- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Headpost Surgery for in vivo Electrophysiological Recording in the Mouse Inferior Colliculus during Locomotion
Published: Vol 10, Iss 23, Dec 5, 2020 DOI: 10.21769/BioProtoc.3840 Views: 3638
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evoked Potential Recordings of Auditory Brainstem Activity in the Mouse: An Optimized Method for the Assessment of Hearing Function of Mice
Neil J. Ingham
Dec 5, 2019 6069 Views

Patch-clamp Recordings and Single Fiber Labeling from Spiral Ganglion Somata in Acutely Prepared Semi-intact Cochleae from Neonatal Rats
Alexander L. Markowitz [...] Radha Kalluri
Jan 5, 2022 4153 Views
Abstract
The inferior colliculus (IC) is a critical midbrain integration center for auditory and non-auditory information. Although much is known about the response properties of the IC neurons to auditory stimuli, how the IC neural circuits function during movement such as locomotion remains poorly understood. Mice offer a valuable model in this respect, but previous studies of the mouse IC were performed in anesthetized or restrained preparations, making it difficult to study the IC function during behavior. Here we describe a neural recording protocol for the mouse IC in which mice are head-fixed, but can run on a passive treadmill. Mice first receive a headpost surgery, and become habituated to head-fixing while being on a treadmill. Following a few days of habituation, neural recordings of the IC neuron activity are performed. The neural activity can be compared across different behavioral conditions, such as standing still versus running on a treadmill. We describe how to overcome the challenges of headpost surgery for awake IC recording, presented by the location and overlying bones. This protocol allows investigations of the IC function in behaving mice, while allowing precise stimulus control and the use of recording methods similar to those for anesthetized preparations.
Keywords: Inferior colliculusBackground
The inferior colliculus (IC) is a critical midbrain auditory integration center where virtually all ascending inputs project to (Winer and Schreiner, 2005). The IC also receives non-auditory inputs including those from somatosensory regions, suggesting multi-modal integration of auditory and non-auditory information (Gruters and Groh, 2012). Mice offer an important model for unraveling the cellular and circuit-level principles of the IC function owing to the broad array of genetic tools available (Ono et al., 2017; Goyer et al., 2019; Chen and Song, 2019; Hoyt et al., 2019; Wong and Borst, 2019; Silveira et al., 2020). However, most neural recording in the mouse IC has been performed in anesthetized or restrained preparations (Muniak et al., 2012; Ayala et al., 2016 ). As a result, relatively little is known about the activity of IC neurons when the animals are engaged in movement such as locomotion (Chen and Song, 2019; Yang et al., 2020). We devised a head-fixed mouse preparation in which neural recordings can be made from IC neurons while a mouse runs on a passive treadmill (Yang et al., 2020). This preparation allows investigation of IC neural activity in behaving mice, while maintaining precise stimulus control. In a similar way to other awake restrained preparations, this protocol would make it easier to combine multi-electrode recordings with pharmacological or optogenetic manipulation of activity, compared to neural recordings with chronically implanted electrodes. This preparation has potential to bring new insights into the functions of the IC by observing how IC circuits operate during behavior such as locomotion or performing an auditory task. Moreover, it should facilitate the investigation of how manipulations of IC circuits or inputs to the IC might influence behavior.
Materials and Reagents
Cotton swabs (e.g., VWR, catalog number: 89031-270 )
Kimwipes (Kimtech, catalog number: 41112 )
1 ml syringe (Korea Vaccine, KOVAX-SYRINGE 1mL )
Slide glass (Matsunami, Frontier HMA-FRC-11 )
Slide cover glass (VWR, catalog number: 48393-219 )
6-8 week-old mice (Orient Bio, stain: C57BL/6 ); headpost size and cementing methods may need to be adjusted for younger mice.
