- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel and Robust Single-cell Trapping Method on Digital Microfluidics
Published: Vol 10, Iss 19, Oct 5, 2020 DOI: 10.21769/BioProtoc.3769 Views: 4060
Reviewed by: Alessandro DidonnaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Multiple Labeling of Compartmentalized Cortical Neurons in Microfluidic Chambers
Guillermo Moya-Alvarado [...] Francisca C. Bronfman
Jan 5, 2024 2701 Views

Microfluidic Cultures of Basal Forebrain Cholinergic Neurons for Assessing Retrograde Cell Death by Live Imaging
Srestha Dasgupta [...] Wilma J. Friedman
Jan 5, 2025 1880 Views

A Model of Breast Cancer Micrometastasis in a Three-Dimensional (3D) Liver Spheroid for Testing an Antimetastatic Therapy
Kseniya V. Nevskaya [...] Elena V. Udut
Sep 20, 2025 3194 Views
Abstract
Due to cell heterogeneity, the differences among individual cells are averaged out in bulk analysis methods, especially in the analysis of primary tumor biopsy samples from patients. To deeply understand the cell-to-cell variation in a primary tumor, single-cell culture and analysis with limited amount of cells are in high demand. Microfluidics has been an optimum platform to address the issue given its small reaction volume requirements. Digital microfluidics, which utilizes an electric signal to manipulate individual droplets has shown promise in cell-culture with easy controls. In this work, we realize single cell trapping on digital microfluidic platform by fabricating 3D microstructures on-chip to form semi-closed micro-wells. With this design, 20% of 30 x 30 array can be occupied by isolated single cells. We also use a low evaporation silicon oil and a fluorinated surfactant to lower the droplet actuation voltage and prevent the drop from evaporation, while allowing cell respiration during the long term of culture (24 h). The main steps for single cell trapping on digital microfluidics, as illustrated in this protocol, include 3D microstructures design, 3D microstructures construction on chip and oil film with surfactant for single cell trapping on chip.
Keywords: Single-cellBackground
Cellular heterogeneity within a large cell population is common. Individual cell analysis will provide more accurate information about the cell-to-cell variation masked by the stochastic average in bulk analysis. Single cell culture, which is the first step of individual cell analysis, becomes very important.
Flow cytometry is a commonly used method for single cell analysis. However, the requisition of large cell numbers (more than 10,000) hurdled its applications in the research of limited precious samples. With the merits of low sample consumption, low analysis cost, microfluidics has been pursued by many scientists, especially in the research field of limited sample acquisition and expensive reagents needed, such as cell-based drug screening. Digital microfluidics, which utilizes electric signal to manipulate individual droplet has shown its promising in cell-culture related research. However, it is difficult to realize single cell capture on a flat electrode on digital microfluidics considering several hundred nanoliter sized droplets. Although several researchers reported that they can isolate single cell on digital microfluidics, the drawbacks hindered their further applications. For example, Gidrol’s group realized single cell isolation by adjusting the concentrations of cell suspensions (Rival et al., 2014). However, the single-cell isolation efficiency was quite low. Lammertyn’s group fabricated a series of micro sized hydrophilic micropatches on an electrode for single cell trapping (Witters et al., 2011). However, the multiple hydrophilic patches resulted in higher droplet transportation voltage, which will not only shorten the chip lifetime, but also cause potential damage to cells.
In our recent work, we developed a single cell culture method for primary tumor drug screening based on microfabrication and digital microfluidic technologies (Zhai et al., 2020). In the work, 3D microstructures were engineered on digital microfluidic chips for single-cell isolation and long-time culture. With novel combination of medium oil and additives, the droplet actuation voltage was lowered to 36 V, 4 times lower than normally used. Following, breast cancer drug screening was run on-chip with the designed microstructures showing consistent results as in conventional 96-well plates.
In the present protocol, we provide detailed steps for this single cell trapping method, including experimental details to perform single cell culture with the digital microfluidic system.
