- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Production and Bioassay of a Diffusible Factor that Induces Gametophyte-to-Sporophyte Developmental Reprogramming in the Brown Alga Ectocarpus
(*contributed equally to this work) Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3753 Views: 4650
Reviewed by: Shweta PanchalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

1-MCP (1-methylcyclopropene) Treatment Protocol for Fruit or Vegetables
Gamrasni Dan [...] Aharoni Asaph
May 20, 2017 16437 Views

Determination of Reduced and Total Glutathione Content in Extremophilic Microalga Galdieria phlegrea
Giovanna Salbitani [...] Simona Carfagna
Jul 5, 2017 22515 Views

14C-paraquat Efflux Assay in Arabidopsis Mesophyll Protoplasts
Jin-Qiu Xia [...] Cheng-Bin Xiang
Sep 20, 2022 1662 Views
Abstract
The brown alga Ectocarpus has a haploid-diploid life cycle that involves alternation between two multicellular generations, the sporophyte and the gametophyte. Life cycle generation is not determined by ploidy but by a genetic system that includes two different three amino acid loop extension homeodomain transcription factors called OUROBOROS and SAMSARA. In addition, sporophytes have been shown to secrete a diffusible factor into the medium that can induce gametophyte initial cells to switch from the gametophyte to the sporophyte developmental program. The protocol presented here describes how to produce sporophyte-conditioned medium containing the diffusible sporophyte-inducing factor and how to assay for activity of the factor using a meio-spore-based bioassay. The protocol, which describes how several steps of these procedures can be optimised, will represent a useful tool for future work aimed at characterising the diffusible factor and investigating its mode of action.
Keywords: Brown algaeBackground
Ectocarpus is a small, filamentous brown alga that has been adopted as a genetic model organism for this group of seaweeds (Peters et al., 2004; Coelho et al., 2012a). The brown algae exhibit a broad range of different life cycles (Cock et al., 2014). Ectocarpus has a haploid-diploid life cycle (Figure 1) that involves alternation between two multicellular generations, the gametophyte and the sporophyte (Müller, 1967; Bothwell et al., 2010). Transition from the sporophyte to the gametophyte generation involves the production of meio-spores, which are released into the surrounding environment and germinate to produce separate male and female, haploid gametophytes. To complete the life cycle, mature female and male gametophytes produce gametes, which fuse to produce a zygote, the initial cell of the diploid sporophyte generation. Life cycle generation (i.e., sporophyte or gametophyte identity) is not determined by ploidy because single gametes are able to develop parthenogenetically, without fusing to produce a zygote, leading to the production of haploid partheno-sporophytes that are phenotypically indistinguishable from diploid sporophytes derived from a zygote (Müller, 1967; Bothwell et al., 2010). The gametophyte and sporophyte generations of Ectocarpus are free-living, exhibit distinct morphologies and functions and show spatio-temporal independence, facilitating the study of the developmental program of each generation and their relationship with the life cycle.
Figure 1. The Ectocarpus life cycle. The diploid sporophyte produces meio-spores via a single meiotic cell division (R! for reduction) followed by multiple mitotic divisions in each unilocular sporangium (US). The meio-spores are released and develop as gametophytes. Gametophytes are either male or female (dioicy) and produce either male or female gametes in plurilocular gametangia (PG). Pairs of male and female gametes fuse (F! for fusion) to form zygotes, which develop as diploid sporophytes to complete the sexual cycle (left). Unfused gametes can undergo parthenogenesis to produce partheno-sporophytes. Partheno-sporophytes produce spores in unilocular sporangia, which develop as gametophytes to complete the parthenogenetic cycle (right). In addition, both diploid sporophytes and partheno-sporophytes produce mito-spores in plurilocular sporangia (PS), which germinate to produce a new sporophyte generation with the same ploidy as its parent (dotted line). Treatment with sporophyte-conditioned medium (+ SCM) induces a subset of meio-spores to switch from the gametophyte to the sporophyte developmental program (grey text). Adapted from Peters et al. (2008).