Dental cement (Sun Medical, model: super bond C&B )
Ground screws (Scitech Korea, self-tapping screw, catalog number: SCW0103 ; diameter = 1 mm; length = 3 mm; pitch ~0.4 mm)
Kwik-Cast (WPI, model: KWIK-CAST , https://www.wpiinc.com/kwik-cast-kwik-cast-sealant; stored at room temperature)
Isoflurane (Hana Pharm, Ifran Solution , stored at room temperature)
2% Lidocaine (Dai Han Pharm Co., Lidocaine HCl Injection 2%, stored at room temperature)
Eye ointment (Samil, Forus, stored at room temperature)
Meloxicam (Boehringer Ingelheim, Metacam, stored at room temperature; 5 mg/Kg)
0.9% Saline (Dai Han Pharm Co., Sterile Normal Saline for Irrigation)
4% Paraformaldehyde (CellNest, catalog number: CNP015-1000 )
Glacial acetic acid (Duksan Reagents, product number: 414 )
Ethanol (Duksan Reagents, 99.9%, product number: 4)
Cresyl violet (Sigma, catalog number: C5042-10G )
Xylene (Duksan Reagents, catalog number: 115 )
Equipment
#5 forceps (Fine Science Tools, catalog number: 11251-20 )
Fine scissors (Fine Science Tools, catalog number: 14090-09 )
Tungsten electrode array (FHC, catalog number: MX61DAW(GK3) )
Stainless steel headpost (Scitech Korea, custom made, Figure 1A)
Headpost holder (SK-HFH11M, Figure 1B)
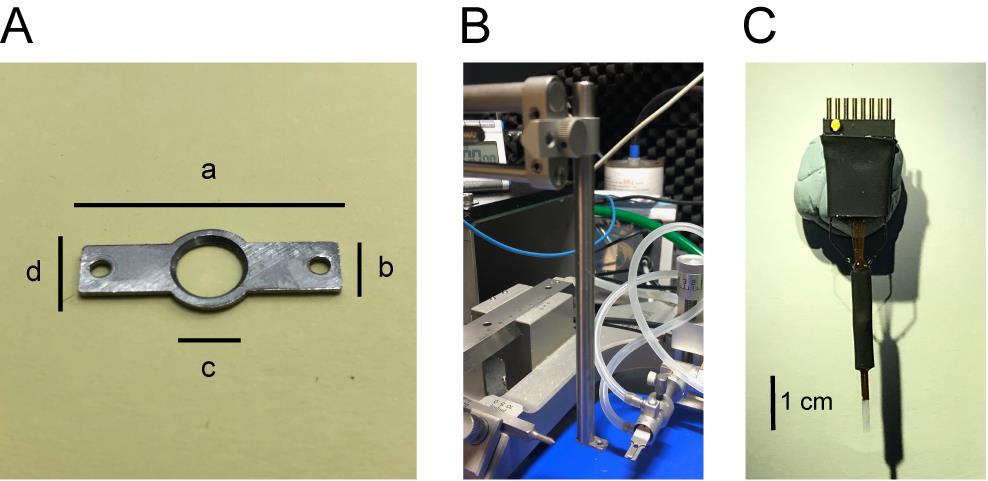
Figure 1. Images of headpost (A), headpost holder (B), and electrode array (C). A. The dimensions of the headpost are: length (a) = 27 mm; width (b) = 6 mm; window inner diameter (c) = 7 mm; window outer diameter (d) = 10 mm; diameter for the holes for screws (M2 x 4mm) = 2 mm; thickness = 1 mm. Shapes and the dimensions of the headpost can be changed as needed. B. A headpost holder attached to stereotaxic frame. C. Tungsten electrode array with electrode spacing ~210 μm.Treadmill with sensor (Scitech Korea, model: SK-DSTR01M)
Our treadmill had a rotary encoder whose voltage output alternates between low and high (range 0-3 V) 240 times per rotation (Figure 2). We mainly used a 28 cm diameter disk, but we find that a 20 cm disk works just as well.
