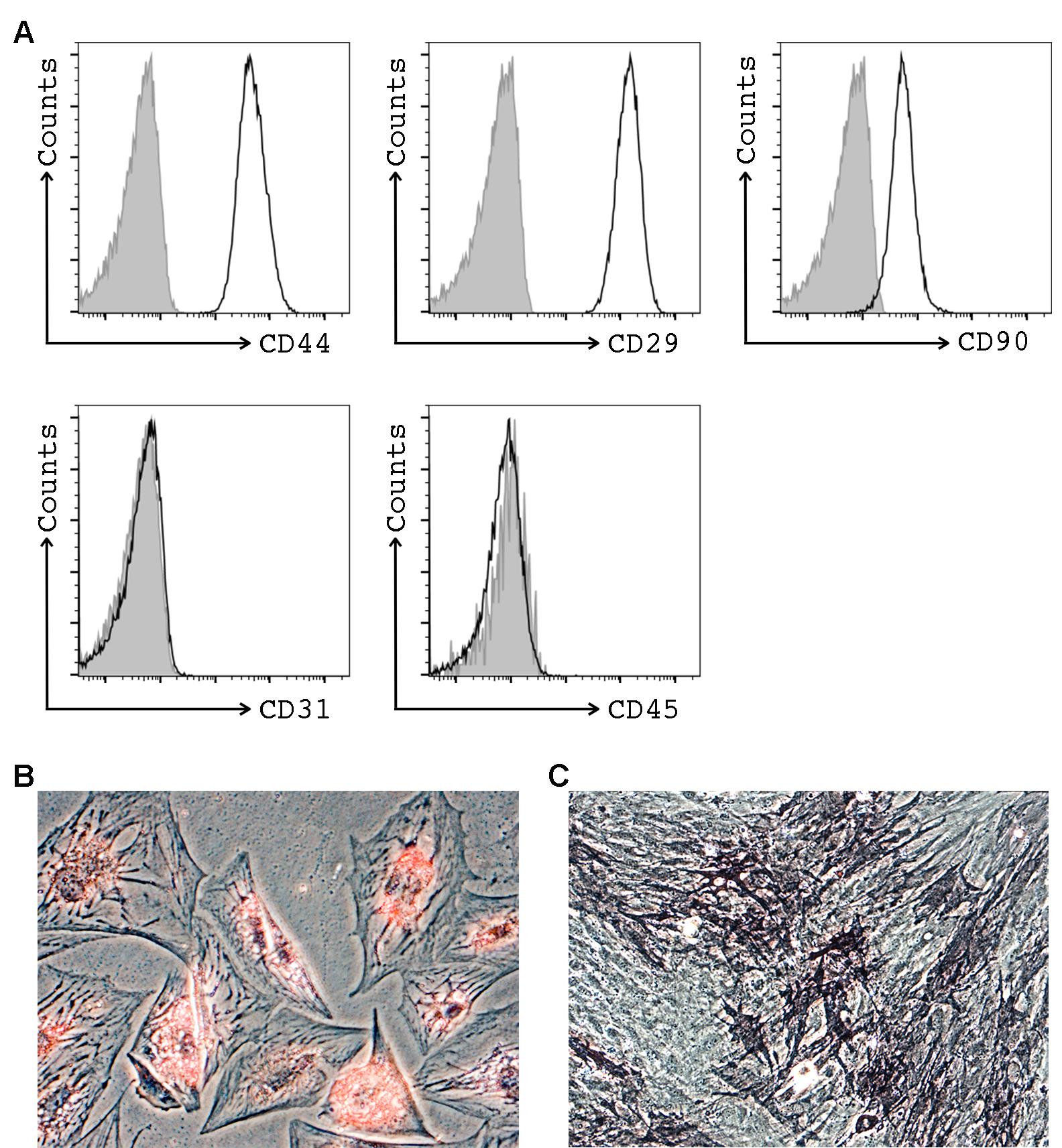
Figure 1. Representative images of MSCs characterization by flow cytometry and multi-lineage differentiation potential. A. Flow cytometry analysis of MSC. Cells were positive for surface stem cell markers (CD44, CD29 and CD90), and negatives for endothelial (CD31), and hematopoietic (CD45) markers. B. Adipogenic differentiation (x200 magnification) as demonstrated showing positivity for oil red staining. C. Osteogenic differentiation (x100 magnification) as demonstrated showing positivity for alkaline phosphatase activity, and was revealed by SigmaFast BCIP/NBT chromogen staining.
