- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A 3D Skin Melanoma Spheroid-Based Model to Assess Tumor-Immune Cell Interactions
Published: Vol 10, Iss 23, Dec 5, 2020 DOI: 10.21769/BioProtoc.3839 Views: 4583
Reviewed by: Lokesh KalekarRon Saar DoverPorkodi PanneerselvamAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Macrophage Polarization by Tumor-induced MDSCs Assay
Felipe Vences-Catalán [...] Shoshana Levy
Aug 20, 2016 14259 Views

Adoptive Transfer of Monocytes Sorted from Bone Marrow
Damya Laoui [...] Jo A Van Ginderachter
Jan 5, 2019 9068 Views

In vivo Imaging of Tumor and Immune Cell Interactions in the Lung
Richard N. Hanna [...] Catherine C. Hedrick
Oct 20, 2016 10736 Views
Abstract
Three-dimensional (3D) tumor spheroids have the potential to bridge the gap between two-dimensional (2D) monolayer tumor cell cultures and solid tumors with which they share a significant degree of similarity. However, the progression of solid tumors is often influenced by the dynamic and reciprocal interactions between tumor and immune cells. Here we present a 3D tumor spheroid-based model that might shed new light on understanding the mechanisms of tumor and immune cell interactions. The model first utilizes the hanging drop assay, which serves as one of the simplest methods for generating 3D spheroids and requires no specialized equipment. Next, pre-established spheroids can be co-cultured either directly or indirectly with an immune cell population of interest. Using skin melanoma, we provide a detailed description of the model, which might hold a significant importance for the development of successful therapeutic strategies.
Keywords: SkinBackground
Three-dimensional (3D) tumor spheroids are spherical self-assembled tumor cell aggregates, which resemble micrometastases and replicate many features of solid tumors. As in the non-proliferating regions of avascular solid tumors, tumor cells within the inner regions of spheroids usually demonstrate perturbed gene and protein expression, altered metabolism, cell cycle arrest and necrotic death (Sant and Johnston, 2017). However, most currently available techniques for generating 3D spheroids are time-consuming, difficult and expensive. A simple, fast and easy method for generating 3D spheroids is through the use of the hanging drop assay (Foty, 2011; Berens et al., 2015). Within the hanging drop, cells have no direct contact with substratum and due to gravity accumulate at the free liquid-air interface to form a single spheroid. In light of the increasingly recognized role of interactions between tumor and immune cells, pre-established spheroids can be co-cultured either directly or indirectly with different populations of immune cells, as shown in Figure 1.
Direct co-culture can be performed by culturing pre-established spheroids with immune cells in close proximity in the same culture environment, for example by layering two components one on top of the other. In contrast, indirect co-culture employs cell culture inserts with porous membranes, to keep immune cells separated from spheroids. Co-culturing pre-established spheroids with immune cells directly can provide information on cell-cell interactions based on physical contact, whereas indirect co-culture can be used to study the effects of secreted signaling molecules.
In our recent work, we generated spheroids from B16 melanoma cells and demonstrated their potential to impair proliferation of recently identified innate immune cells, group 2 innate lymphoid cells (ILC2s), through the production of lactic acid (Moro et al., 2010 and 2015; Wagner and Koyasu, 2019; Wagner et al., 2020). Here we provide a detailed workflow necessary for the generation of 3D spheroids from B16 melanoma cells that can be co-cultured with an immune cell population of interest. Using a hanging drop assay, B16 spheroids were generated over a period of 7 days, as shown in Figure 2. The optimal number of uniformly shaped spherical tumor cell aggregates with a smooth surface was obtained using cultures initiated with 10,000 cells per 25 μl. At least 24 spheroids can be produced per dish. Pre-established spheroids can be co-cultured with an immune cell population of interest to assess an impact of tumor cells on immune cell effector functions and vice versa. In order to address specific research questions, alterations to this condition including cell transfection, transduction and cell culture media modification with different therapeutic agents can be easily performed.