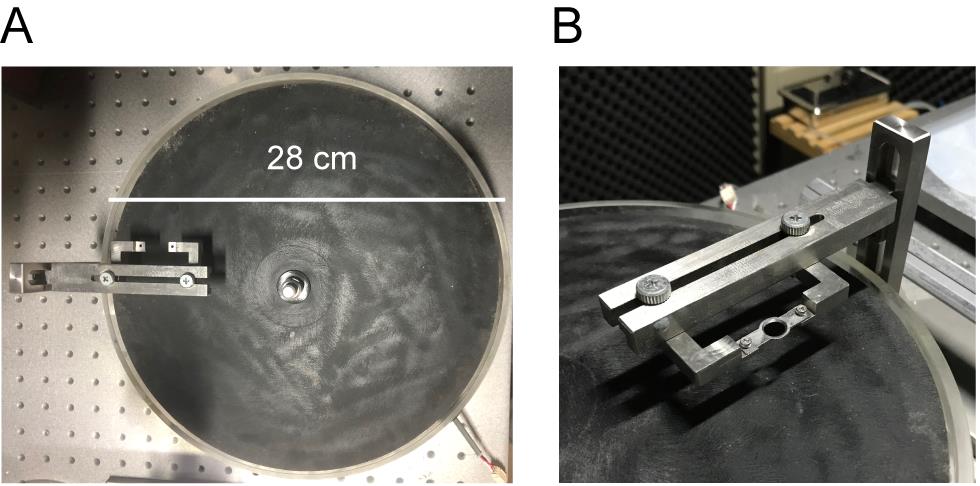
Figure 2. Treadmill with a head-fixing frame. A. Top view. We used a treadmill plate with a diameter of 28 cm, but it can be smaller. Mice were placed ~10 cm away from the center, and this distance was used to convert rotation to speed. B. Head-fixing frame with a headpost in place.Head-fixing frame (Scitech Korea, model: SK-HFH02M )
Single axis motorized micro-manipulator (Scientifica, model: IVM Mini )
Stereotaxic manipulator (Narishige, models: SM-15L and SM-15M-2 )
Stereotaxis frame (David Kopf Instruments, model: Model 900 ) with mouse gas anesthesia head holder (David Kopf Instruments, model: Model 923-B )
Dental drill (Saeshin, model: Forte400s )
Heating pad (Harvard Apparatus, catalog number: 55-7020 )
Infrared video camera (e.g., https://tinyurl.com/yc7uszzf)
Digital-to-analog converter (National Instruments, model: PCIe-6343 )
Ultrasonic speaker (Avisoft, catalog number: 60108 )
Power amplifier (Avisoft, catalog number: 70103 )
Headstage (Intan Technologies, catalog number: C3334 )
Open Ephys data acquisition board (Open Ephys)
Stimulator (A-M Systems, model: Model 2100)
Sound booth (Joongang Medics, single wall single room audiobooth)
Peristaltic pump (Harvard Apparatus, model: P-1500 )
Cryostat (Leica, model: CM1950 )
Software
MATLAB (Mathworks, https://www.mathworks.com)
Open Ephys GUI (Open Ephys, https://open-ephys.org/gui)
Procedure
Headpost surgery
Place mice on a heating pad in the stereotaxic frame under Isoflurane anesthesia (1-4%).
Apply eye ointment to prevent dryness.
Inject meloxicam subcutaneously around the neck (5 mg/kg).
Clean the scalp by wiping with 70% ethanol.
Inject 2% lidocaine underneath the scalp as a local anesthetic.
Make an incision to expose the top of the skull.
Gently remove the tissue around the posterior end of the skull over the IC and cerebellum with forceps.
Clean the skull surface and let it dry completely.
Mark the target area on the skull with a pen (AP: ~5.0 mm from bregma; ML: ~1.0 mm).
Choose a location for the ground screw toward the anterior end of the skull (Figure 3).