EVs isolation by ultracentrifugation
When the cells are approximately 80% confluence, aspirate the supernatant and add 6 ml of PBS (pre-warmed to 37 °C) to wash the residual FBS. Repeat washing step one more time.
Replace PBS with 15 ml of RPMI1640 deprived of FBS (pre-warmed to 37 °C) and incubate for 16 h.
Harvest the MSC conditioned serum free medium and transfer to 50 ml conical tube and keep at 4 °C.
Trypsinize MSCs and determine cell number and viability in an automated cell counter following manufacturer’s instructions (repeat Steps A1-A3).
Our estimation at time of collection was ± 1.5 x 106 MSCs and ± 95% of live cells.
Centrifuge MSC conditioned serum free medium at 3,000 x g for 20 min at 4 °C and micro filtrate with 0.22 μm pore filter membranes to remove cell debris and apoptotic bodies.
Transfer cell-free supernatants into a 38.5 ml capacity open-top thin wall ultra-clear tube. Balance opposite tubes within 10 mg of each other (see Notes 3-4).
Ultracentrifuge at 100,000 x g for 1 h, 4 °C, using SW32 Ti rotor.
Carefully remove tubes from the rotor.
Remove supernatant by aspiring carefully with a Pasteur pipette.
Resuspend EVs pellets with a 200 µl of freezing medium and froze them at -80 °C for later use (see Notes 5-6).
EVs characterization
See Note 7.
EVs quantification:
Nanosight Tracking Analysis (NTA)
Particle size distribution and concentration measurement:
Take 1 ml of EVs solution (dilution factor 1:40 in PBS 0.1 µm filtered) and vortex.
With a syringe without needle, inject EVs solution slowly (avoiding bubbles) into the chamber of particle size analyzer for visualization. It is important to have approximately 20-60 particles in the field of view, to obtain an accurate concentration and size values (Video 1).
Video 1. EVs moving under Brownian motion recorded by NTA. This video visualizes the expected Brownian motion of the EVs in liquid suspension illuminated by a laser light source. The EVs size distribution and concentration are measured using the combination of the light scattering properties with Brownian motion. The real time monitoring helps to determine changes in the characteristics of vesicle populations.Take triplicate readings during 60 s at 30 frames per second (fps), camera level at 16 and manual monitoring of temperature. Figure 2A shows representative results obtained by NanoSight from EVs produced by MSCs.
Protein quantification:
Determination of protein concentration (A280nm) was checked by NanoDrop using 2 µl of EVs solution following the manufacturer's instructions.
Assessment of absence of contaminants by Electron Microscopy
Use a holey carbon support film on a 400-mesh copper grid. Glow discharge the grid and place 3 µl of the EVs sample (from Step B10) on a plunger (Leica EM GP) and blot it with Whatman No. 1 filter paper.