The possibility to reproduce the complex interactions between tumor and immune cells represents an important area of unfulfilled need towards the design of efficient therapeutic strategies. This protocol describes a simple, fast, and easy method for generating 3D spheroids that can be used to study the mechanisms of tumor and immune cell interactions.
Figure 1. Scheme displaying the principle of the hanging drop assay. Tumor cells per drop of cell culture medium are placed on the lid of a PBS containing dish and incubated for 7 days. Pre-established spheroids are collected and co-cultured directly or indirectly with an immune cell population of interest.
Materials and Reagents
75 cm2 cell culture flasks (Thermo Fisher, catalog number: 156499 )
10 cm dishes (Thermo Fisher, catalog number: 150464 )
15 ml conical tubes (Corning, catalog number: 352196 )
FalconTM permeable support for 24-well plate with 0.4 µm transparent PET membrane (Corning, catalog number: 353095 )
FalconTM 24-well TC-treated cell polystyrene permeable support companion plate (Corning, catalog number: 353504 )
Mus musculus B16F10 skin melanoma cells (ATCC, catalog number: CRL-6475 )
PBS, pH 7.4 (Gibco, catalog number: 10010023 )
0.25% Trypsin-EDTA, phenol red (Gibco, catalog number: 25200072 )
Trypan Blue solution (0.4%) (Thermo Fisher, catalog number: 15250061 )
RPMI-1640 medium with L-glutamine and sodium bicarbonate, liquid, sterile-filtered (Sigma-Aldrich, catalog number: R8758 )
Heat inactivated Fetal Bovine Serum (Japan Bioserum, catalog number: S1820-500 ) (Testing of different batches is recommended)
MEM Non-essential Amino Acid solution (100×) (Sigma-Aldrich, catalog number: M7145 )
Sodium Pyruvate (100 mM) (Gibco, catalog number: 11360070 )
HEPES solution (1 M) (Sigma-Aldrich, catalog number: H3537 )
Penicillin-Streptomycin (10,000 U/ml) (Gibco, catalog number: 15140122 )
2-Mercaptoethanol (Gibco, catalog number: 21985023 )
L-Glutamine (200 mM) (Lonza, catalog number: 17-605E )
Complete cell culture medium (see Recipes)
Equipment
Pipettes
Hemocytometer
Incubator (37 °C, 5% CO2)
Water bath (37 °C)
Refrigerated benchtop centrifuge for spinning conical tubes
Inverted microscope
Procedure
Single Cell Suspension Preparation
Plate early (3-4) passage of B16F10 cells in a 75 cm2 cell culture flask in complete cell culture medium. Incubate at 37 °C until cells reach 80-90% confluency.
Remove and discard cell culture medium.
Briefly rinse the monolayer with PBS to remove all traces of serum since it contains the trypsin inhibitor.
Detach adherent cells from the surface of a cell culture flask by adding 1.0 to 2.0 ml of 0.25% Trypsin-EDTA solution to the flask. Incubate at 37 °C until the monolayer is dispersed (usually within 5-10 min). Monitor cells under an inverted microscope.
As soon as cells have detached, neutralize the Trypsin-EDTA solution by adding 8.0 to 9.0 ml of complete cell culture medium (shown below). Gently pipette up and down the mixture until cells are in suspension.
After spinning down, count cells using a hemocytometer and perform a dilution to allow for the seeding of 10,000 cells per 25 μl of complete cell culture medium.
Generation of Spheroids
Remove the lid from the 10 cm dish and carefully deposit 25 μl drops on the inner side of the lid.
Fill the bottom of the dish with 10 ml PBS to humidify the culture chamber after distribution of the drops.
Flip the lid in a confident, yet controlled manner. Cells under the force of gravity will be aggregated at the bottom of the hanging drop. Make sure to place the drops sufficiently apart from each other and not too close to the dish edges.
Carefully place the dish in the incubator and preferably avoid touching or moving for the first 2 days.
