- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Agrobacterium-mediated Transformation of Sweet Basil (Ocimum basilicum)
Published: Vol 10, Iss 22, Nov 20, 2020 DOI: 10.21769/BioProtoc.3828 Views: 5695
Reviewed by: Sriema L. WalawageAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Agrobacterium-mediated Transformation of Japonica Rice Using Mature Embryos and Regenerated Transgenic Plants
Ammar Elakhdar [...] Takahiko Kubo
Sep 20, 2021 6215 Views

Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Alka Srivastava [...] Praveen C. Verma
May 20, 2023 4333 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1968 Views
Abstract
Sweet basil (Ocimum basilicum) is a popular herb with high economic value and is currently threatened by a severe oomycete disease. An efficient transformation method is a prerequisite for gene functional analysis to accelerate molecular breeding and deploy effective disease management strategies, and breeding through genetic engineering. Here we present a detailed protocol for a highly efficient Agrobacterium tumefaciens-mediated transformation method for sweet basil, which was established based on a previously reported method by other researchers, with modifications on several aspects, including growth of sweet basil, age of plants used for explants, preparation and concentration of Agrobacteria. This protocol allows researchers in academia and agroindustry to generate transgenic sweet basil plants in an easy, quick and highly reproducible manner. In addition, this protocol may be applicable to transform other species within the genus Ocimum.
Keywords: Sweet basilBackground
Sweet basil (Ocimum basilicum) is a popular herb, holding high economic value for its significant medicinal, culinary and cosmetic properties. Its global production is hampered by severe downy mildew disease caused by the oomycete pathogen Peronospora belbahrii. Traditional breeding involving interspecific hybridization in sweet basil has been a hassle with F1 sterility and difficulty in segregating out undesirable traits. On the other hand, genetic engineering of sweet basil offers a platform to study molecular biology, broaden sources of desirable traits and accelerate breeding process. A highly efficient CRISPR/Cas9-mediated gene editing system for targeted mutagenesis of ObDMR1 gene was established for sweet basil using Agrobacterium-mediated stable transformation (Navet and Tian, 2020). Two independent constructs were used for transformation to express gene-editing reagents. With NPTII gene as the selectable marker, we obtained 34 and 48 kanamycin-resistant regenerated plants from 176 and 238 explants, yielding a transformation efficiency of 19.3% and 20.2% respectively. All kanamycin-resistant plants were tested positive for transgene integration. The transformation method we described (Navet and Tian, 2020) differs from the previous Agrobacterium-mediated transformation of sweet basil established by Deschamps and Simon (2002), which utilized the regeneration protocol by Phippen and Simon (2000), in certain aspects: mainly, growth of sweet basil, age of plants selected for explants, preparation of Agrobacteria, and Agrobacterium concentration used to infect explants. We established the protocol using sweet basil cultivar Genoveser. To shorten and simplify the process we used leaf discs from small young leaves (contrary to fully expanded leaves) as explants and a simple method to prepare Agrobacteria. Our lab has been successful in transforming sweet basil with diverse plant binary vectors following this method. Here we provide a detailed protocol for this convenient and reliable transformation method through which transgenic sweet basil plants with stable inheritance of the transgene can be quickly generated. This protocol is expected to enable researchers in academia and agroindustry to meet their needs in basil functional genomics and breeding for desirable traits.