Vitrify the suspension by rapid immersion in liquid ethane (-179 °C) and mount the grid on a Gatan 626 cryo-transfer system and insert it into the microscope.
Take images using cryo-electron microscope operates at 200 kV, record on a GatanUltrascan US1000 CCD camera and analyze with a Digital Micrograph 1.8 (n = 3 per group) (Figures 2B-2C).
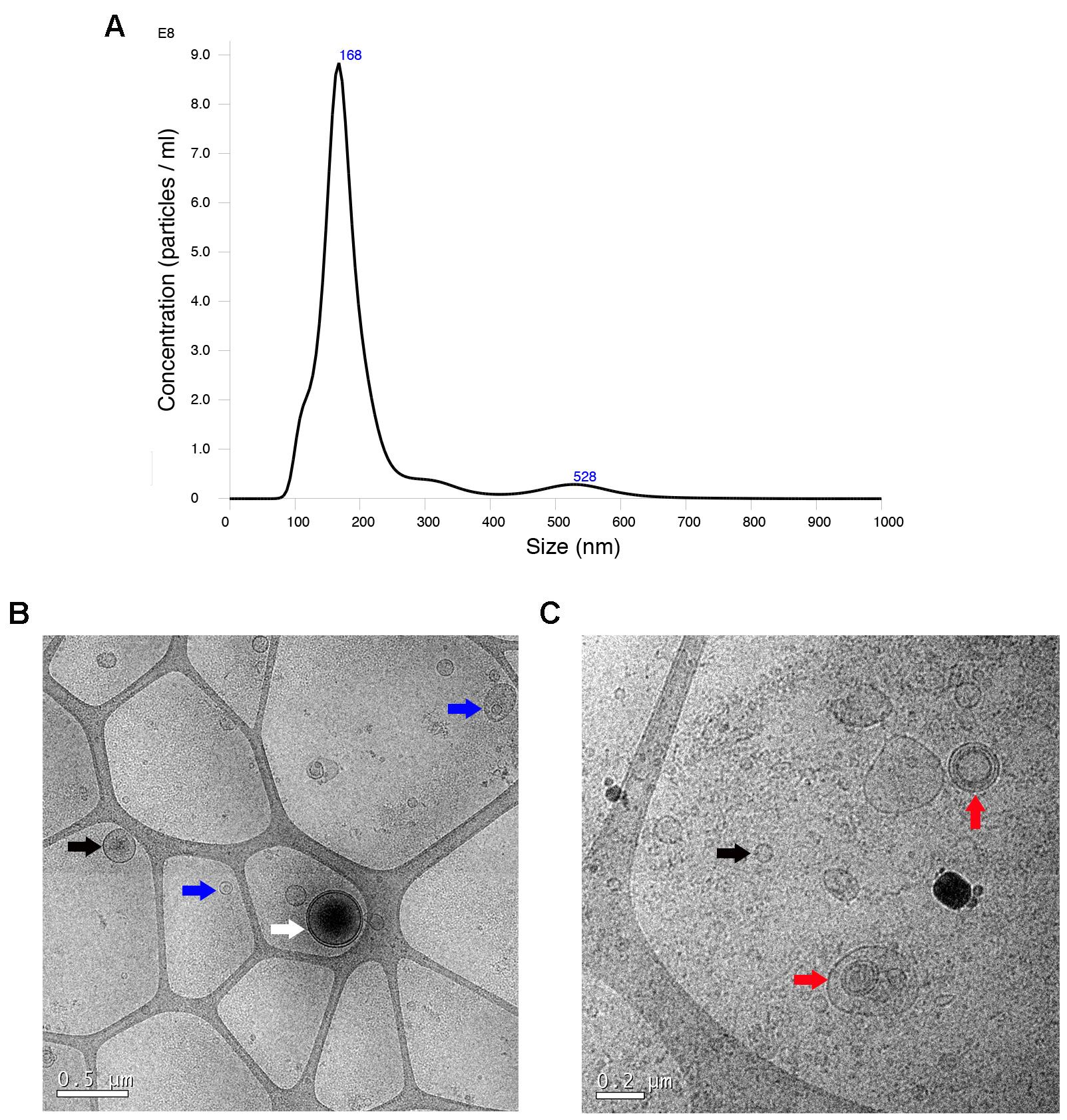
Figure 2. Characterization of EVs by electron microscopy and Nanoparticle Tracking Analysis (NTA). A. NTA measurement shows the concentration and size distribution. The mean EVs size was 201.9 ± 101.4 nm. B-C. Representative cryo-electron microscopy images of EVs (scale bars 0.5 and 0.2 µm, respectively). Cryo-electron microscopy allows to visualize the grid with an irregular distribution of hole sizes and shapes, and inside of them EVs of various sizes with lipid bilayers. The white arrow points a vesicle with a double membrane (white arrow). Most vesicles have an intact membrane and a round shape. Some vesicles are single, double and multilayer, in the case that contains inside another smaller or two and more (black, blue and red arrow, respectively).
Data analysis
MSCs characterization by flow cytometry: Flow cytometric data obtained from BD FACS Canto II cytometer were analyzed with FlowJo software following the gating strategy described by Ramírez-Bajo et al., 2020b.
Nanosight tracking analysis: Data were analyzed with the Nanosight NTA Software version 3.1 (build 3.1.46), with detection threshold set to 5, and blur, min track length, and max jump distance set to auto.
Notes
According to standardized criteria defined by ISCT’s guidelines, MSCs must a) be plastic adherent when kept under standard culture conditions, b) positively expressed MSC markers (CD29, CD44, and CD90) and negatively endothelial (CD31) and hematopoietic lineage (CD45) markers, and c) retain a multipotent phenotype with the ability to differentiate in adipogenic and osteogenic lineage under the standard differentiation conditions (Figures 1B-1C).
In 2018, the International Society for Extracellular Vesicles (ISEV) published and update of the MISEV2014 guidelines with different recommendations to improve the reproducibility of published EVs results. The ISEV checklist summarizes the major aspects to follow in EV science (Lotvall et al., 2014).
The estimation of number of cells/ml or /surface area and % of live/dead cells at time of collection (or at time of seeding with estimation at time of collection) is a mandatory requirement of ISEV Checklist.
To maintain sterility of EVs: Firstly, buckets must be cleaned with ethanol and let dry in the hood. Secondly, insertion or removal of the tubes from buckets has to be done also in the hood.