Change cell culture medium on the 3rd and 6th day in each drop. To this end, carefully remove the lid from the dish and replace approximately 10 μl of used media with 10-15 μl of fresh media in each drop, in order to maintain constant osmolality and nutrient supply. Replace the medium from the edge of the drop. Avoid touching spheroids. Flip the lid in a confident, yet controlled manner.
Monitor the drops under an inverted microscope. Pay attention not to disturb the droplets while moving the dish.
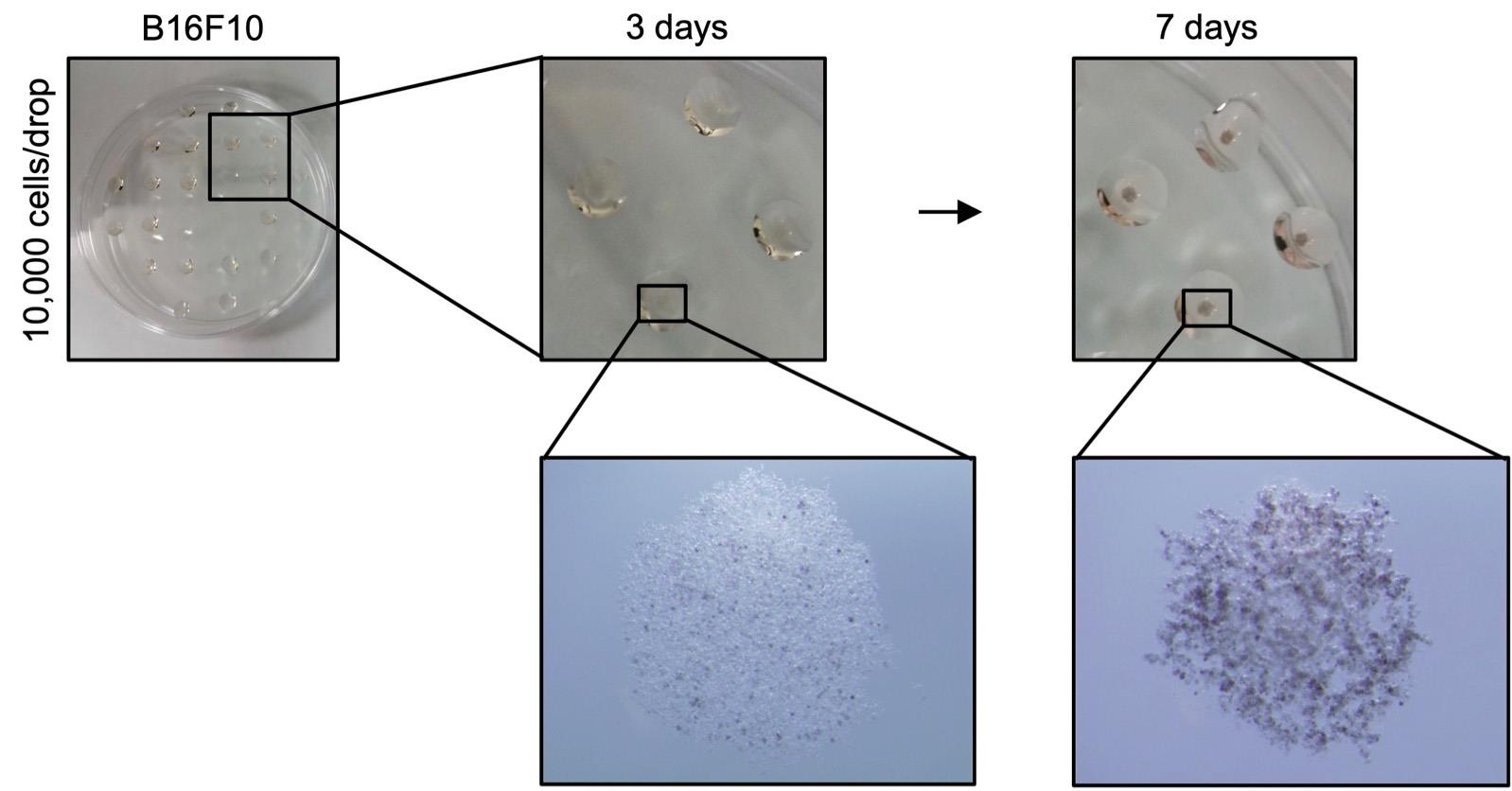
Figure 2. Representative photographs of skin melanoma spheroids. B16F10 melanoma cells (10,000 cells per 25 ul drop of cell culture medium) placed on the lid of a PBS containing dish and incubated for 3 and 7 days. Objective magnification: 3.2x (lower panel).
