- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bacterial Adhesion Kinetics in a High Throughput Setting in Seconds-minutes Time Resolution
Published: Vol 11, Iss 2, Jan 20, 2021 DOI: 10.21769/BioProtoc.3844 Views: 2761
Reviewed by: Amit Kumar DeyAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Biosynthesis and Genetic Encoding of Non-hydrolyzable Phosphoserine into Recombinant Proteins in Escherichia coli
Philip Zhu [...] Richard B. Cooley
Nov 5, 2023 2512 Views

Mobilization of Plasmids from Bacteria into Diatoms by Conjugation Technique
Federico Berdun [...] Eduardo Zabaleta
Mar 5, 2024 1917 Views

Artificial Metalloenzymes in Artificial Sanctuaries Through Liquid–Liquid Phase Separation
Kaixin Wang [...] Tong Wu
Oct 5, 2025 1566 Views
Abstract
Bacterial surface adhesion, the first step in many important processes including biofilm formation and tissue invasion, is a fast process that occurs on a time scale of seconds. Adhesion patterns tend to be stochastic and spatially heterogeneous, especially when bacteria are present in low population densities and at early stages of adhesion to the surface. Thus, in order to observe this process, a high degree of temporal resolution is needed across a large surface area in a way that allows several replicates to be monitored. Some of the current methods used to measure bacterial adhesion include microscopy, staining-based microtiter assays, spectroscopy, and PCR. Each of these methods has advantages in assaying aspects of bacterial surface adhesion, but none can capture all features of the process. In the protocol presented here, adapted from Shteindel et al., 2019, fluorescently-labeled bacteria are monitored in a multi-titer setting using a standard plate fluorimeter and a dye that absorbs light in the fluorophore excitation and emission wavelengths. The advantage of using this dye is that it restricts the depth of the optic layer to the few microns adjacent to the bottom of the microtiter well, eliminating fluorescence originating from unattached bacteria. Another advantage of this method is that this setting does not require any preparatory steps, which enables reading of the sample to be repeated or continuous. The use of a standard multi-titer well allows easy manipulation and provides flexibility in experimental design.
Keywords: Bacterial adhesionBackground
Bacterial adhesion is the first step of many important processes such as pathogen tissue invasion, symbiont recruitment and biofilm formation. Adhesion is a dynamic process, changing on a time scale of seconds (Fletcher, 1977; Vadillo-Rodríguez et al., 2004), therefore continuous, kinetic measurements are needed to study these events. Sampling a large surface area is also helpful, as adhesion tends to be heterogeneous and stochastic at a microscopic scale. In addition to having an assay that can make kinetic measurements, it is desirable to have an assay that is simple, cost-effective, and high throughput because it makes it possible to have multiple replicates and to test a wide range of experimental parameters.
While many methods for the measurement of adhesion have been reported - each one with advantages and disadvantages - none of the methods capture every aspect of this dynamic process. Microscopy allows detailed and direct measurement of adhesion but the main drawback of microscopy is a small field size. As a result, sampling of many different fields is needed to obtain statistical validity but sampling in this way makes it difficult to get high resolution kinetic data. This contradiction is highlighted in early time points of adhesion kinetics, when the number of cells per surface unit is low, causing a large stochastic effect due to small sample sizes (Vadillo-Rodríguez et al., 2004). Another popular method for bacterial attachment measurement is using crystal violet dye in microtiter plates (O'Toole, 2011). While this method is high throughput and inexpensive, it does not allow repeated measurements, making kinetics complicated and unreliable. Furthermore, sample preparation is not fast which makes this assay inadequate for monitoring events that occur at seconds-minutes timescales. Nano plasmonics is an effective way to make real time, kinetic measurements without the need for fluorescent labeling, but this method is expensive and requires highly specialized equipment, fabrication capabilities and expertise (Funari et al., 2018, Abadian and Goluch, 2015). The protocol presented here uses fluorescent labeling and is able to provide real time, kinetic measurements of bacterial adhesion in different culture densities, salt concentrations and different surfaces, using a variety of bacterial species (Shteindel et al., 2019), as well as changes in bacterial adhesion behavior under the effect of quorum sensing signals and in the different bacterivores (in preparation). All graphics in this protocol are adapted from Shteindel et al., 2019.