Materials and Reagents
Biological Materials
Seeds of sweet basil cultivar Genoveser (Enza Zaden)
Agrobacterium tumefaciens strain EHA105 harboring binary vector pKSE401 derived plasmids, which contain NPTII as a selectable marker gene
Chemicals
Kanamycin sulfate (VWR International, catalog number: 89149-900 )
Rifampicin (Santa Cruz, catalog number: sc-200910 )
Luria-Bertani (LB) Broth, Miller (Fisher Scientific, catalog number: BP1426-2 )
Agar (VWR International, catalog number: AAA10752-36 )
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
3′,5′-Dimethoxy-4′-hydroxyacetophenone (Acetosyringone) (Sigma-Aldrich, catalog number: D134406 )
Murashige and Skoog (MS) basal medium (Sigma-Aldrich, catalog number: M5519 )
Sucrose (Sigma-Aldrich, catalog number: S7903 )
Phytoagar (PlantMedia, catalog number: 40100072-1 )
Thidiazuron (TDZ) (PlantMedia, catalog number: 30631018-2 )
1-Naphthaleneacetic acid (NAA) (PlantMedia, catalog number: 30631016-1 )
Timentin (PlantMedia, catalog number: 42010012-3 )
Clorox Regular Bleach Disinfectant with Cloromax (Sodium hypochlorite 6%) (Clorox)
MilliQ water, generated using Synergy Water Purification System (EMD Millipore)
Glassware, plasticware and other consumables
Sterile Petri dishes for bacterial growth (100 x 15 mm, BD Falcon, catalog number: 351029 )
1.5 ml micro-centrifuge tubes
Spectrophotometer cuvettes
Syringe Filter (0.2 µm) (VWR International, catalog number: 28145-477 )
Sterile pipette tips (20, 200, 1,000 µl)
Sterile 15 ml and 50 ml conical tubes (VWR International, catalog numbers: 82050-276 and 82050-346 )
Glass media bottles
Cork borer (Number #2)
Sterile paper towel (Scott® C-Fold Towels) (VWR International, catalog number: 10815-570 )
Sterile forceps and scalpel (sterilized by autoclaving)
Cell culture dishes (100 x 20 mm, Greiner Bio-One, catalog number: 664160-TRI )
Plant culture containers with lid (190 ml, Greiner Bio-One, catalog number: 967161 )
3MTM MicroporeTM Surgical Tape (3M Healthcare, catalog number: 1530-0 )
Aluminium foil
3.5” square plant pots
Soil Mix (e.g., SunGro Horticulture Sunshine Mix #4)
Plastic flats (1020 Trays, Heavy Duty) and dome (7” Vented Humidity Dome for 1020 Tray) for plant growth (Greenhouse Megastore, catalog numbers: CN-FLHD and PR-DOME7 )
Media and solutions
Stock solutions of antibiotics, plant growth regulators and other solutions
Kanamycin (50 mg/ml) (see Recipes)
Rifampicin (25 mg/ml) (see Recipes)
Timentin (200 mg/ml) (see Recipes)
TDZ (1 mg/ml) (see Recipes)
NAA (0.5 mg/ml) (see Recipes)
Acetosyringone (AS) (0.2 M) (see Recipes)
12% (v/v) Clorox Regular Bleach Disinfectant solution (see Recipes)
Media for Agrobacterium
LB agar (see Recipes)
Agrobacterium Inoculation media (IN) (see Recipes)
Media for sweet basil regeneration
Callus and shoot induction (SI) media (see Recipes)
Root induction (RI) media (see Recipes)
Equipment
-80 °C freezer
Autoclave (any model that allows a temperature of 121 °C)
Plant growth chamber (any model with temperature and light control)
Incubator shaker (e.g., New Brunswick Scientific, model: Excella E25 )
pH meter (e.g., ThermoFisher, Orion Star A111 )
Laminar flow hood (e.g., LABCONCO, model: 36125 )
Pipetman® 20, 200,1000 µl (e.g., RANIN, Pipet-Lite XLS )
Spectrophotometer (e.g., Eppendorf, 6131 23550 )
Orbital shaker (e.g., Heidolph Rotamax 120 Orbital Platform Shaker)
Plant tissue culture room with controlled light and temperature
Procedure
Plant Growth
Plant sweet basil seeds in square pots filled with Sunshine Mix 4 and allow it to grow for 3 weeks in a plant growth chamber at 25 °C with a photoperiod of 12 h.
Plants used for transformation are at two-true leaf stage.
The first two true leaves, with a length usually ranging from 1.5-2.0 cm, are used to excise explants for stable transformation.
Agrobacterium preparation
Note: Perform under sterile conditions in a laminar flow hood.
Three days prior to transformation, streak Agrobacterium tumefaciens EHA105 carrying the desired plasmid, which is stored at -80 °C, on a LB agar plate with appropriate antibiotics. We used the plasmids derived from pKSE401 vector (Xing et al., 2014) for targeted mutagenesis of sweet basil ObDMR1 using CRISPR/Cas9 (Navet and Tian, 2020). The LB agar plates in our experiments are supplemented with 15 μg/ml rifampicin (for selection of A. tumefaciens EHA105) and 50 μg/ml kanamycin (for selection of the plasmid). Incubate the plate at 28 °C for two days.
Note: This plate can be stored at 4 °C and used within one week.The day before transformation, scoop bacteria from the above plate using the bottom of a sterile 1.5 ml micro-centrifuge tube and spread on a new LB agar plate with appropriate antibiotics (containing kanamycin and rifampicin in our study). Incubate the plate overnight at 28 °C.
On the day of transformation, scrape a portion of bacterial cells with a sterile 1 ml pipette tip and resuspend in 1 ml Agrobacterium Inoculation media (IN) by pipetting the solution up and down until a homogeneous suspension is observed. Transfer the suspension to a 15 ml tube containing 5 ml IN media, and mix well.
