- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and ex vivo Expansion of Human Limbal Epithelial Progenitor Cells
Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3754 Views: 5084
Reviewed by: Ralph Thomas BoettcherVishal S ParekhZheng Zachory Wei

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2235 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1462 Views
Abstract
Limbal stem cell transplantation has been used successfully to treat patients with limbal stem cell deficiency all over the world. However, long term clinical results often proved less satisfactory due to the low quality of the graft or inadequate properties of transplanted cells. To enhance the ex vivo expansion of human limbal epithelial stem or progenitor cells (LEPC) by preserving stem cell phenotype and to improve subsequent transplantation efficiency, cell-matrix interactions ex vivo should mimic the condition in vivo. The laminin isoforms preferentially expressed in the limbal niche can be used as a culture matrix for epithelial tissue engineering. We recently published the expansion of LEPC on various laminin isoforms and observed that laminin alpha 5-derived matrices support the efficient expansion of LEPC compared to tissue culture plates and other laminin isoforms by preserving stem/progenitor cell phenotype. Here, we describe an optimized protocol for the isolation of LEPC from cadaveric corneal limbal tissue by collagenase digestion and efficient expansion of LEPC using recombinant human laminin-511 E8 fragment (LN-511E8) as culture substrate.
Keywords: Limbal epithelial progenitor cellsBackground
Limbal epithelial stem/progenitor cells (LEPC), responsible for the continuous renewal of the corneal epithelium, reside in a highly specialized and complex environment. It comprises specific extracellular matrix components and supporting limbal niche cells at the corneo-scleral limbus (Schermer et al., 1986; Cotsarelis et al., 1989; Ordonez et al., 2012; Mei et al., 2012). Damage or injury to this stem/progenitor cell reservoir can lead to corneal neovascularization, chronic inflammation, and stromal scarring associated with corneal opacity and loss of vision (Kenyon and Tseng, 1989; Sangwan and Tseng, 2001; Dua et al., 2010). Cultured limbal epithelial transplantation (CLET) has been applied as a therapeutic approach in various clinical centers and the overall success rates of autologous CLET for unilateral cases with a follow-up period of at least 24 months were reported to amount to 72-76% (Rama et al., 2010; Tsai et al., 2010; Utheim et al., 2013; Holland et al., 2015). In the case of bilateral, 34 months of follow-up showed a 