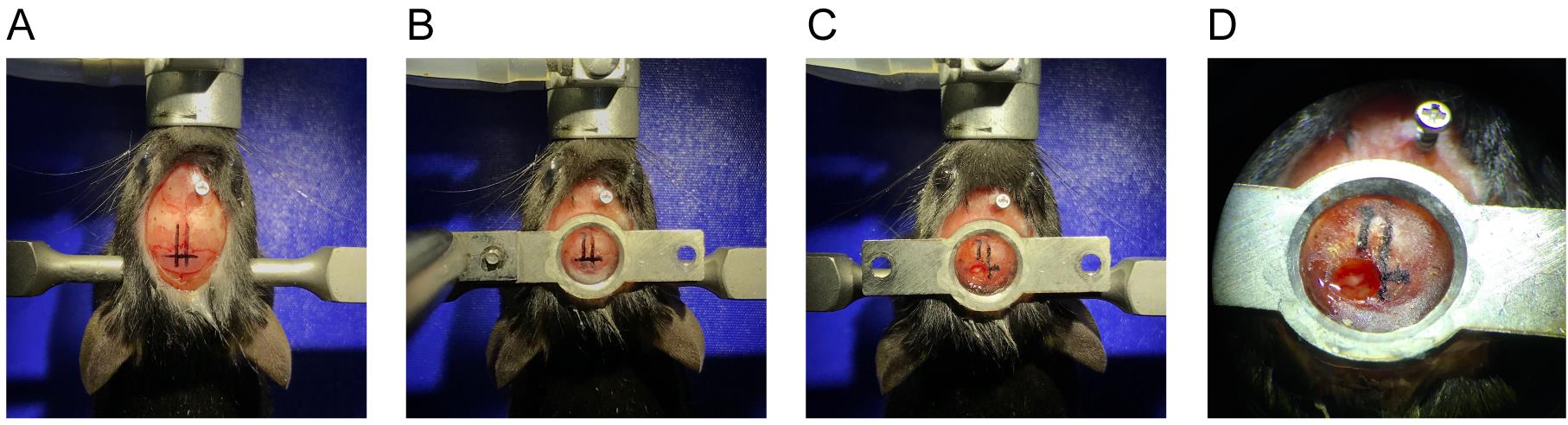
Figure 3. Headpost surgery and craniotomy. A. Exposed skull during headpost surgery. At this point, a ground screw has been secured, and 3 marking lines have been drawn based on stereotaxic measurements (the two rostrocaudal lines mark the midline and 1 mm lateral to the midline, respectively; the mediolateral line marks the location 5 mm posterior to the bregma). B. Headpost has been cemented. C-D. Craniotomy performed on the day of recording. (A-B) on the day of headpost surgery, and (C-D) on the day of recording.Carefully screw a ground screw into the skull using a screw driver. Take care not to go too deep into the brain. The depth can be assessed by examining the dent in the brain surface when removing the brain from the skull for histology. In the case of the screw listed here, we find that about 3 turns (~1.2 mm) give consistently good placement. Apply a small amount of dental cement only at the bottom portion of the screw.
Position a headpost using a holder attached to the stereotaxic manipulator, such that IC is accessible for future craniotomy (~2 mm x 1 mm). Make sure the skull area posterior to the target area is sufficiently exposed and free of tissue to allow cementing of the posterior side of the headpost circle.
Apply dental cement to secure the headpost. To prevent the headpost from coming off, it is critical to prepare the skull surface as dry as possible. Use swabs and wipes to absorb any liquid and wait until the surface becomes dry. Lift up the headpost and apply cement to its bottom surface, then bring it down to the skull. Apply cement to fill any space left out between the headpost and the skull. The skull surface posterior to the target area, below the posterior rim of the headpost circle, runs vertically, creating large space between the skull and the headpost. It may take several rounds of cementing to fill in this space to form a thick, stable base. Going around the inner rim of the headpost circle, extend cement from the skull over the top surface, encasing the rim. Keep the target area for craniotomy as free of cement as possible. Prevent cement from touching the skin because this may weaken the cementing.
Turn off the isoflurane and let the animal recover.
Habituation to head fixation and treadmill
Note: We kept our mice on a reverse light-dark cycle, and our behavior and recording sessions occurred during the dark period.
Wait at least one day after the head-post surgery.
Place the mice on a treadmill and hold them by the headpost and screw in the headpost to the head-fixing frame.
Adjust the height of the head-fixing frame and the angle of the treadmill plate (~10 degrees) so that the mice can move as naturally as possible.
Keep the mice head-fixed for ~30 min per day. Repeat the procedure for > 3 days. For habituation, we looked at the following behavioral changes: 1) a reduction in urination and defecation while on the treadmill, and 2) the mice stop struggling and begin to run voluntarily.
Surgery on the day of neural recording
Place the mice in the stereotaxic frame using the headpost under isoflurane anesthesia.
Based on the markings made during the headpost surgery, perform craniotomy (~2 mm x 1 mm) using a dental drill. The opening should be large enough for the electrode array. The skull overlying the IC tends to be thick and vascular, so drill slowly, applying saline to the region periodically. Also, take care not to damage the transverse sinus, which can be included in the craniotomy. We recommend performing durotomy later right before the electrodes are lowered into the brain (Procedure D, step 3).
Cover the opening with Kwik-Cast.
Let the mice recover.