Note: Cover this tube with aluminium foil to avoid light as IN media contains light-sensitive acetosyringone.
Measure the OD600 of the above bacterial suspension using a spectrophotometer with IN media as blank, and adjust the final OD600 to ~0.6, a concentration used for inoculating explants. Approximately 20 ml of Agrobacterium suspension is needed for 100-150 explants. Incubate the suspension in a sterile 50 ml conical tube at room temperature (RT) on an orbital shaker at 70 rpm for 2 h in dark.
Note: Loosen the lid of the 50 ml conical tube to allow aeration and then cover the tube with aluminium foil.
Preparation of explants
Note: Perform Steps C2, C3 and C4 in sterile conditions in a laminar flow hood.
Prepare the explants while the incubation of Agrobacterium suspension is taking place. From three-week-old sweet basil plants pluck the first pair of true leaves, and surface sterilize the leaves for 5 min by immersing them in 12% (v/v) Clorox Regular Bleach Disinfectant solution in a 500 ml media bottle. This step should be done gently to avoid leaf damage. For each transformation, we usually handle ~100 leaves.
Immediately after surface sterilization, wash the leaves in sterile MilliQ water for 3 min by gently inverting the bottle several times. Repeat this washing step three more times. A total of four washes are required to remove any trace of bleach sticking to the leaves.
Transfer the leaves onto a sterile paper towel. Excise two round leaf discs from each leaf from regions close to leaf base along the midrib using a sterile #2 cork borer, as shown in Figure 1.
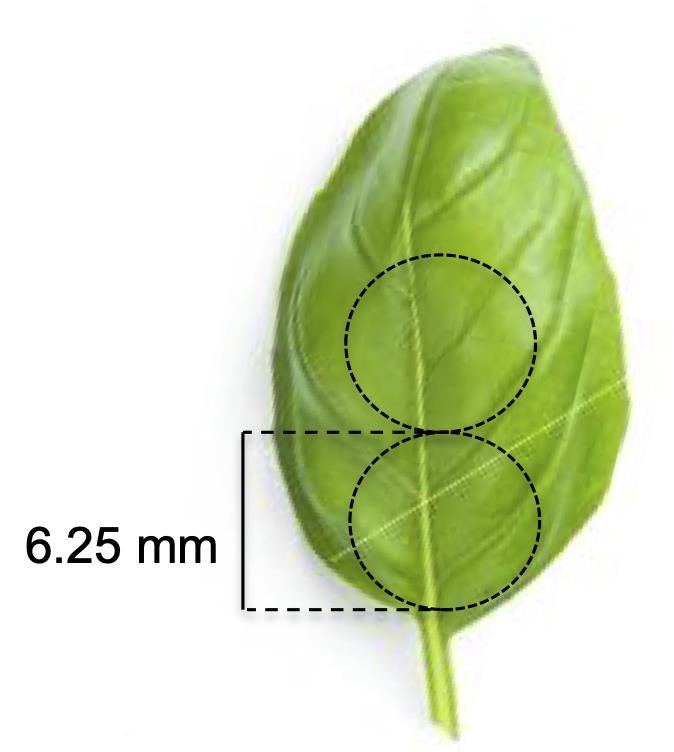
Figure 1. Excision of two explants (leaf discs of 6.25 mm diameter) from a surface-sterilized true sweet basil leafAfter excising the explants from each leaf, immediately place them in a sterile Petri dish filled with sterile MilliQ water as shown in Figure 2. This keeps the leaf discs fresh until explants are extracted from all leaves.

Figure 2. Explants (leaf-discs) floating in sterile MillQ water
Inoculation of explants with Agrobacterium and their co-cultivation
Note: Perform under sterile conditions.
After the completion of Agrobacterium incubation and explants preparation, immerse all the explants in the Agrobacterium suspension for 30 min (Approximately 20 ml of Agrobacterium suspension is needed for 100-150 explants), as shown in Figure 3. Keep the tube away from light by covering it with aluminium foil. Gently invert the tube 3-4 times every 10 min to ensure that all explants get submerged in the suspension.