Balance the weight within the buckets. Thinwall tube needs to be full to provide tube wall support in the bucket. If the volume is too low it may collapse. For this reason, it is important to fill the tube with supernatant or adding PBS 0.1 μm sterile filtered.
Using SW32 Ti requires balancing the weight of the tubes with the buckets following Beckman’s recommendation’s: https://www.beckman.com/resources/fundamentals/principles-of-centrifugation/balancing-your-rotor.
In order to resuspend the EVs pellets, you have to pipette them up and down with a 200 μl pipette but not too roughly, because it is better to avoid producing air bubbles.
EVs can be stored for 2 days at 4 °C. Storing samples at -80 °C is recommended for extended period.
Following ISEV recommendations it requires quantification by at least 2 methods. In our case, we determined particle number by Nanosight and protein count by NanoDrop Spectrophotometer. These results have to be expressed per volume of initial fluid or number of producing cells.
Recipes
Complete alpha-MEM medium
Alpha-MEM
10% FBS
1x L-Glutamine
1x P/S
Filter sterilize the solution and store at 4 °C
Freezing medium
RPMI 1640
1% DMSO
Filter sterilize the solution and store at 4 °C
Note: Filter all the solutions using a 20 ml syringe and a 0.22 μm filter into a new sterile tube.
Acknowledgments
This study has been partially funded by the research grant from Sociedad Española de Nefrología (2014, JC), Redes Temáticas de Investigación Cooperativa En Salud, REDINREN (RD12/0021/0028 and RD16/0009/0023) co-funded by ISCIIISubdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa,” and Secretaria d’Universitats i Recerca and CERCA Programme del Departament d’Economia i Coneixement de la Generalitat de Catalunya (2017-SGR-1331). This work has been developed in the context of AdvanceCat with the support of ACCIÓ (Catalonia Trade and Investment; Generalitat de Catalunya) under the Catalonian ERDF operational program (European Regional Development Fund) 2014-2020. This work has been developed at the Centre de Recerca Biomèdica Cellex, Barcelona, Spain. Our protocol has been based on a previous protocol described by Herrera et al. (2010).
Competing interests
The authors declare no conflicts of interests.
Ethics
Bone marrow-MSCs were isolated from femurs of Lewis and Fisher rats (200g) (Ramírez-Bajo et al., 2020b). The study was approved by and conducted according to the guidelines of the local animal ethics committee (Comitè Ètic d’Experimentació Animal, CEEA, Decret 214/97, Catalonia, Spain).
References
- Azmi, A. S., Bao, B. and Sarkar, F. H. (2013). Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 32(3-4): 623-642.
- Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O. and Skog, J. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2: 180.
- Bartholomew, A., Sturgeon, C., Siatskas, M., Ferrer, K., McIntosh, K., Patil, S., Hardy, W., Devine, S., Ucker, D., Deans, R., Moseley, A. and Hoffman, R. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30(1): 42-48.
- Ben-Ami, E., Miller, A. and Berrih-Aknin, S. (2014). T cells from autoimmune patients display reduced sensitivity to immunoregulation by mesenchymal stem cells: role of IL. Autoimmun Rev 13(2): 187-196.
- Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., Morando, L., Busca, A., Falda, M., Bussolati, B., Tetta, C. and Camussi, G. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20(5): 1053-1067.
- Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V. and Biancone, L. (2010). Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78(9): 838-848.
- Camussi, G., Deregibus, M. C. and Tetta, C. (2010). Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens 19(1): 7-12.
- Ebrahimi, B., Eirin, A., Li, Z., Zhu, X. Y., Zhang, X., Lerman, A., Textor, S. C. and Lerman, L. O. (2013). Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One 8(7): e67474.
- Fevrier, B. and Raposo, G. (2004). Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16(4): 415-421.
- Grange, C., Bellucci, L., Bussolati, B. and Ranghino, A. (2020). Potential Applications of Extracellular Vesicles in Solid Organ Transplantation. Cells 9(2).
- Gregorini, M., Corradetti, V., Pattonieri, E. F., Rocca, C., Milanesi, S., Peloso, A., Canevari, S., De Cecco, L., Dugo, M., Avanzini, M. A., Mantelli, M., Maestri, M., Esposito, P., Bruno, S., Libetta, C., Dal Canton, A. and Rampino, T. (2017). Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J Cell Mol Med 21(12): 3381-3393.
- Guescini, M., Guidolin, D., Vallorani, L., Casadei, L., Gioacchini, A. M., Tibollo, P., Battistelli, M., Falcieri, E., Battistin, L., Agnati, L. F. and Stocchi, V. (2010). C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res 316(12): 1977-1984.