70% success rate after allogeneic central penetrating limbo-keratoplasty in conjunction with conjunctivoplasty, mitomycin C, and amniotic membrane transplantation (Eberwein et al., 2012). However, long-term corneal regeneration often proved less satisfactory. This might be caused by the low quality of the graft or inadequate properties of transplanted progenitor cells (Daya et al., 2005; Rama et al., 2010; Pellegrini et al., 2014; Nakamura et al., 2016). These limitations underscore the need for developing novel standardized LEPC culture techniques that ensure the preservation of the stem/progenitor cell phenotype and function during cultivation and after transplantation.
Laminins (LNs) are the best-characterized extracellular matrix constituents present in basement membranes (BM) of adult stem cell niches, including the limbal stem cell niche, where they influence cell behaviors, such as cell adhesion, differentiation, and phenotype stability. Various reports have shown heterogeneity of LN isoform expression in BMs of ocular surface epithelia. Especially LN chains α5, β1, β2, and γ1 are preferentially localized to the BM of the limbal epithelium compared to that of the corneal epithelium (Ljubimov et al., 1995; Schlötzer-Schrehardt et al., 2007; Polisetti et al., 2017) (Figure 1). We have reported earlier that LN alpha 5-containing isoforms as well as the bioactive fragment of human LN-511 (LN-511 E8) support efficient ex vivo expansion of LEPC (Polisetti et al., 2017).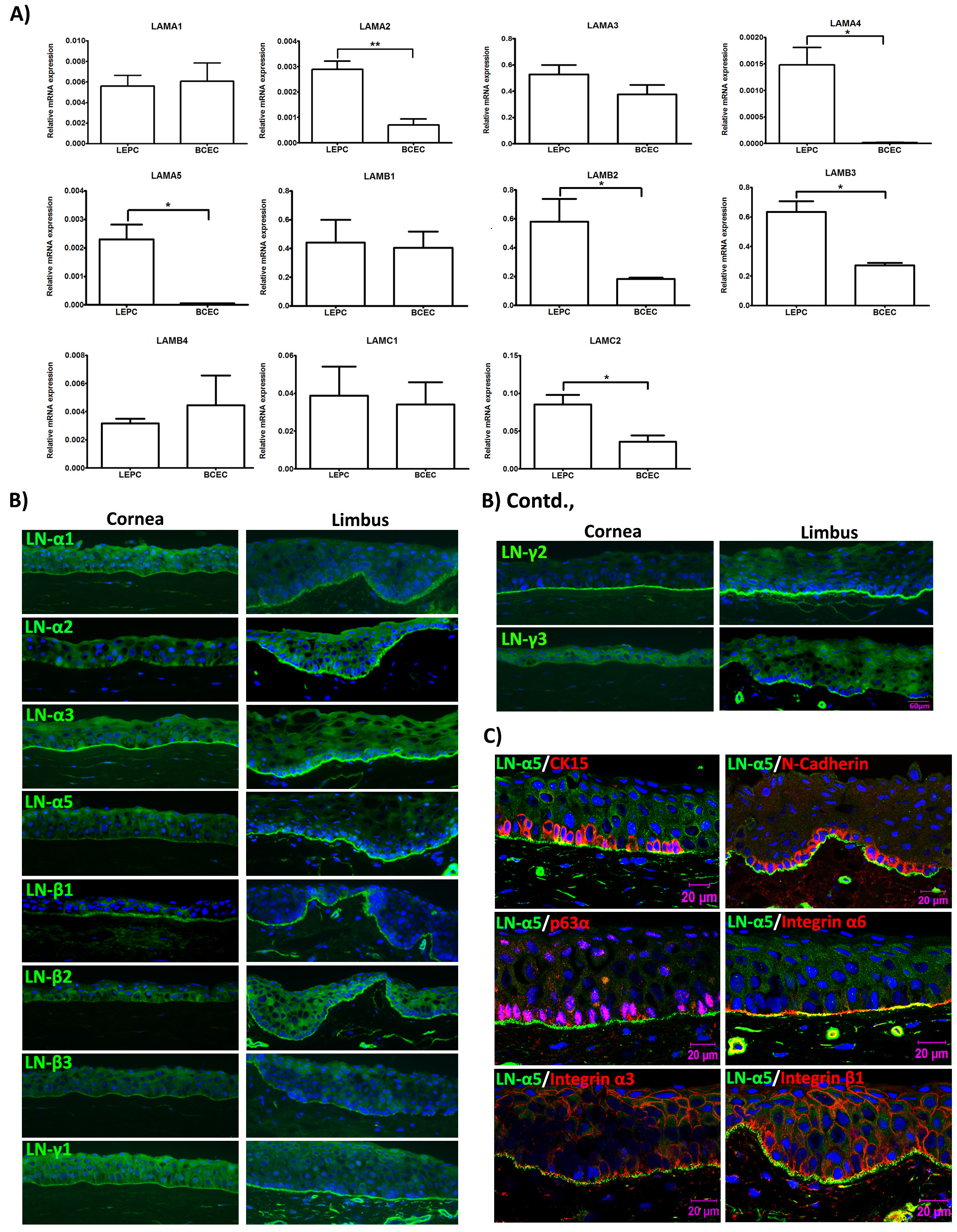
Figure 1. Expression of laminin chains in the limbal stem cell niche in situ. A. Quantitative real-time polymerase chain reaction (qRT-PCR) primer assays showing higher expression levels of laminin α2 (LAMA2), α4 (LAMA4), α5 (LAMA5), β2 (LAMB2), β3 (LAMB3), and γ2 (LAMC2) in microdissected limbal epithelial stem/progenitor cell (LEPC) clusters compared with basal corneal epithelial cell (BCEC) populations; laminin α1 (LAMA1), α3 (LAMA3), β1 (LAMB1), β4 (LAMB4), γ1 (LAMC1) showed no differential expression patterns. B. Immunofluorescence analyses of corneoscleral tissue sections showing differential staining patterns of laminin α2, α5, β2, β3, γ2, and γ3, but similar staining patterns of laminin α1, α3, β1, and γ1 in the basement membranes of corneal and limbal epithelia; laminin α4 was largely negative in epithelial basement membranes. C. Immunofluorescence double labeling of laminin (LN) α5 (green) and cytokeratin (CK)15, N-Cadherin, p63α, integrin α6, integrin α3, and integrin β1 (red); nuclear counterstaining with DAPI (blue); scale bar = 20 µm. Reprinted from Polisetti et al., 2017, licensed under a CC BY 4.0.