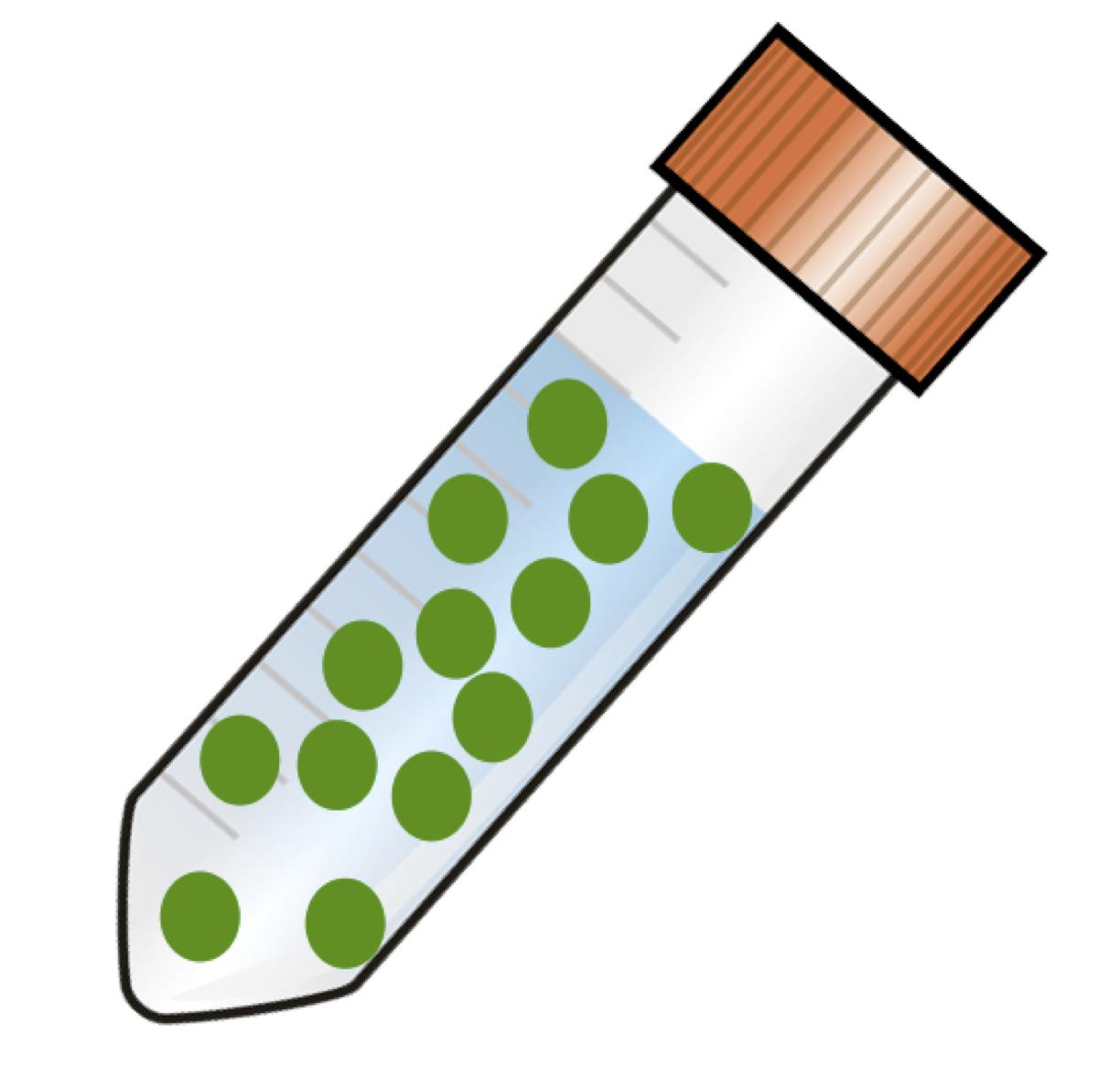
Figure 3. Diagrammatic representation showing leaf discs immersed in Agrobacterium suspensionBlot dry the leaf discs by gently pressing them between two layers of sterile paper towel to remove excess suspension.
Co-cultivation: Transfer the Agrobacterium-inoculated explants using sterile forceps onto callus and shoot induction (SI) media plates supplemented with 200 µl AS with abaxial side touching the media (20 explants per Petri dish) (Figure 4A). Seal the plates with MicroporeTM surgical tape and wrap with aluminium foil. Incubate the plates for three days in plant tissue culture room at 25 °C under dark conditions.
Regeneration of transgenic plants
Transfer the Agrobacterium-infected explants to SI media plates supplemented with 200 μg/ml timentin (to inhibit the growth of Agrobacterium tumefaciens EHA105) and 30 μg/ml kanamycin for induction and selection of transgenic calli. Seal the plates with MicroporeTM surgical tape tape, wrap with aluminium foil and incubate the plates at 25 °C under dark conditions for 4-6 weeks with regular sub-culturing onto fresh media every two weeks.