Materials and Reagents
For 3D microstructures fabrication on chip
- Adhesive tape (3M, catalog number: 1183 )
- Chromium patterned bottom plate (Changsha Shaoguang Chromium Edition Company, China)
- ITO top plate (Zhuhai Kaivo Optoelectronic Technology Company, China)
- Isopropanol, abbreviate as IPA (Fisher Chemical, catalog number: 182060 )
- Acetone (Fisher Chemical, catalog number: 183005R )
- Milli-Q water (18.2 MΩ)
- SU-8 3010 (MicroChem, catalog number: 17120963 )
- SU-8 2050 (MicroChem, catalog number: 17070500 )
- SU-8 developer (MicroChem, catalog number: 18090763 )
- Amorhpous Fluoroplastics Solution (6% Teflon solution) (Chemours, catalog number: 1706ES0014 )
- N2 (Junyu company, China)
- FC-40 (3M, catalog number: 20051 )
For cell suspensions preparation
- Pipette tips10 μl (Eppendorf, catalog number: 0 224930000 )
- Pipette tips 200 μl (Eppendorf, catalog number: 0 22491296 )
- Silicone oil (Clearco, CAS number: 107-51-7 )
- Breast cancer cell lines MDA-MB-231 (ATCC, catalog number: BNCC245095 )
- RPMI 1640x basic medium (Gibco, catalog number: 11875-093 )
- Fetal bovine serum (FBS) (Gibco, catalog number: 10270-106 )
- Trypsin (Gibco, catalog number: 25200-056 )
- Pluronic® F-127, abbreviate as F127 (Sigma-Aldrich, CAS number: 9003-11-6 )
- Cisplatin (Sigma, catalog number: P4394 )
- EthD-1 (Thermo Fisher Scientific, catalog number: E1169 )
- Cell Counting Kit-8 (CCK-8) (Dojindo, Shanghai)
For single cell trapping on chip
- Pipette tips10 μl (Eppendorf, catalog number: 0 224930000 )
- Pipette tips 200 μl (Eppendorf, catalog number: 0 22491296 )
- Silicone oil (Clearco, CAS number: 107-51-7 )
Equipment
- Plasma cleaner (ABM, model: MR-200S )
- UV exposure (ABM, model: ABM-350J )
- Hot plate (Qiangyuan Instrument, model: BX-1 )
- Laser cutting machine (ZKJ Laser, Shang Hai)
- Clean bench (Lansi Purification, model: LANSI-GZ-01 )
- Cell incubator (Thermo Fisher, model: Steri-Cycle i160 CO2 incubator )
- Pipette (Eppendorf, Research plus single channel adjustable range pipette)
- Microscope (Olympus, model: BX63F )
- Cellometer (Mini, Dakewe, China)
- Microplate reader (Thermo Fisher, ID number: IED0501003M )
- Spin coater (KW-4A, SETCAS Electronics Company, China)
Software
- Auto CAD 2017 (Autodesk company)
- ImageJ (NIH government)
Procedure
The single cell trapping protocol can be divided into 4 parts: 3D microstructure design, 3D microstructures construction on chip, cell suspensions preparation, and oil film with surfactant for single cell trapping on chip. The final fraction is done by the digital microfluidic system. The detailed protocols for each part are as following:
- 3D microstructure design
We use AutoCAD to design the microstructures. The microstructures include repetitive structure arrays (Figure 1). The yellow frame in Figure 1 shows the single structure, which consisted of four walls (rectangular frames), each one with a width of 7 μm, a length of 20 μm, and a small gap of 2.5 μm (Figure 1, right) between the ends of each wall, forming a semi-closed well between the walls. The designed microstructures were used to produce a mask for the following photolithography experiments.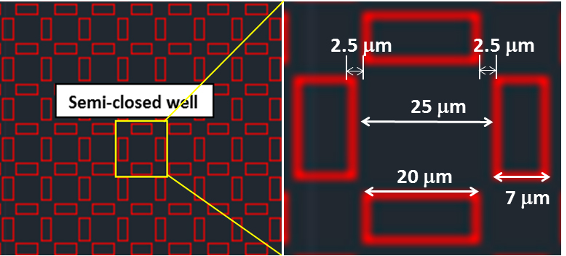