Here, we present an optimized protocol for isolation and expansion of LEPC. The isolation protocol is similar to the protocol provided by Chen et al. (2011) with few modifications, but we expanded LEPC on LN-511E8-coated plates to improve proliferation and preservation of a stem/progenitor cell phenotype (Polisetti et al., 2017).
Materials and Reagents
- Micropipette tips (0.5-20 µl, 100-200 µl, 1,000 µl) (Greiner Bio-One)
- 60 mm cell culture dish (Corning, Falcon®, catalog number: 353004 )
- 100 mm cell culture dish (Corning, Falcon®, catalog number: 353003 )
- Syringe filter 0.2 μm (VWR, catalog number: 28145-501 )
- Disposable Scalpel blades No. 10 (pfm Medical ag, Feather®, catalog number: 201000010 )
- Serological pipettes (5 ml, 10 ml) (Corning, StripetteTM)
- 15 ml conical tubes (Greiner Bio-One, catalog number:188271)
- T75 flasks (Corning, catalog number: CLS430641 )
- Reversible cell strainers (Stem Cell Technologies, catalog number:27215)
- Cell filter 20 µm (Sysmex Partec GmbH, Görlitz, Germany)
- Collagenase A (Sigma-Aldrich, Roche Diagnostics, catalog number: 10103578001 )
- Dulbecco’s Phosphate buffered saline (DPBS) (Pan Biotech, catalog number: P04-361000 )
- 0.25% Trypsin-EDTA (Thermo Fisher Scientific, Gibco®, catalog number: 25200056 )
- 0.05% Trypsin-EDTA (Thermo Fisher Scientific, Gibco®, catalog number: 25300054 )
- Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 11960044 )
- Fetal Bovine Serum (FBS) (Thermo Scientific, GibcoTM, catalog number: 10082147 )
- Penicillin-Streptomycin/Amphotericin B Mixture (Pan Biotech, catalog number: P06-07300 )
- Keratinocyte serum-free medium (KSFM) (Life Technologies, catalog number: 10724-011 )
- Keratinocyte supplements, contains bovine pituitary extract, human recombinant EGF (Life Technologies, catalog number: 37000-015 )
- iMatrix 511(Nippi, catalog number: 892012 )
- 70% Ethanol
- Cytokeratin (CK) 15 (Invitrogen, catalog number: MA5-11344 )
- p63α (Abcam, catalog number: ab32353 )
- CCAAT/enhancer-binding protein delta (Abcam, catalog number: ab139730 )
- CK19 (Abcam, catalog number: ab52625 )
- CK3 (Invitrogen, catalog number: MA1-5763 )
- CK12 (Abcam, catalog number: ab185627 )
- Connexin-43 (Abcam, catalog number: ab235282 )
- Collagenase solution (see Recipes)
- KSFM complete medium (see Recipes)
- Laminin coated plates (see Recipes)
Equipment
- Pipette aid
- Micropipette (P20, P200, P1000)
- Microdissection Scissors
- Forceps
- Scissors
- Hemocytometer
- Biosafety cabinet
- CO2 incubator
- Centrifuge
- Phase contrast inverted microscope with a camera (Objectives 4x, 10x, 20x)
- Freezer -20 °C
- Refrigerator 2-8 °C
Software
- CapturePro 2.10.0.1 (JENOPTIC Optical systems GmbH)
Procedure
- Dissection of limbus
- Organ-cultured corneoscleral tissue with appropriate research consent and ethics approval may be provided by a cornea bank if the corneas are not suitable for transplantation due to low corneal endothelial cell density (< 2,200 cells/mm2), or the presence of neurological disorders or malignancies in the donor. Donor cornea remnants after corneal endothelial transplant preparation are also a valuable source if appropriate research consent and approval have been obtained.
- Place the corneoscleral tissue in a 60 mm culture dish and wash with DPBS twice. Cut the tissue into 12 three-clock hour sectors using a scalpel blade and forceps.