Materials and Reagents
2 x 96 well plate, clear polystyrene, Flat bottom, untreated (JETbiofil, China, catalog number: TCP001096 )
1.5 ml plastic tube
LB agar plate, 300 μg/ml Carbenicillin
Eight channel pipetor reservoirs
Pseudomonas aeruginosa PAO1 with the pMRP9-1 plasmid (Carbenicillin resistance, constitutive GFPmut2) freezer stock
Di-ionized water
Allura red AC powder (Sigma, catalog number: 458848 )
Crystal violet powder (Sigma, catalog number: C6158-100G )
Acetic acid (Sigma, catalog number: 27225-500ML-R )
Allura red AC solution (see Recipes)
NaCl solution (see Recipes)
M9 medium (see Recipes)
Crystal violet (see Recipes)
Acetic acid 30% v/v (see Recipes)
Equipment
Shaker-incubator
Micro-centrifuge
Multichannel pipetor
Multimode plate reader (Synergy HT, Biotek, USA)
Software
Gen5 (platereader software)
Procedure
Bacterial culture
- Inoculate frozen stock culture of bacteria (e.g., GFP tagged PA01) onto LB-Carbenicillin plate and incubate 24 h at 37 °C.
- Pick one colony from the plate and inoculate an Erlenmeyer flask, containing 50 ml of M9 medium, 0.4% glucose, 200 µg/ml Carbenicillin.
- Incubate at 37 °C, 120 RPM, 16 h.
Preparation for kinetics and kinetic measurement of bacterial adhesion
Prepare plate (first 6 columns) according to the following table using NaCl solution, de-ionized water, 8 channel pipetor and reservoir, in 50 μl volume per well. These concentrations will be halved when the bacterial culture is added.
Note: Using final salt concentrations greater than 500 mM is not recommended as it causes dye aggregation.
Table 1. Plate plan for bacterial adhesion in various salt concentrations experiment
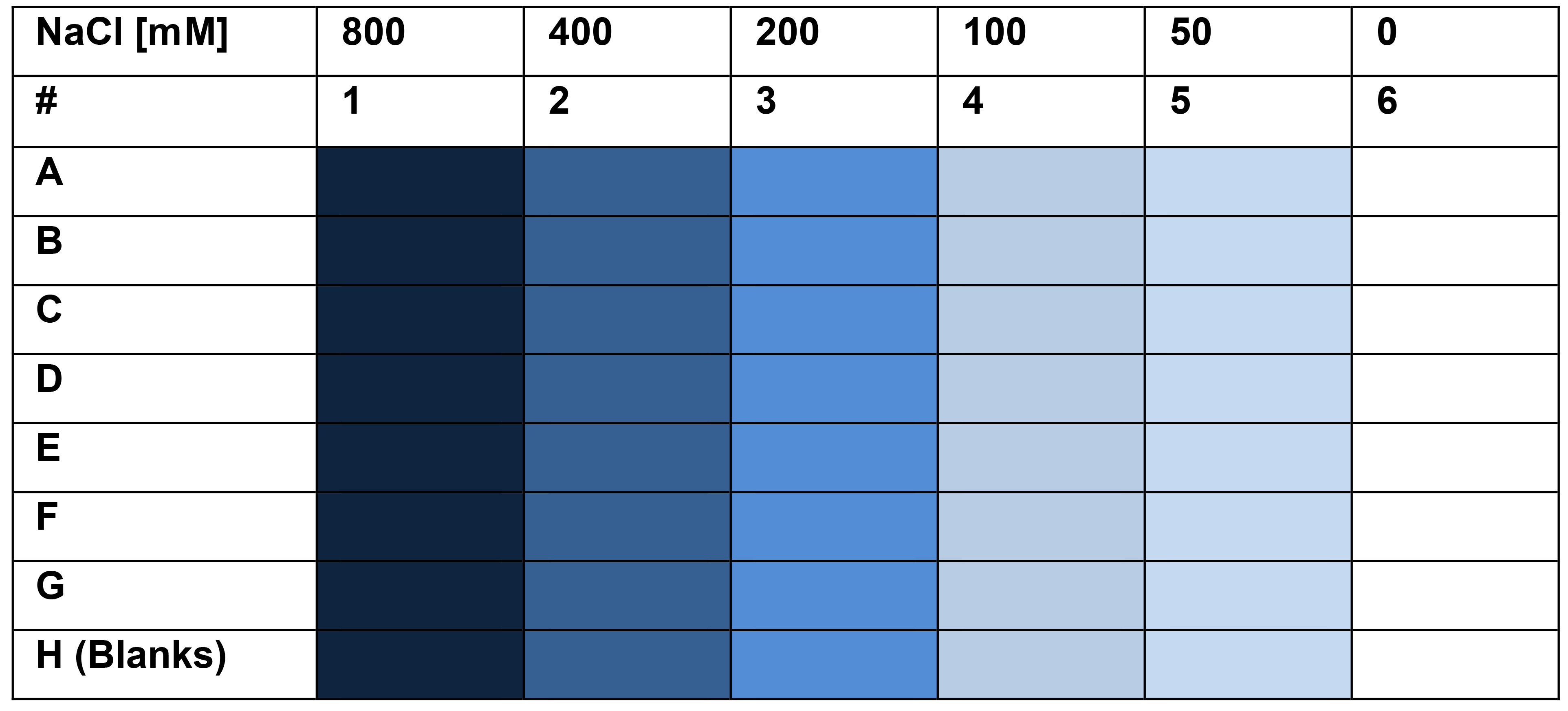
To 984 μl of di-ionized water, add 16 μl of Allura red AC solution. Pipet 50 μl per well to wells 1-6 of row H, these well will serve as blanks.