Alternation of generations in Ectocarpus has been shown to be controlled by two homeodomain transcription factors (HD TFs) of the three amino acid loop extension (TALE) class, OUROBOROS (ORO) and SAMSARA (SAM), which are necessary for the initiation of the sporophyte program (Coelho et al., 2011; Arun et al., 2019). In addition, the sporophyte has been shown to secrete a non-cell-autonomous, diffusible factor that can cause receptive cells to switch from the gametophyte to the sporophyte developmental program (Arun et al., 2013 and 2019). This diffusible sporophyte-inducing factor is only effective on the single-cell stage of the gametophyte generation. Developing meio-spores became resistant to the factor at the same point in time as they synthesise a cell wall, about 24-48 h after release from unilocular sporangia (Arun et al., 2013). This observation suggests that the cell wall may play a role in locking the individual into the developmental program that has been initiated. oro and sam mutants do not respond to treatment with the diffusible factor, suggesting that ORO and SAM are part of the signalling network that detects the factor.
Preparations of the diffusible sporophyte-inducing factor are produced by filtering seawater medium in which Ectocarpus sporophytes have been cultivated, resulting in cell-free, sporophyte-conditioned medium (SCM). Procedures to produce and assay the diffusible factor were described briefly in Arun et al. (2013) and Arun et al. (2019). Activity of the diffusible factor can be assayed either by treating freshly released meio-spores or by treating gametophyte-derived protoplasts (Arun et al., 2013). The protocol below describes treatment of meio-spores, which are easier to obtain than gametophyte-derived protoplasts. For information about the method using gametophyte-derived protoplasts, please refer to Arun et al. (2013). The meio-spore-based procedure is described in detail and some specific tips to improve both SCM production and the bioassay for the diffusible factor are provided. This detailed protocol is currently being employed in experiments aimed at biochemically characterising the diffusible factor.
Materials and Reagents
- 55-mm diameter Petri dishes (Corning, catalogue number: CLS430589 )
- 150-mm diameter Petri dishes (Corning, catalogue number: CLS430597 )
- 10 L polycarbonate carboys (Nalgene, catalogue number: 2251-0020 )
- Parafilm (Sigma-Aldrich, catalogue number P7793-1EA )
- Coverslips (Knittel, catalogue number: MS0009 )
- Large size cell strainer (for example a 12.5 cm Finlandek permanent coffee filter)
- 40 µm nylon cell strainer (Falcon, product number: 352340)
- 0.22 µm Millex-GP polyethersulphone membrane filter (Millipore, catalogue number: SLGP033RS )
- Glass bottles (Fisherbrand, catalogue number: 15476113 )
- A closed (e.g., metal) box to keep Petri dishes in the dark
- Sterile, plugged, glass Pasteur pipettes (Sigma-Aldrich, catalogue number: S6143 )
- Sterile, 10 ml plastic pipettes (Corning, catalogue number: 4488 )
- 22 x 30 mm coverslips
- Sporophytes and gametophytes of Ectocarpus male strain Ec32 (reference CCAP 1310/4 in the Culture Collection of Algae and Protozoa, Oban, UK)
- H3BO3
- FeCl3
- MnSO4·H2O
- ZnSO4·7H2O
- CoSO4·7H2O
- EDTA
- Vitamin B12
- TRIS (Trisma base)
- (NH4)2Fe(SO4)2·6H2O
- NaNO3
- C3H7Na2O6P·5H2O
- Provasoli solution (see Recipes)
Solution 1
Solution 2
Solution 3
Solution 4
Solution 5 - Provasoli-enriched natural seawater (PES) (see Recipes)
Equipment
- Neubauer chamber Bürker (0.1 mm depth, 0.0025 mm2)
- Fine dissecting forceps (Dumont, catalogue number: 0103-27-PO )
- Thermostatically-controlled, illuminated growth cabinet (Pol-Eko-Aparatura, model: KK 240 FIT P ) or growth room
- Binocular dissecting microscope (Olympus, model: SZ61 )
- Inverted light microscope (Olympus, model: CKX41 )
Software
- Excel, version 15.32 (Microsoft)
Procedure
- Preparation of SCM
- Preparation of Ectocarpus strains
- Prepare sporophytes and gametophytes of Ectocarpus male strain Ec32.
- Maintain the sporophytes and gametophytes separately by growing in 10 ml of Provasoli-enriched seawater (PES, i.e., natural seawater enriched with Provasoli supplement; Starr and Zeikus, 1993) in 55-mm diameter Petri dishes or in 100 ml PES in 150-mm diameter Petri dishes under standard growth conditions: 13 °C, 12 h:12 h (light:dark) and 20 µM photons/m2/s (Coelho et al., 2012b). The edge of the Petri dish is wrapped with a band of Parafilm, which allows sufficient passive aeration for algal growth.