Co-cultures with Immune Cells
Collect spheroids by the wide end of the tip (100 or 1,000 μl pipette tip cut widely 5-6 mm from the end) and transfer to an appropriate cell culture plate.
Co-culture spheroids with immune cells in complete cell culture medium by either layering two components one on top of the other in the same well of the cell culture plate or by employing cell culture inserts with porous membranes, to keep immune cells separated from spheroids.
Notes
Antibiotics (and antimycotics) may be added to PBS in order to minimize bacterial (and fungal) contamination during the period of spheroid generation.
Always prepare a surplus number of spheroids in case some of the drops detach from the lid or slide on the surface of the lid during the change of the medium process.
The longer time of incubation is possible, however the cell viability should be assessed. We have observed around 90% cell viability following 7 days of incubation.
For the use of other types of cancer cells, the number of cells and the volume of the drops should be adjusted.
Recipes
Complete cell culture medium
RPMI-1640 medium
10% Fetal Calf Serum
100 μM MEM Non-essential Amino Acid
10 mM HEPES
100 U/ml Penicillin-Streptomycin
1 mM Sodium Pyruvate
50 μM 2-mercaptoethanol
400 μM L-Glutamine
Acknowledgments
This work was supported by the FRIPRO Mobility Grant Fellowship from the Research Council of Norway (302241) to M.W., and by a Grant-in-Aid for Scientific Research (A) (20H00511) from the Japan Society for the Promotion of Science, to S.K. This protocol was adapted from the original research paper published in Wagner et al. (2020).
Competing interests
The authors declare no competing interests.
References
- Berens, E. B., Holy, J. M., Riegel, A. T. and Wellstein, A. (2015). A cancer cell spheroid assay to assess invasion in a 3D setting. J Vis Exp (105): 53409.
- Foty, R. (2011). A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp (51): 2720.
- Moro, K., Yamada, T., Tanabe, M., Takeuchi, T., Ikawa, T., Kawamoto, H., Furusawa, J., Ohtani, M., Fujii, H. and Koyasu, S. (2010). Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463(7280): 540-544.
- Moro, K., Ealey, K. N., Kabata, H. and Koyasu, S. (2015). Isolation and analysis of group 2 innate lymphoid cells in mice. Nat Protoc 10(5): 792-806.
- Sant, S. and Johnston, P. A. (2017). The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol 23: 27-36.
- Wagner, M., Ealey, K. N., Tetsu, H., Kiniwa, T., Motomura, Y., Moro, K. and Koyasu, S. (2020). Tumor-derived lactic acid contributes to the paucity of intratumoral ILC2s. Cell Rep 30(8): 2743-2757 e2745.
- Wagner, M. and Koyasu, S. (2019). Cancer immunoediting by innate lymphoid cells. Trends Immunol 40(5): 415-430.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wagner, M. and Koyasu, S. (2020). A 3D Skin Melanoma Spheroid-Based Model to Assess Tumor-Immune Cell Interactions. Bio-protocol 10(23): e3839. DOI: 10.21769/BioProtoc.3839.
Category
Cancer Biology > Tumor immunology > Tumor microenvironment > Immunosuppression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