Transfer PAO1 culture into 12 x 1.5 ml microcentrifuges (1.5 ml per tube), centrifuge once to separate the culture (5,000 x g, 1 min, 25 °C). Resuspend each pellet in 500uL of de-ionized water. Transfer the resuspended bacteria into an 18 mm glass test tube.
Note: Bacterial adhesion behavior is effected by long incubation periods in de-ionized water, thus it is important to minimize the amount of time bacteria are in water.Turn on the plate-reader, enter the following protocol: Temperature, 25 °C, start kinetics (60 min, read every 30 s), Read parameters: Fluorescence (Excitation 485/20nm, Emission 528/20nm, Bottom, Gain 60). Validate the protocol and start reading to allow the lamp to heat (180 s), do not start the kinetics yet.
Note: If you wish to follow culture density in parallel to adhesion, you can measure OD650nm to avoid dye absorbance at 600nm. This reading values of correspond to 80% of the OD600nm measurement. Culture density data is interesting, (in this experiment, there is a reverse correlation between adhesion culture density as attached bacteria contribute less to optical density at OD650nm). But these measurements should be interpreted with caution because bacterial aggregation, for instance, will elevate optical density dramatically. If you choose to incorporate an OD650nm reading, set the reading frequency to 1 measurement every 1 min.Determine culture density using a new plate, by adding 100 μl of the culture to well A1 of a multi-titter plate, and read for OD600nm against blank.
Add the required amount of de-ionized water and dye to prepare 6 ml of bacterial culture OD600nm = 0.1, dye concentration of 1.6 mg/ml (see Table 2 for calculation example)
Table 2. Calculation example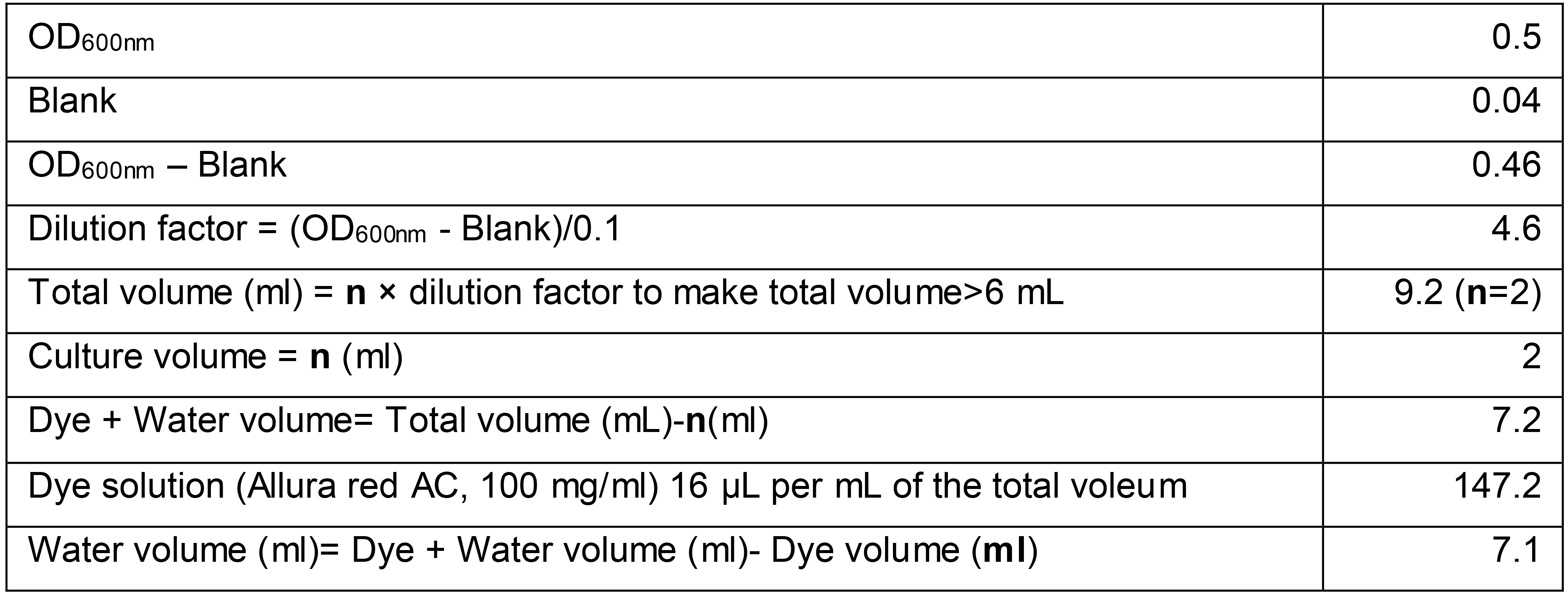
Using multichannel pipetor add 50 μl of the bacterial suspension (diluted, with dye) to wells 1-6 of rows A-G.