- Growth of partheno-sporophytes for the production of SCM
- Grow cultures of Ectocarpus strain Ec32 gametophytes in 150 mm Petri dishes (about 0.13 g per Petri dish) for 2 weeks under low light conditions (2-3 µM photons/m2/s, 12 h:12 h light: dark) in PES at 13 °C.
- Induce the release of gametes by grouping 20 to 30 gametophytes together in a small volume of medium (to simulate low tide conditions) and incubate in the dark in a closed box for four hours at 13 °C (Figure 2).
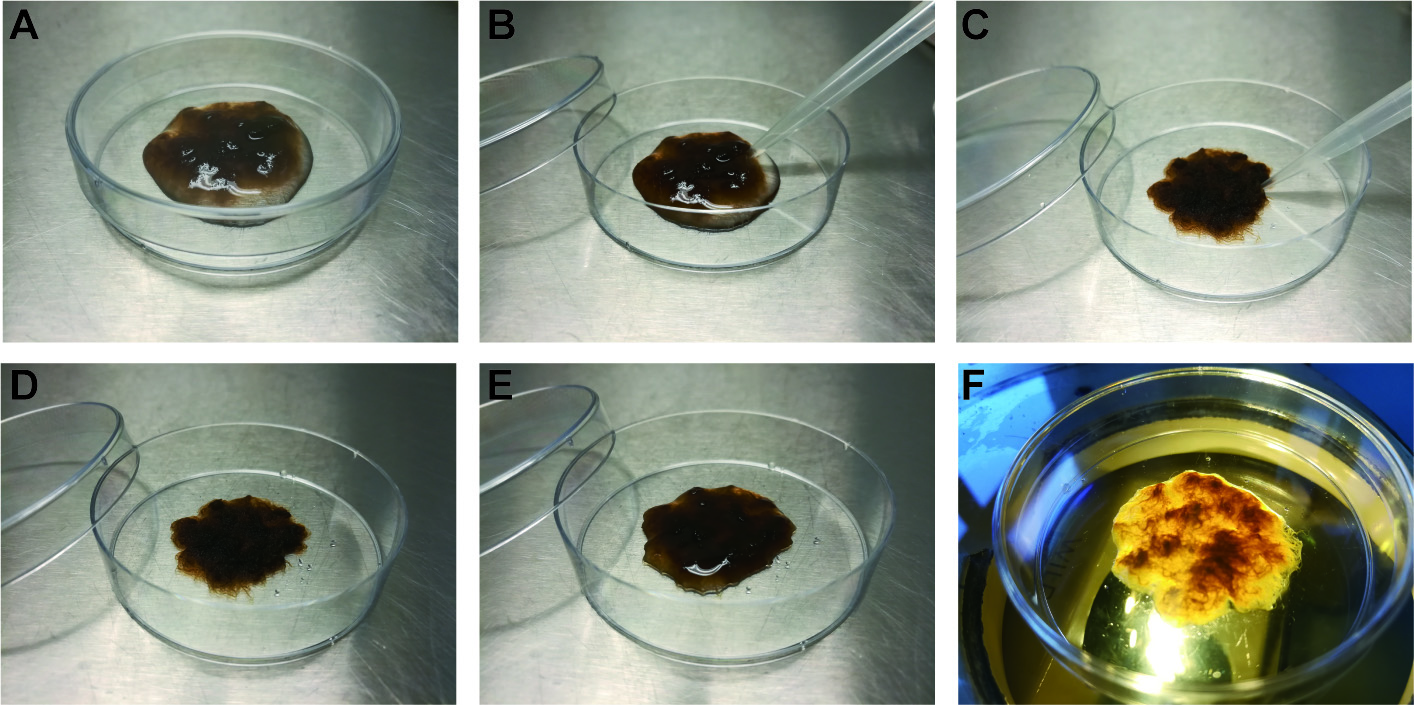
Figure 2. Induction of gamete release. A. Gametophytes grouped into a small volume of PES. B and C. Removal of excess PES with a pipette. D. Gametophytes after removal of excess PES. E. After incubation in the dark for four hours, 300 µl of PES is added. F. Gametophytes are placed under strong light to induce gamete release. - Add 300 µl PES and place under strong light (25-30 µM photons/m2/s) to induce gamete release. Synchronous release of large numbers of gametes should be observed within ten minutes of illumination with strong light (Video 1).Video 1. Release of Ectocarpus gametes from a plurilocular gametangium and swarming gametes
- Estimate the number of gametes released using a Neubauer chamber. Mix 9 µl of the released gametes in PES with 1 µl of 10% glutaraldehyde. After 10 min at room temperature pipette the mix onto the grid of the Neubauer chamber and cover with a coverslip. Count the number of gametes in a 0.04 mm2 square on the Neubauer chamber grid, then repeat the counting at least nine times and calculate the average of these counts (= X). The number of gametes per µL is calculate by dividing the average number of gametes counted per 0.04 mm2 square by the surface area (mm2) x chamber depth (mm) and then correcting for the dilution (i.e., X/(0.04 x 0.1) x 10/9).