During this process, we observe several key regeneration stages, including: swelling-up of leaf discs in irregular fashion within 1 week (Figure 4B), callus formation in another 1-2 weeks (Figure 4C), followed by the sprouting of tiny shoots usually as pale/yellow-colored (since plates are incubated under dark conditions) (Figure 4D) and occasionally green-colored (Figure 4E). A regular check on plates every week is recommended to observe callus/shoot formation. Do not allow the calli/shoots to turn dark (brown/black) and sub-culture onto fresh media if and when required. While sub-culturing clean the calli/shoots by removing any dead/decaying (brown/black) tissues and then place on fresh media.
Note: Shoots may emerge inside the media as well, so flip the side when performing fresh sub-culturing to allow proper aeration.
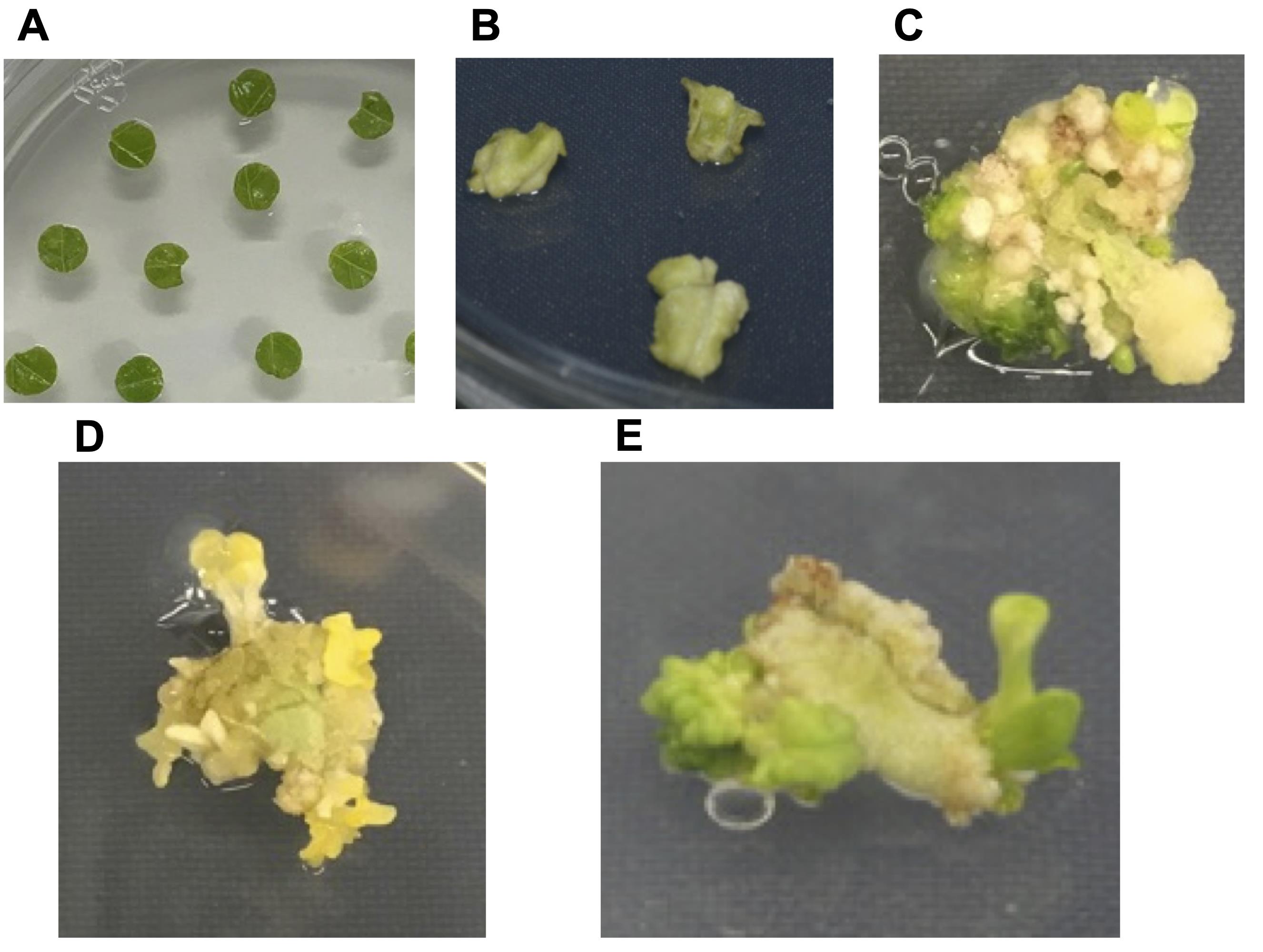
Figure 4. Regeneration stages on callus and shoot induction (SI) media. A. Co-cultivation of leaf explants with Agrobacteria on SI media containing acetosyringone. B. Swelling-up of leaf-discs after 1 week on SI media supplemented with timentin and kanamycin. C. Callus formation after 2 weeks. D. Pale/yellow-colored shoots emerging from growing calli. E. Green-colored shoots.Once the shoots develop on calli, cut individual shoots (length approximately 0.5 cm and above) with a sterile scalpel and transfer to plant culture containers containing root induction (RI) media supplemented with 200 μg/ml timentin and 30 μg/ml kanamycin (Figure 5A). Seal the containers with MicroporeTM surgical tape, cover with aluminium foil and incubate the containers for 1 week in dark at 25 °C.