- Make incisions at 1 mm before and beyond the anatomical limbus to get limbal segments (see Video 1).Video 1. Limbal Epithelial Progenitor Cell Isolation
- Isolation of limbal epithelial progenitor cells
- Place the limbal segments into a 60 mm dish containing 4 ml of collagenase A (2 mg/ml) and cut the limbal segments into smaller pieces with the scalpel blade. Incubate at 37 °C with 5% CO2 overnight to digest the stromal collagen (get limbal epithelial cell clusters).
- After overnight incubation, triturate several times with 1 ml pipette and observe for the presence of cell clusters and single cells in the microscope (Figure 2). The cell clusters are supposed to consist of limbal epithelial progenitor cells, stromal, and melanocyte niche cells.
Note: In case of incomplete digestion of limbal segments after overnight incubation and trituration, re-incubate for 2 more hours in the same solution at 37 °C with 5% CO2 for complete digestion. On the other hand, over digestion of tissue might adversely affect cell viability and the quality of cells.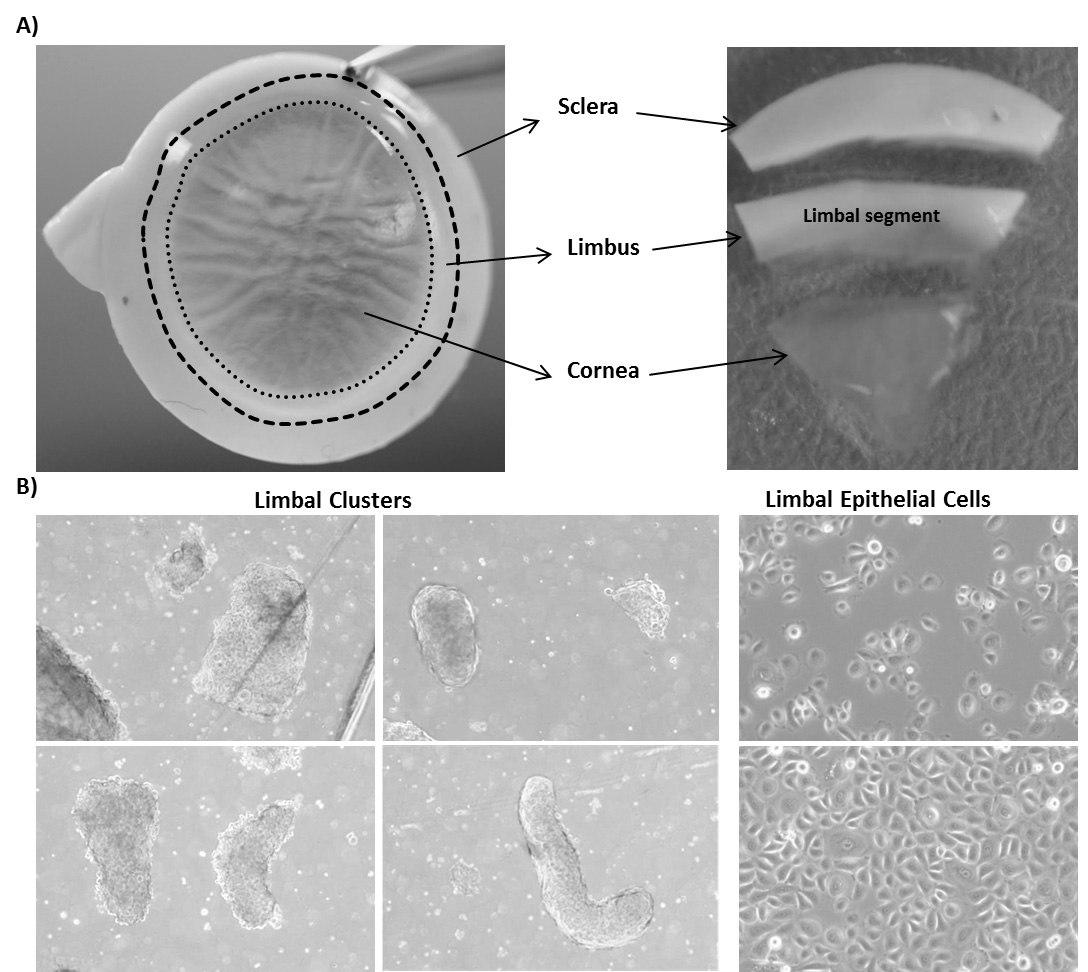
Figure 2. Isolation of limbal epithelial progenitor cells. A. The corneal scleral rim (left) was cut into sectors and each sector was trimmed off 1mm before and after the limbal region (right). B. Different sizes of limbal clusters and single cells (left) formed after overnight incubation of limbal segments in collagenase solution (x40 magnification). Limbal cluster-derived limbal epithelial cells cultured in KSFM media at 50% confluence (right, top) and 100% confluence (right, bottom) (x40 magnification). - Separate cell clusters from single cells by using reversible cell strainers with a pore size of 37 µm. The single cells pass through the cell strainer and the clusters are retained. Wash the strainers with PBS twice to remove single cells. Reverse the strainer and place it on a 15 ml Falcon tube or on a 60 mm dish. Add 0.25% trypsin-EDTA (5 ml) to flush clusters into petri dish or tube and incubate at 37 °C for 10-15 min to dissociate the clusters into single cells.