Neural recording
Position the mice on the treadmill and secure their head in the head fixing frame.
Remove Kwik-Cast over the craniotomy.
Perform durotomy using a 30 g needle and fine forceps.
Position the electrodes at target locations, set the depth to zero at surface, and lower them into the brain. To minimize the interference by line noise, we performed neural recording in a shielded walk-in sound booth, had the light source for surgery turned off during recording, and kept the electrode connector and the headstage as close as possible. Certain type of animal movements such as hunching can introduce artifacts into neural recording. Movement-related artifacts are often similar across electrodes, so when necessary, they can be digitally subtracted using a reference electrode or using one of the recording channels without spiking activity.
With sufficient habituation as mentioned in Procedure B, recording sessions can last up to ~4 h (Video 1). If the mice are not well habituated, they may struggle to pull themselves off the head-fixing frame, or they raise their tail toward the head, ending up hitting the electrodes. The treadmill plate needs to be cleaned with tissue as needed. Add saline periodically to the recording area to prevent the brain tissue from drying. We did not provide water during recording, but this may be helpful.
At the end of the recording, make marking lesions at the desired electrodes (30 μA for 10 s).
Video 1. Example video recording of a neural recording session
Perfusion and histology
Perform perfusion and histological processing for Nissl staining as needed according to the standard procedures (see for example, https://doi.org/10.1038/protex.2015.022).
Data analysis
The sensor output of the treadmill alternates between low and high voltage values, which can be recorded as through a digital input channel. To convert this into speed, count the ticks in millisecond time bins and the resulting histogram can be smoothed (we used a 200-ms wide hanning window in MATLAB). In our device, one tick corresponded to 1/240th of a rotation, yielding 2π x (radius of ~10 cm)/240 ~0.26 cm. If needed, running periods can be defined by thresholding the speed (we used 2 cm/s) (Figure 4).
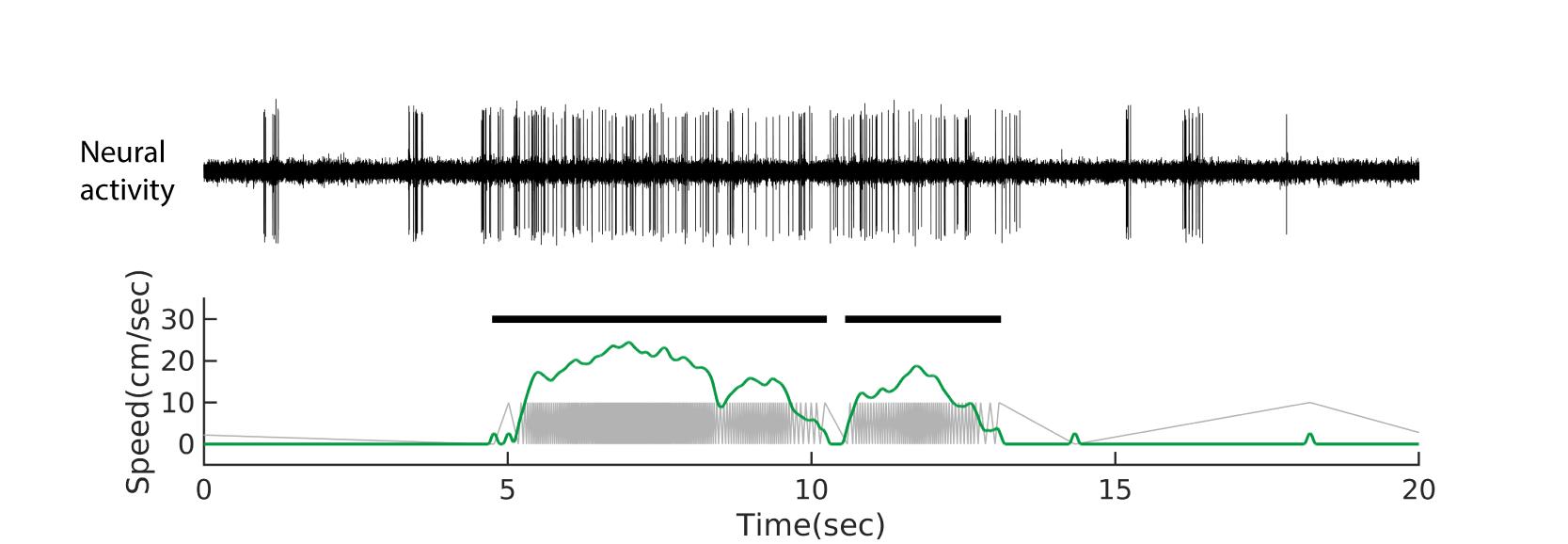
Figure 4. Example neural recording and running speed plot. Example spiking activity of an IC neuron (top) and the corresponding speed (bottom, green) are shown. In the bottom plot, treadmill sensor signal (grey) is also shown. The horizontal bars in black indicate running periods determined based on the speed. Adapted from Yang et al., 2020 .