Note: Do not overcrowd the container with young shoots; allow half-centimeter spacing while placing the shoots.After 1 week, remove the foil and incubate the containers under light with a 12-hour photoperiod at 25 °C for 4-8 weeks with regular sub-culturing every two weeks onto fresh RI media supplemented with timentin and kanamycin. During sub-culturing to fresh media, make sure to remove any dead or decaying tissue, and only transfer the well-differentiated green shoot stalks (Figure 5B). Transfer each well-differentiated shoot stalk separately into a tissue culture container to allow root establishment and full development of the transgenic plant (Figures 5C-5D). It usually takes 2-3 weeks after providing light conditions for a shoot stalk to develop into a transgenic plant with robust roots. At this stage (Figures 5C-5D), leaf tissue can be excised from the plants growing in the container to test for transgene integration by PCR.
Notes:
Keep a close observation on containers and perform sub-culturing when required;
Discard shoots that do not show signs of root formation within one month.

Figure 5. Shoot and root development on root induction (RI) media. A. Shoots with emerging stalks transferred from SI to RI media for further development. B. Developing shoots on RI media after 2 weeks under light. C-D. Plantlet with well-established shoot (C) and root (D) system.
Acclimatization
Remove the transgenic plants with well-developed shoot & root system from media container and thoroughly wash the roots with sterile MilliQ water to remove residue media.
Plant them in moistened soil mix (Figure 6A) and grow under 100% relative humidity for 3-4 days in a tray covered with a plastic dome in a growth chamber set at 25 °C with a 12-hour photoperiod.
Adapt the plants to ex vitro conditions by gradually reducing the humidity over the next 2-3 days through partial opening of the plastic dome, and finally removing the plastic dome.
Once the plant attains a certain height (12.0-15.0 cm), repot the plants in larger pots and transfer them to a greenhouse at 25-30 °C under natural light to propagate seeds (Figure 6B).
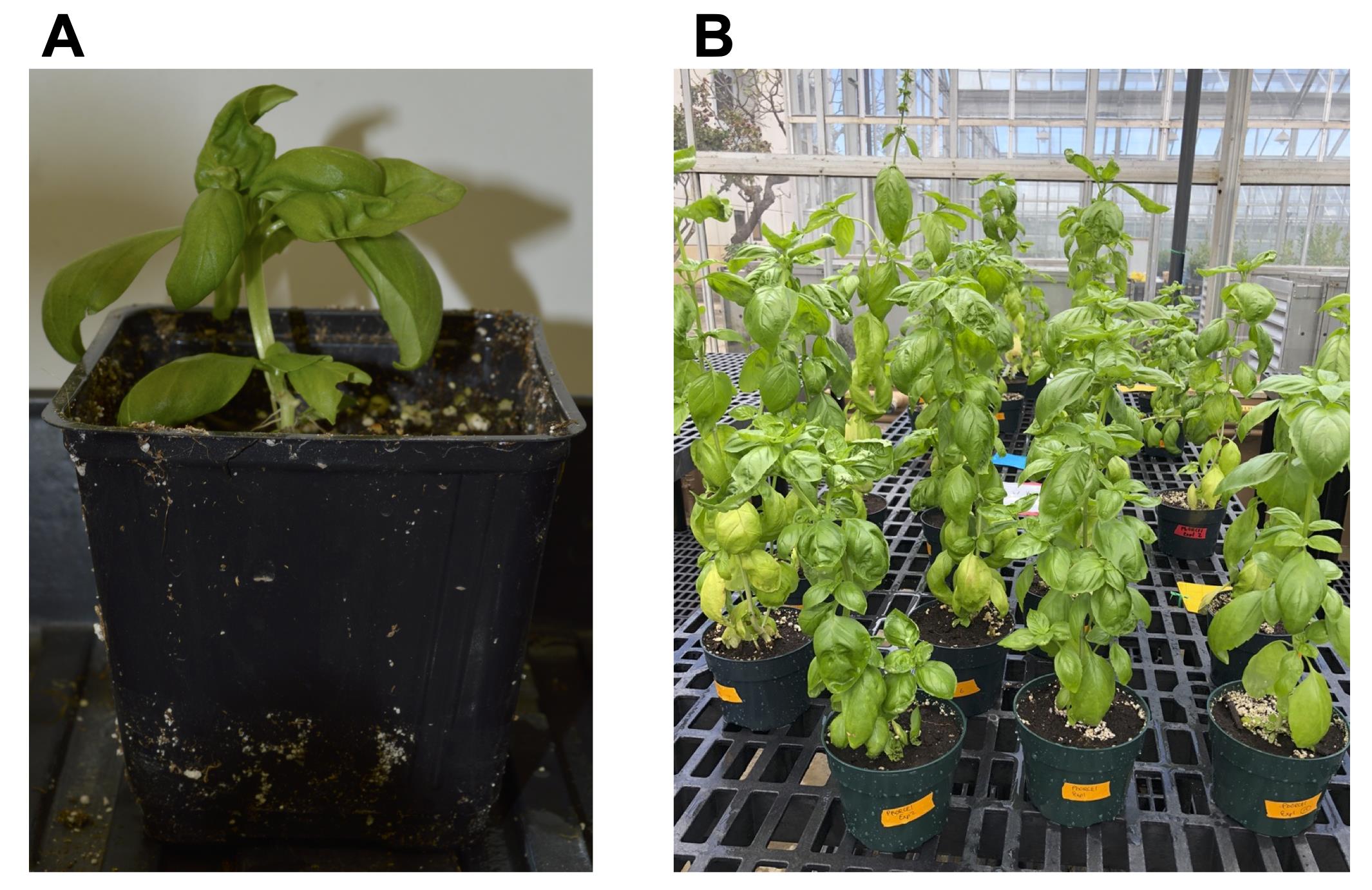
Figure 6. Acclimatization of regenerated transgenic plant in soil (A) and continued growth of transgenic plants in greenhouse for seed production (B)
Notes
Do not use older sweet basil leaves for transformation (as it decreases the efficiency).
Use freshly prepared acetosyringone (AS) solution in order to increase efficiency of transformation.
Limit the exposure of solution/media containing AS to light as much as possible.
Handle plucked leaves gently while surface sterilizing and washing since vigorous shaking may damage leaf.