Figure 1. The designed structures for single cell culture with Auto CAD - For 3D microstructures fabrication on chip
The DMF chip is composed of three parts: the bottom plate, the top plate, and the conductive adhesive tape as spacer to connect the top plate and bottom plate. We used autoCAD to design the electrode patterns. Then chromium electrodes (Cr) were patterned on glass plates by and purchased from Changsha Shaoguang Chromium Edition Company, China. ITO top plates were purchased from Zhuhai Kaivo Optoelectronic Technology Company, China. Figure 2 showed the process for the bottom and top plates fabrication and the assembling of bottom plate and top plate with spacer.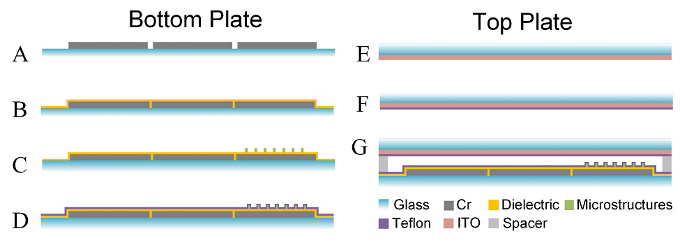
Figure 2. The process for the bottom (A-D) and top plate (E-F) fabrication. (G) The assembling of bottom plate and top plate with spacer to form a DMF chip
The detailed protocols are as following:- Coat 10 μm SU-8 layer on the bottom plate as the dielectric layer.
- Plasma treatment for the designed chromium patterned bottom plate for 1 min (The plate is placed in the chamber of plasma cleaner equipment. First, the plate is treated with vacuum and then oxygen. Then the surface of the plate will be ionized. This process is called plasma treatment. The plasma treated plate will promote the bonding between SU-8 and plate. Plasma treatment is a very common used technique in the microfabrication field.).
- Pour SU-8 3010 (4 ml) on the bottom plate to cover about 1/3 area of the chip.
- Spin coat at 500 rpm, 5 s and sequentially 3,000 rpm, 30 s with spin coater (The procedure is as follows: excess SU-8 solution was poured to the glass substrate, which is rotated at high speed to spread the SU-8 solution by centrifugal force until the solution spins off the edges of the glass substrate and the desired thickness of the SU-8 film is achieved).
- Bake the chip for 3 min at 65 °C, 5 min at 95 °C on hot plate.
- Precise patterning of the dielectric layer with mask aligner via UV exposure for 18.5 s.
- Post-bake the chip at 65 °C for 3 min and 95 °C for 3 min on hot plate.
- Develop the chip with SU-8 developer for 1 min (The plate was immersed in the SU-8 developer solution. The transparent parts on the mask after UV exposure will help UV light irradiate the plate directly and strengthen the bonding between SU-8 and the plate. After developing, the pattern of the transparent parts on the mask will be printed as SU-8 film on the plate, while the pattern of the opaque parts on the mask will not be printed and washed off.).
- Plasma treatment for the designed chromium patterned bottom plate for 1 min (The plate is placed in the chamber of plasma cleaner equipment. First, the plate is treated with vacuum and then oxygen. Then the surface of the plate will be ionized. This process is called plasma treatment. The plasma treated plate will promote the bonding between SU-8 and plate. Plasma treatment is a very common used technique in the microfabrication field.).
- Coat 60 μm thickness SU-8 on the chip as fences to prevent droplets from drifting.
- Plasma treatment for the bottom plate for 1 min.
- Pour SU-8 2050 (4 ml) on the bottom plate to cover about 1/3 area of the chip.
- Spin coat at 500 rpm, 5 s and sequentially 2,500 rpm, 30 s with spin coater.