Note: In place of reversible cell strainers, 20 µm cell filters can be used. In the video, we used 20 µm filters. - After incubation, triturate the cell suspension with a 1ml pipette. Inhibit trypsin digestion using 5ml of DMEM containing 10% FBS. Transfer the cell suspension into a 15 ml Falcon tube and centrifuge at 200 x g for 5 min.
- After centrifugation, resuspend the cell pellet in 1 ml of KSFM complete media for feeder-free cultures. These cells can also be used for clonal assays on feeder layers.
- Expansion of limbal epithelial progenitor cells
- Seed the limbal cluster-derived single cells (1 x 103 cells/cm2) onto LN511E8-coated plates (flasks or plates depending on cell number obtained. Preparation of laminin-coated plates described in Recipe 3.
Note: Do not allow the laminin-coated flasks to dry. - Cultivate for 7 to 10 days at 37 °C with 5% CO2 in KSFM complete medium to expand limbal epithelial cells. Change media every 2 days.
Note: If any stromal fibroblast contamination occurs after 7 days in culture, remove the stromal fibroblasts, which tend to adhere less firmly to the LN matrix, by using 0.05% trypsin-EDTA. Remove the media and wash it with DPBS. Incubate with 4 ml of 0.05% trypsin-EDTA for 2 min. Wash the flasks with DMEM containing 10% FBS followed by one wash with KSFM. Add 12 ml of KSFM complete medium and continue with culture - To verify the expanded population for the presence of stem/progenitor cell population, various stem/progenitors markers (Cytokeratin (CK) 15, p63α, CCAAT/enhancer-binding protein delta, CK19) and differentiated markers (CK3, CK12, Connexin-43) can be used by means of Reverse Transcription Polymerase chain reaction and by immunocytochemistry.
- Seed the limbal cluster-derived single cells (1 x 103 cells/cm2) onto LN511E8-coated plates (flasks or plates depending on cell number obtained. Preparation of laminin-coated plates described in Recipe 3.
- Sub-cultivation of limbal epithelial progenitor cells
- At 70 to 80% confluency, remove the media and wash once with DPBS.
- Add 4 ml of trypsin-EDTA (0.05%) and incubate at 37 °C with 5% CO2 for 5 min.
- After incubation, add 4 ml of DMEM containing 10% FBS to inhibit trypsin action and mix well.
- Transfer the cell suspension into 15 ml tube and centrifuge at 200 x g for 5 min. Dissolve the cell pellet in KSFM complete medium and use cells for the application of choice or for subculturing.
Note: Over confluence (more than 80%) and prolonged trypsin digestion (more than 5 min) adversely affect cell viability and the quality of cells during sub-culturing. Always passage cells at 70 to 80% confluence. Avoid prolonged incubations in trypsin.
Data analysis
The conditions provided in this protocol have been optimized to isolate and expand LEPCs. A detailed analysis of the isolation and expansion of the limbal epithelial progenitor cells can be found at Polisetti et al., 2017.