Cleaning of calli and young shoots by removing dark/decaying tissues should be performed during regular sub-culturing.
Do not overcrowd tissue culture Petri dishes or containers with developing tissues as it may limit the nutrient supply for organ differentiation and growth.
Check the tissue culture plates/containers regularly for fungal and bacterial contamination, especially at 2 days after sub-culturing to new media. Once contamination is found, rescue the non-contaminated tissues by removing the fungal/bacterial colony (if still very small) carefully, or transferring the clean tissues to new media right away.
Recipes
Kanamycin (50 mg/ml)
Dissolve 500 mg kanamycin sulfate in 10 ml MilliQ water
Filter sterilize using a 0.2 µm syringe filter
Store at -20 °C in 1 ml aliquots
Rifampicin (25 mg/ml)
Dissolve 250 mg rifampicin in 10 ml DMSO
Store at -20 °C in 1 ml aliquots wrapped in aluminium foil to protect from light
Timentin (200 mg/ml)
Dissolve 2 g timentin in 10 ml MilliQ water
Filter sterilize using a 0.2 µm syringe filter
Store at -20 °C in 1 ml aliquots
Thidiazuron (TDZ) (1 mg/ml)
Dissolve 50 mg of TDZ in 1 ml 1 N NaOH and gradually dilute with sterile MilliQ water to make a final volume of 50 ml
Store at 4 °C and is good to use for several months
NAA (0.5 mg/ml)
Dissolve 25 mg NAA in 1 ml 1 N NaOH. Bring the volume to 50 ml using sterile MilliQ water by constantly stirring the solution
Store at 4 °C and is good to use for several months
Acetosyringone (AS) (0.2 M)
Dissolve 40 mg acetosyringone in 1 ml DMSO by vortexing to dissolve it completely
Wrap the tube in aluminium foil; Make fresh on the day of the experiment
12% (v/v) Clorox Regular Bleach Disinfectant solution (300 ml)
Clorox Regular bleach 36 ml
MilliQ water 264 ml
Mix well
LB agar supplemented with kanamycin (50 µg/ml) and rifampicin (15 µg/ml), 250 ml
LB Broth, Miller 6.25 g
Agar 3.75 g
Make up the volume to 250 ml using MilliQ water
Autoclave and once media cools to 50 °C, add 250 μl kanamycin (50 mg/ml) and 150 µl lrifampicin (25 mg/ml), swirl the media to mix well and pour in 100 x 15 mm Petri dishes
Store the plates at 4 °C and use within one month
Agrobacterium Inoculation media (IN) [4.3 g/L Murashige and Skoog basal medium (MS), 3% sucrose, 16.8 μM thidiazuron (TDZ), pH 5.7, supplemented with 200 μM acetosyringone (AS)], 100 ml
MS basal medium 0.43 g
Sucrose 3 g
TDZ (1 mg/ml) 400 µl
Dissolve above in 80 ml MilliQ water, adjust pH to 5.7 with 0.5 N NaOH, and make up the volume to 100 ml using MilliQ water
Autoclave, add 100 μl AS (0.2 M) when it cools down to room temperature, cover with aluminium foil to avoid light.