- Bake the chip for 3 min at 65 °C, 10 min at 95 °C on hot plate.
- Precise patterning with mask aligner via UV exposure for 13.5 s.
- Post-bake the chip at 65 °C for 3 min and 95 °C for 5 min on hot plate.
- Develop the chip with SU-8 developer for 5 min.
- Plasma treatment for the bottom plate for 1 min.
- Coat 10 μm thickness SU-8 layer as a microstructure array to perform single-cell culture.
- Plasma treatment for the bottom plate for 1 min.
- Pour SU-8 3010 (4 ml) on the bottom plate to cover about 1/3 area of the chip.
- Spin coat at 500 rpm, 5 s and sequentially 3,000 rpm, 30 s with spin coater.
- Bake the chip for 3 min at 65 °C, 5 min at 95 °C on hot plate.
- Precise patterning with mask aligner via UV exposure for 18.5 s.
- Post-bake the chip at 65 °C for 3 min and 95 °C for 3 min on hot plate.
- Develop the chip with SU-8 developer for 1 min.
- Plasma treatment for the bottom plate for 1 min.
- Coat Teflon on both the bottom plate and top plate.
- Prepare 0.5%Teflon solution 100 ml (use FC-40 to dilute 6% Teflon solution to 0.5%).
- Wash the bottom plate and top plate with 5 ml IPA and 5 ml MilliQ-water.
- Blow dry the bottom plate and top plate with N2.
- Dry off the top plate and bottom plate with hot plate at 100 °C.
- Cool down the top plate and bottom plate at the room temperature.
- Use pipette to transfer adequate amount of 0.5% teflon solution (about 1 ml) to cover the bottom plate and top plate.
- Spin coat the bottom plate and top plate for 500 rpm, 10 s and sequentially 1,000 rpm, 60 s with spin coater.
- Bake the bottom plate and top plate for 10 min at 185 °C on hot plate.
- Prepare 0.5%Teflon solution 100 ml (use FC-40 to dilute 6% Teflon solution to 0.5%).
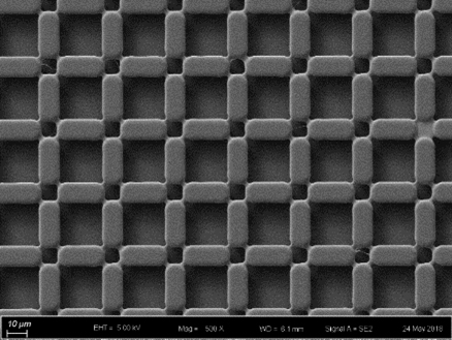
Figure 3. The SEM image result for the designed 3D microstructures - Coat 10 μm SU-8 layer on the bottom plate as the dielectric layer.
- Chip assembly
The DMF chip consists of the bottom plate, a spacer (made of adhesive tapes) to connect the two plates, and a top plate, as shown in the insert real image of Figure 4. The bottom plate is a main part of the DMF chip. A series of chromium electrodes are designed and fabricated on a glass plate, which is used for droplet operation by charging the electrodes or not. We also call it as bottom plate. A 1.5 mm diameter hole is drilled on the top plate by laser cutting machine for sample loading (the inserted image in Figure 4). The scheme of the chip pattern is shown in Figure 4, including sample loading position, the electrodes with microstructures for cell culture and other electrodes aid for the sample moving to the cell culture spots. All the cell culture spots in Figure 4 contain the semi closed well system illustrated by the Figure 3 SEM image. The grey droplet in Figure 4 illustrates the travel of a drug or cell culture suspension from the pipette tip to one of the cell culture compartments.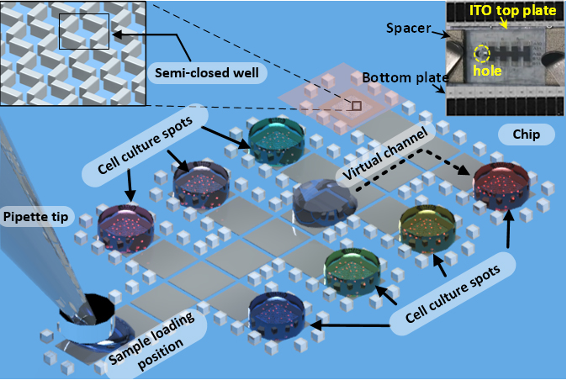
Figure 4. The scheme of the chip pattern and the image of the assemble chip - Cell suspensions preparation
- Discard the medium when the MDA-MB-231 cells grow to 90% density in the 6-well plates.