Acknowledgements
This work was supported by the Institute for Basic Science in Korea (IBS-R015-D1) and Korea Brain Research Institute basic research program (20200082). This protocol has been adapted from Yang et al. (2020) . We thank Dr. Oliver James for helpful suggestions on earlier versions of the manuscript.
Competing interests
The authors declare no competing financial interests.
Ethics
All experiments performed were approved by the Institutional Animal Care and Use Committee of Sungkyunkwan University in accordance with the National Institutes of Health guidelines (protocol approval ID: SKKUIACUC2020-02-01-1, validity period: 4/22/2020-1/14/2021).
References
- Ayala, Y. A., Perez-Gonzalez, D., Duque, D., Palmer, A. R. and Malmierca, M. S. (2016). Extracellular Recording of Neuronal Activity Combined with Microiontophoretic Application of Neuroactive Substances in Awake Mice. J Vis Exp (111): e53914.
- Chen, C. and Song, S. (2019). Differential cell-type dependent brain state modulations of sensory representations in the non-lemniscal mouse inferior colliculus. Commun Biol 2: 356.
- Goyer, D., Silveira, M. A., George, A. P., Beebe, N. L., Edelbrock, R. M., Malinski, P. T., Schofield, B. R. and Roberts, M. T. (2019). A novel class of inferior colliculus principal neurons labeled in vasoactive intestinal peptide-Cre mice. Elife 8: e43770.
- Gruters, K. G. and Groh, J. M. (2012). Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus. Front Neural Circuits 6: 96.
- Hoyt, J. M., Perkel, D. J. and Portfors, C. V. (2019). Dopamine Acts via D2-Like Receptors to Modulate Auditory Responses in the Inferior Colliculus. eNeuro 6(5): ENEURO.0350-19.2019.
- Muniak, M. A., Mayko, Z. M., Ryugo, D. K. and Portfors, C. V. (2012). Preparation of an awake mouse for recording neural responses and injecting tracers. J Vis Exp (64): e3755.
- Ono, M., Bishop, D. C. and Oliver, D. L. (2017). Identified GABAergic and Glutamatergic Neurons in the Mouse Inferior Colliculus Share Similar Response Properties. J Neurosci 37(37): 8952-8964.
- Silveira, M. A., Anair, J. D., Beebe, N. L., Mirjalili, P., Schofield, B. R. and Roberts, M. T. (2020). Neuropeptide Y Expression Defines a Novel Class of GABAergic Projection Neuron in the Inferior Colliculus. J Neurosci 40(24): 4685-4699.
- Winer, J. A. and Schreiner, C. E. (2005). The Inferior Colliculus. New York, Springer.
- Wong, A. B. and Borst, J. G. G. (2019). Tonotopic and non-auditory organization of the mouse dorsal inferior colliculus revealed by two-photon imaging. Elife 8: e49091.
- Yang, Y., Lee, J. and Kim, G. (2020). Integration of locomotion and auditory signals in the mouse inferior colliculus. Elife 9: e52228.
Article Information
Copyright
Yang and Kim. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yang, Y. and Kim, G. (2020). Headpost Surgery for in vivo Electrophysiological Recording in the Mouse Inferior Colliculus during Locomotion. Bio-protocol 10(23): e3840. DOI: 10.21769/BioProtoc.3840.
- Yang, Y., Lee, J. and Kim, G. (2020). Integration of locomotion and auditory signals in the mouse inferior colliculus. Elife 9: e52228.
Category
Neuroscience > Sensory and motor systems > Auditory system
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