Note: Adhesion kinetics is fast, try to finish pipetting as fast as possible, within 30 s or so, or you will lose a lot of data.
Load the plate onto the plate reader and start kinetics.
Note: Black plates are often used in fluorescence, to prevent crosstalk between wells. In the case of the method presented here, read fluorescence is emitted from a shallow layer adjacent to the bottom of the well, eliminating crosstalk which originated from light traveling diagonally through the wall into adjacent wells, similar to elimination of crosstalk by black side plates.
Post kinetic preparation and post kinetics
While the plate reader is running the kinetics program, mix 12 ml of 0.8 mg/ml dye solution in a multichannel pipetor reservoir. This solution will be used to read fluorescence of the attached bacteria in the absence of unattached bacteria, as a control for the kinetic measurement.
When the kinetic measurement ends, use the pipetor to gently remove the liquid from all wells.
Add 100 μl of the 0.8 mg/ml dye solution to each well and read fluorescence once more using the same parameters (not kinetics).
Note: Make sure to keep wells empty for the shortest possible time, GFP matures in the presence of high oxygen concentration leading to a dramatic increase in fluorescence if left dry for too long.
Optional step: Perform crystal violet staining on the same sample according to O'Toole 2011.
Export kinetic and post kinetic reading results to Excel, sort and average according to treatment, calculate standard deviation, graph.
Data analysis
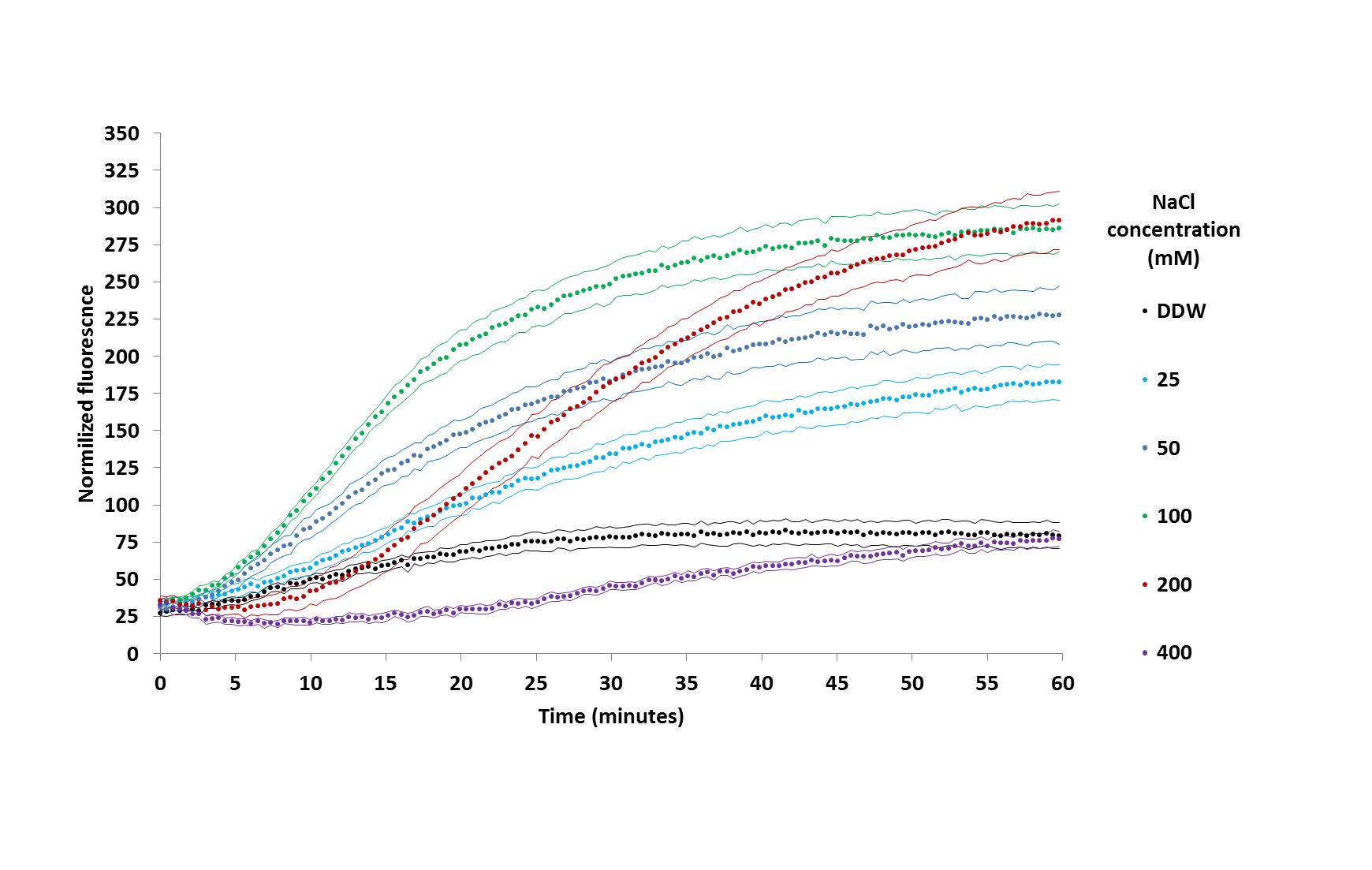
Figure 1. Pseudomonas aeruginosa adhesion kinetics in several salt concentrations (adapted from Shteindel et al., 2019) each dotted curve represents adhesion in a different salt concentration, symbols stand for measurement times, flanking curves stand for ± 1SD, n = 7 for each treatment.