- Add 0.5 ml trypsin to the well plate and shake to wet the whole well, then discard the trypsin incubate at 37 °C for 1 min in the cell incubator.
- Add 0.5 ml RPMI 1640 medium (supplement with 10% FBS) to the well plate.
- Use a pipette to transfer the cell suspensions to 1.5 ml sterile centrifuge tube.
- Count the cell concentration with Cellometer and adjust the cell concentration to 8 x 105 cells/ml with RPMI 1640 medium.
- Add F127 to the MDA-MB-231 cell suspension with the percent of 0.01%.
- Oil film with surfactant for single cell trapping on chip
- Fill up the space between the bottom and top plates of the chip with oil via a pipette.
- Pipette 0.6 μl droplets (8 x 105 MDA-MB-231 cells/ml) containing 0.01% Pluronic F127 into the holes and move the droplets of cells to the virtual chambers by charging the adjacent electrodes sequentially (Figure 5 showed the DMF system. The electronic control system was used to control the charge of the DMF chip. Each electrode is connected to a contact electrode pad patterned at the edge of the DMF chip through chromium lines patterned on chip, and further connected to the control electronics panel by jumper wires connector. A power supply is connected to the contro electronics. Physical relays on the control board are used as the switches to turn on or turn off the charging of each electrode. The commands are sent from a computer software via Bluetooth to the control electronics). All the operations were carried out in clean bench.
- Place the chip in a humidified incubator (37 °C, 5% CO2) for a long-time culture (24 h).
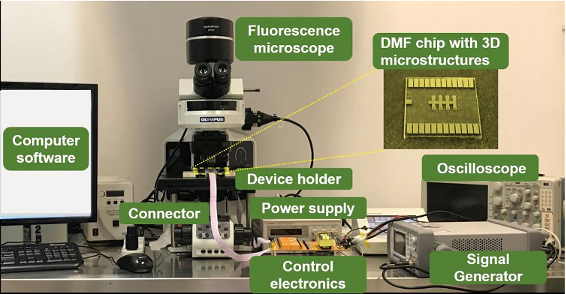
Figure 5. System setup. The DMF system was consisted of four parts: a DMF chip, electronic control system (power supply, signal generator, PCB board and connectors), a self-written control software and a fluorescence microscope.
Figure 6 is an example image of a compartment of the "cell culture spots" in Figure 4. Each circle is a cell in this image. The restriction of the 3D microstructures beneath each droplet would force the droplet to form a curved interface due to the interfacial tension, which promotes the single cell trapping. The cells sit in the semi closed well, as shown in Figure 6.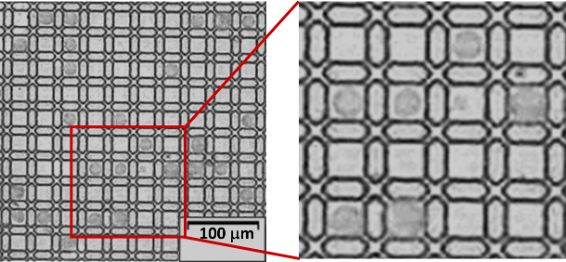
Figure 6. The image result for single cell culture with the designed 3D microstructures
- Example on breast cancer drug toxicity test
Drug toxicity test on DMF chip- Fill up the space between the bottom and top plates of the chip with oil using a pipette.