Recipes
- Collagenase solution
Reagent Collagenase A
Components and Preparation 5 mg Collagenase A
220 ml of DMEM High Glucose
25 ml of fetal calf serum
5 ml of Penicillin-Streptomycin/Amphotericin B Mix
Mix well by inverting
Method of Sterilization Sterile Filter with 0.2 µm filter
Notes Prepare 10 ml aliquots
Storage -20 °C - KSFM Media
Medium Keratinocyte Serum-Free Medium (KSFM) complete
Components and Preparation 500 ml of KSFM Basal Medium
25 mg of BPE (add entire amount)
2.5 µg of EGF (add entire amount)
5 ml of Penicillin-Streptomycin/Amphotericin B Mix
Mix well by inverting
Method of Sterilization None
Notes Prepare aliquots if needed
Protect from light
Storage 1 month at 2-8 °C - Laminin coated plates
- Dilute 75 µl of iMatrix-511 in 8 ml of 1x DPBS
- Coat the T75 flask with 8 ml of dilute iMatrix-511 (0.5 µg/cm2) solution for 1 h at 37 °C, 5% CO2 in the incubator, or at 4 °C overnight
- After incubation, remove the iMatrix-511 solution and add 10 ml of KSFM complete medium
Acknowledgments
This work is supported by DFG Grant INST 410/45-1 FUGG. This protocol was adapted from previous work (Polisetti et al., 2017).
Competing interests
The authors declare that they have no competing interests.
References
- Chen, S. Y., Hayashida, Y., Chen, M. Y., Xie, H. T. and Tseng, S. C. (2011). A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods 17(5): 537-548.
- Cotsarelis, G., Cheng, S. Z., Dong, G., Sun, T. T. and Lavker, R. M. (1989). Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57(2): 201-209.
- Daya, S. M., Watson, A., Sharpe, J. R., Giledi, O., Rowe, A., Martin, R. and James, S. E. (2005). Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 112(3): 470-477.
- Dua, H. S., Miri, A. and Said, D. G. (2010). Contemporary limbal stem cell transplantation – a review. Clin Exp Ophthalmol 38(2): 104-117.
- Eberwein, P., Bohringer, D., Schwartzkopff, J., Birnbaum, F. and Reinhard, T. (2012). Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin C, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology 119(5): 930-937.
- Holland, E. J. (2015). Management of limbal stem cell deficiency: a historical perspective, past, present, and future. Cornea 34 Suppl 10: S9-15.
- Kenyon, K. R. and Tseng, S. C. (1989). Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96(5): 709-722.
- Ljubimov, A. V., Burgeson, R. E., Butkowski, R. J., Michael, A. F., Sun, T. T. and Kenney, M. C. (1995). Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72(4): 461-473.
- Mei, H., Gonzalez, S. and Deng, S. X. (2012). Extracellular matrix is an important component of limbal stem cell niche. J Funct Biomater 3(4): 879-894.
- Nakamura, T., Inatomi, T., Sotozono, C., Koizumi, N. and Kinoshita, S. (2016). Ocular surface reconstruction using stem cell and tissue engineering. Prog Retin Eye Res 51: 187-207.
- Ordonez, P. and Di Girolamo, N. (2012). Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells 30(2): 100-107.
- Pellegrini, G., Rama, P., Di Rocco, A., Panaras, A. and De Luca, M. (2014). Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells 32(1): 26-34.
- Polisetti, N., Sorokin, L., Okumura, N., Koizumi, N., Kinoshita, S., Kruse, F. E. and Schlotzer-Schrehardt, U. (2017). Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci Rep 7(1): 5152.
- Rama, P., Matuska, S., Paganoni, G., Spinelli, A., De Luca, M. and Pellegrini, G. (2010). Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363(2): 147-155.
- Sangwan, V. S. and Tseng, S. C. (2001). New perspectives in ocular surface disorders. An integrated approach for diagnosis and management. Indian J Ophthalmol 49(3): 153-168.
- Schermer, A., Galvin, S. and Sun, T. T. (1986). Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103(1): 49-62.
- Schlötzer-Schrehardt, U., Dietrich, T., Saito, K., Sorokin, L., Sasaki, T., Paulsson, M. and Kruse, F. E. (2007). Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res 85(6): 845-860.
- Tsai, R. J., Li, L. M. and Chen, J. K. (2000). Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 343(2): 86-93.
- Utheim, T. P. (2013). Limbal epithelial cell therapy: past, present, and future. Methods Mol Biol 1014: 3-43.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Polisetti, N., Schlunck, G., Reinhard, T., Kruse, F. E. and Schlötzer-Schrehardt, U. (2020). Isolation and ex vivo Expansion of Human Limbal Epithelial Progenitor Cells. Bio-protocol 10(18): e3754. DOI: 10.21769/BioProtoc.3754.
Category
Stem Cell > Pluripotent stem cell > Cell pluripotency
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