- Harding, C., Heuser, J. and Stahl, P. (1984). Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35(2): 256-263.
- Herrera, M. B., Fonsato, V., Gatti, S., Deregibus, M. C., Sordi, A., Cantarella, D., Calogero, R., Bussolati, B., Tetta, C. and Camussi, G. (2010). Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 14(6B): 1605-1618.
- Koch, M., Lemke, A. and Lange, C. (2015). Extracellular Vesicles from MSC Modulate the Immune Response to Renal Allografts in a MHC Disparate Rat Model. Stem Cells Int 2015: 486141.
- Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V. and Laktionov, P. P. (2018). Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int 2018: 8545347.
- Kunter, U., Rong, S., Boor, P., Eitner, F., Muller-Newen, G., Djuric, Z., van Roeyen, C. R., Konieczny, A., Ostendorf, T., Villa, L., Milovanceva-Popovska, M., Kerjaschki, D. and Floege, J. (2007). Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18(6): 1754-1764.
- Lotvall, J., Hill, A. F., Hochberg, F., Buzas, E. I., Di Vizio, D., Gardiner, C., Gho, Y. S., Kurochkin, I. V., Mathivanan, S., Quesenberry, P., Sahoo, S., Tahara, H., Wauben, M. H., Witwer, K. W. and Thery, C. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913.
- Mittelbrunn, M., Gutierrez-Vazquez, C., Villarroya-Beltri, C., Gonzalez, S., Sanchez-Cabo, F., Gonzalez, M. A., Bernad, A. and Sanchez-Madrid, F. (2011). Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2: 282.
- Momen-Heravi, F., Balaj, L., Alian, S., Mantel, P. Y., Halleck, A. E., Trachtenberg, A. J., Soria, C. E., Oquin, S., Bonebreak, C. M., Saracoglu, E., Skog, J. and Kuo, W. P. (2013). Current methods for the isolation of extracellular vesicles. Biol Chem 394(10): 1253-1262.
- Ramírez-Bajo, M. J., Martín-Ramírez, J., Bruno, S., Pasquino, C., Banon-Maneus, E., Rovira, J., Moya-Rull, D., Lazo-Rodriguez, M., Campistol, J. M., Camussi, G. and Diekmann, F. (2020). Nephroprotective Potential of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Murine Model of Chronic Cyclosporine Nephrotoxicity. Front Cell Dev Biol 8: 296.
- Ramírez-Bajo, M. J., Rovira, J., Lazo-Rodriguez, M., Banon-Maneus, E., Tubita, V., Moya-Rull, D., Hierro-Garcia, N., Ventura-Aguiar, P., Oppenheimer, F., Campistol, J. M. and Diekmann, F. (2020). Impact of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Rat Model of Kidney Rejection. Front Cell Dev Biol 8: 10.
- Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A. and Ratajczak, M. Z. (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20(9): 1487-1495.
- Rigo, F., De Stefano, N., Navarro-Tableros, V., David, E., Rizza, G., Catalano, G., Gilbo, N., Maione, F., Gonella, F., Roggio, D., Martini, S., Patrono, D., Salizzoni, M., Camussi, G. and Romagnoli, R. (2018). Extracellular Vesicles from Human Liver Stem Cells Reduce Injury in an Ex Vivo Normothermic Hypoxic Rat Liver Perfusion Model. Transplantation 102(5): e205-e210.
- Stone, M. L., Zhao, Y., Robert Smith, J., Weiss, M. L., Kron, I. L., Laubach, V. E. and Sharma, A. K. (2017). Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res 18(1): 212.
- Thery, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F. and Atkin-Smith, G. K., et al. (2018). Minimal information for studies of extracellular vesicles 2018(MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1): 1535750.
- Thery, C., Zitvogel, L. and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8): 569-579.
- Togel, F., Hu, Z., Weiss, K., Isaac, J., Lange, C. and Westenfelder, C. (2005). Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289(1): F31-42.
- Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J. and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6): 654-659.
- Wang, Y., Han, Z. B., Song, Y. P. and Han, Z. C. (2012). Safety of mesenchymal stem cells for clinical application. Stem Cells Int 2012: 652034.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ramirez-Bajo, M. J., Banon-Maneus, E., Rovira, J., Campistol, J. M. and Diekmann, F. (2020). Isolation of Extracellular Vesicles Derived from Mesenchymal Stromal Cells by Ultracentrifugation. Bio-protocol 10(24): e3860. DOI: 10.21769/BioProtoc.3860.
Category
Stem Cell > Adult stem cell > Mesenchymal stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link