Note: The proper statistical test for this dataset is a repeated measurement one-way ANOVA. Sixty measurements points over six treatments carry a heavy significance penalty due to reuse of data over multiple tests, making it unusable. Normalization of this dataset allowing the use of parametric tests is also not trivial. The curves representing different treatments are separated by several standard deviations over most of the kinetics–i.e., between groups differences are larger than within group differences, making them statistically significant.
Bacterial adhesion is often described by the DELVO model, predicting low adhesion at low and high salt concentrations, and high adhesion in median concentrations (Hermansson, 1999). Adhesion at the last time point in different salt concentrations agree with this description, but the kinetic data reveal details which are not captured in a single measurement. While 100 and 200 mM salt concentrations produce similar adhesion outcomes after one hour, adhesion kinetics at 100 mM is higher than at 200mM. Similarly, 400 and 0 mM salt concentrations produce comparable adhesion at the end of experiment, but a higher kinetics is seen at the 400 mM treatment.
In order to verify that the test measures adhering bacteria, and not unattached bacteria near the bottom of the plate, or sedimented bacteria, we introduced a post kinetics measurement. In this measurement we remove the liquid in each well and replace it with sterile buffer, removing the vast majority of un-attached bacteria and diluting any remaining un-attached cells. Preforming a second bottom fluorescence reading allow us to evaluate the fraction of fluorescence originating from attached bacteria at the last kinetic time point. Post kinetics values are practically identical, suggesting all fluorescence read at the last time point originated from attached bacteria (Figure 2). The only statistics used is Pearson correlation calculated using the Excel automatic function.