- Pipette 0.6 μl droplets (8 x 105 MDA-MB-231 cells/ml) containing 0.01% Pluronic F127 and 2 μM EthD-1 and cisplatin solution (0.6 μl) in a series of concentrations into the holes and move to the virtual chambers by charging the adjacent electrodes sequentially and mix on the DMF chip. All the operations were carried out on a clean bench.
- Place the chip in a humidified incubator (37 °C, 5% CO2) for a long-term culture (24 h).
- Measure the red fluorescence of the cells under the Microscope.
Drug toxicity test off-chip
- Seed MDA-MB-231 cells (1.0 x 104 cells/well, 100 μl RPMI 1640 medium supplied with 10% FBS) in a 96-well plate for 12 h.
- Discard the medium and pipette a series of cisplatin concentrations (100 μl) with each concentration in triplicates to the 96-well plate and culture for 24 h.
- Add 10 μl CCK-8 solution to each well and incubate for 0.5 h.
- Measure 450 nm absorbance of each well using a microplate reader.
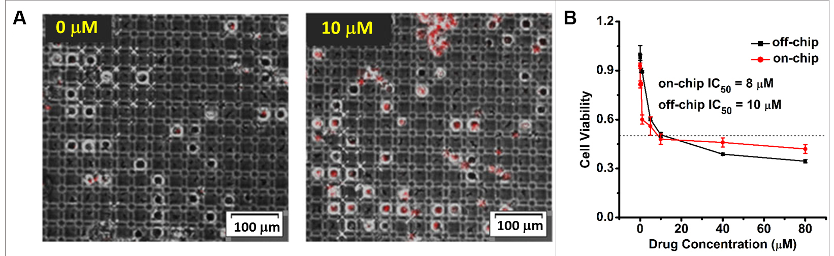
Figure 7. The Cisplatin (Cis) drug toxicity test on MDA-MB-231 breast cancer cells. A. The on-chip fluorescence image results for 0 μM, 10 μM Cis-treated MDA-MB-231 cells for 24 h. B. The drug toxicity test results for Cis-treated MDA-MB-231 cells on /off-chip (96-well plate). - Fill up the space between the bottom and top plates of the chip with oil using a pipette.
Acknowledgments
This work was supported by Macau Science and Technology Development Fund (FDCT) [FDCT110/2016/A3, FDCT 0053/2019/A1, AMSV SKL Fund]; University of Macau [MYRG2017-00022-AMSV, MYRG2018-00114-AMSV].
Competing interests
The authors declare that they have no conflict of interest.
References
- Rival, A., Jary, D., Delattre, C., Fouillet, Y., Castellan, G., Bellemin-Comte, A. and Gidrol, X. (2014). An EWOD-based microfluidic chip for single-cell isolation, mRNA purification and subsequent multiplex qPCR. Lab Chip 14(19): 3739-3749.
- Witters, D., Vergauwe, N., Vermeir, S., Ceyssens, F., Liekens, S., Puers, R. and Lammertyn, J. (2011). Biofunctionalization of electrowetting-on-dielectric digital microfluidic chips for miniaturized cell-based applications. Lab Chip 11(16): 2790-2794.
- Zhai, J., Li, H., Wong, A. H. H., Dong, C., Yi, S., Jia, Y., Mak, P. I., Deng, C. X. and Martins, R. P. (2020). A digital microfluidic system with 3D microstructures for single-cell culture. Microsystems Nanoengineering 6(1): 6.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhai, J., Li, H., Wong, A. H. H., Dong, C., Yi, S., Jia, Y., Mak, P., Deng, C. and Martins, R. (2020). A Novel and Robust Single-cell Trapping Method on Digital Microfluidics. Bio-protocol 10(19): e3769. DOI: 10.21769/BioProtoc.3769.
Category
Cancer Biology > General technique > Drug discovery and analysis
Cell Biology > Cell isolation and culture > Microfluidic culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