Always make fresh on the day of the experiment
Callus and shoot induction (SI) media (4.3 g/L MS, 3% sucrose, 0.8% phytoagar, 16.8 μM TDZ, pH 5.7) supplemented with 200 μM AS, 500 ml
MS basal medium 2.15 g
Sucrose 15 g
TDZ (1 mg/ml) 2 ml
Dissolve above in 400 ml MilliQ water, adjust pH to 5.7 with 0.5 N NaOH, and make up the volume to 500 ml using MilliQ water
Add 4 g Phytoagar
Autoclave, add 500 μl AS (0.2 M) when it cools down to about 50 °C, mix well and pour in cell culture dishes/Petri-plates, cover with foil to avoid light
Always make fresh on the day of the experiment
Callus and shoot induction (SI) media supplemented with timentin (200 µg/ml) and kanamycin (30 µg/ml), 500 ml
Prepare SI media as above (9)
After autoclaving when the media cools down to 50 °C, add 500 µl timentin (200 mg/ml) and 300 µl kanamycin (50 mg/ml), swirl the media and pour in cell culture dishes
Media plates can be stored at 4 °C for two weeks
Root induction (RI) media [4.3 g/L MS, 3% sucrose, 0.8% phytoagar, 0.054 μM NAA, pH 5.7] supplemented with timentin (200 µg/ml) and kanamycin (30 µg/ml), 500 ml
MS 2.15 g
Sucrose (3%) 15 g
NAA (0.5 mg/ml) 10 μl
Dissolve above in 400 ml MilliQ water, adjust pH to 5.7 with 0.5 N NaOH, and make up volume to 500 ml using MilliQ water
Add phytoagar 4 g
Autoclave, once media cools down to 50 °C, add 500 µl timentin (200 mg/ml) and 300 µl kanamycin (50 mg/ml), swirl the media and pour in plant tissue culture containers
Media containers can be stored at 4 °C for two weeks
Acknowledgments
This protocol was derived from the original research paper by Navet and Tian (2020). It was modified based on the methods developed for sweet basil regeneration described by Phippen and Simon (2000) and Agrobacterium-mediated transformation of sweet basil previously reported by Deschamps and Simon (2002). This work was supported by NIFA HATCH (Accession No. 1003536 and 1020611) and the USDA-ARS Agreement No. 58~5320~4~020, entitled, “Control of Pests and Diseases and Adding Value to Specialty Crops”, managed by the College of Tropical Agriculture and Human Resource, University of Hawaii at Manoa.
Competing interests
The authors declare no conflict of interest.
References
- Deschamps, C. and Simon, J. (2002). Agrobacterium tumefaciens-mediated transformation of Ocimum basilicum and. O. citriodorum. Plant Cell Rep 21: 359-364.
- Navet, N. and Tian, M. (2020). Efficient targeted mutagenesis in allotetraploid sweet basil by CRISPR/Cas. Plant Direct 4(6): e00233.
- Phippen, W. B. and Simon, J. E. (2000). Shoot regeneration of young leaf explants from basil (Ocimum basilicum L.). In Vitro Cell Dev-PL 36(4): 250-254.
- Xing, H. L., Dong, L., Wang, Z. P., Zhang, H. Y., Han, C. Y., Liu, B., Wang, X. C. and Chen, Q. J. (2014). A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14: 327.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Navet, N. and Tian, M. (2020). Agrobacterium-mediated Transformation of Sweet Basil (Ocimum basilicum). Bio-protocol 10(22): e3828. DOI: 10.21769/BioProtoc.3828.
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > Transformation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