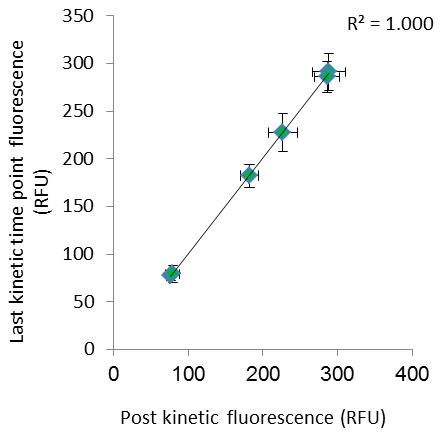
Figure 2. Last kinetic time point fluorescence VS post kinetic fluorescence (adapted from Shteindel et al., 2019) n = 7 for each data point, error bars stand for ± 1SD.
To allow comparison of this method to a more traditional approach, crystal violet staining was applied to the same sample after the performance of the post kinetic reading (Figure 3). Crystal violet results show the same basic pattern of effect, with higher error, due to the low sensitivity of this method.
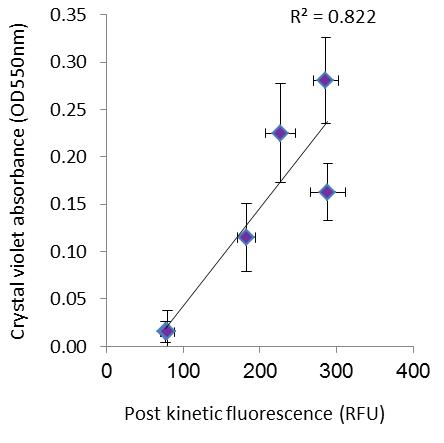
Figure 3. Post kinetic fluorescence vs. crystal violet staining (adapted from Shteindel et al., 2019) n = 7 for each data point, error bars stand for ± 1SD.
Recipes
Allura red AC solution
Measure approximately 100 mg of powder in a 1.5 ml plastic tube
Add 10 μl of the media intended for the adhesion experiment for every mg of powder
Do not mix by pipetting, use a vortex, mix until completely dissolved
NaCl solution
Add 2.338 g of NaCl into 50 ml of di-ionized water, mixed until dissolved
M9 medium
Prepared according to thelabrat.com, 0.4% glucose, 200 μg/ml Carbenicillin 50 ml in a 100 ml Erlenmeyer bottle
Crystal violet
Add 50 mg of crystal violet powder to a 50 ml plastic test tube
Seal test tube, vortex until dissolved
Acetic acid 30% v/v (for crystal violet staining)
In a chemical hood measure 15 ml of acetic acid in a 50 ml plastic test tube and add 35 ml of di-ionized water
Acknowledgments
This protocol is adapted from Shteindel et al., 2019.
Competing interests
No competing interests, financial or other are associated with this protocol.
References
- Abadian, P. N. and Goluch, E. D. (2015). Surface plasmon resonance imaging(SPRi) for multiplexed evaluation of bacterial adhesion onto surface coatings. Analytical Methods 7(1): 115-122.
- Fletcher, M. (1977). The effects of culture concentration and age, time, and temperature on bacterial attachment to polystyrene. Can J Microbiol 23(1): 1-6.
- O'Toole, G. A. (2011). Microtiter dish biofilm formation assay. J Vis Exp (47): e2437.
- Shteindel, N., Yankelev, D. and Gerchman, Y. (2019). High-throughput quantitative measurement of bacterial attachment kinetics on seconds time scale. Microb Ecol 77(3): 726-735.
- Vadillo-Rodríguez, V., Busscher, H. J., Norde, W., de Vries, J. and van der Mei, H. C. (2004). Relations between macroscopic and microscopic adhesion of Streptococcus mitis strains to surfaces. Microbiology (4): 1015-1022.
- Funari, R., Bhalla, N., Chu, K. Y., Soderstrom, B. and Shen, A. Q. (2018). Nanoplasmonics for real-time and label-free monitoring of microbial biofilm formation. ACS sensors 3(8): 1499-1509.
- Hermansson, M., (1999). The DLVO theory in microbial adhesion. Colloids Surf B Biointerfaces 14(1-4): 105-119.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Shteindel, N. and Gerchman, Y. (2021). Bacterial Adhesion Kinetics in a High Throughput Setting in Seconds-minutes Time Resolution. Bio-protocol 11(2): e3844. DOI: 10.21769/BioProtoc.3844.
Category
Microbiology > Microbial biofilm
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