- Use each batch of released gametes to inoculate two 150 mm Petri dishes (about 106 gametes per Petri dish) and cultivate the resulting partheno-sporophyte germlings under low light conditions for 14 days.
- Inoculate a 10 L carboy of PES with 0.5 g of partheno-sporophyte thalli and grow for between four and eleven weeks at 13 °C under low light conditions. The carboy culture should be aerated by pumping air through a 0.22 µm filter. The entry and exit tubes for the air pass through holes in the top of the carboy and a 10 ml sterile pipette is used to deliver the air bubbles to the bottom of the carboy (Video 2).Video 2. Culture of Ectocarpus partheno-sporophytes in a 10 L carboy
- Collection and storage of the SCM
- Use a large size cell strainer to remove the bulk of the partheno-sporophyte tissue from the culture and then filter the SCM through a Falcon 40 µm cell strainer and a 0.22 µm filter to remove any remaining cells.
- Store the SCM in glass bottles (prepared beforehand by cleaning with 70% ethanol) in a cold room at 4 °C. The SCM can be stored for at least eight weeks at 4 °C without loss of activity.
- Preparation of Ectocarpus strains
- Bioassay of the diffusible sporophyte-inducing factor
- Production of unilocular sporangia containing meio-spores
- The bioassay of the diffusible sporophyte-inducing factor is carried out using meio-spores produced by fertile sporophytes. Depending on the strain of Ectocarpus, meio-spores can exhibit different levels of heteroblasty, i.e., spontaneous initiation of the sporophyte program instead of the gametophyte program (Müller, 1967). To assay the activity of the diffusible factor, it is important to use a strain that exhibits a low level of spontaneous heteroblasty, such as the strain Ec32.
- Induce the release of gametes from 45 to 60 mature gametophytes by grouping the material together in a small volume of PES (to simulate low tide conditions) and incubating in the dark at 13 °C for four hours (follow the procedure described in Steps A2a-A2c above).
- Allow the gametes to germinate parthenogenetically to produce partheno-sporophytes under standard growth conditions: light intensity of 20 µM photons/m2/s, 12 h:12 h light:dark at 13 °C in 150-mm Petri dishes (Coelho et al., 2012b).
- Change the culture medium regularly (once every 2 weeks) until the partheno-sporophyte filaments produce upright filaments. When upright filaments are produced, remove individual partheno-sporophytes and culture separately. Note that cultures grown at high density will not produce unilocular sporangia. Plurilocular sporangia should develop after about 1 to 2 weeks and the unilocular sporangia appear about one week later.
- Alternatively, unilocular sporangia can be produced on cultured upright filaments, for example by transferring previously dissected upright filaments with unilocular sporangia that have released meio-spores back into culture. These filaments will adhere to the bottom of a 55-mm Petri dish and produce new upright filaments in a few days under standard culture conditions. The upright filaments will produce many unilocular sporangia after about one week. Cultivation of upright filaments results in the production of fewer (or no) plurilocular sporangia than cultivation of partheno-sporophytes (reducing the risk of contaminating meio-spore preparations with mito-spores). This method of producing unilocular sporangia is more rapid than cultivation of whole partheno-sporophytes.
- Bioassay of the diffusible factor by treatment of meio-spores
- Under a binocular microscope, dissect a piece of sporophyte upright filament that bears one or more unilocular sporangia using a sterile glass Pasteur pipette that has been broken to create a sharp, cutting point (Figure 3).
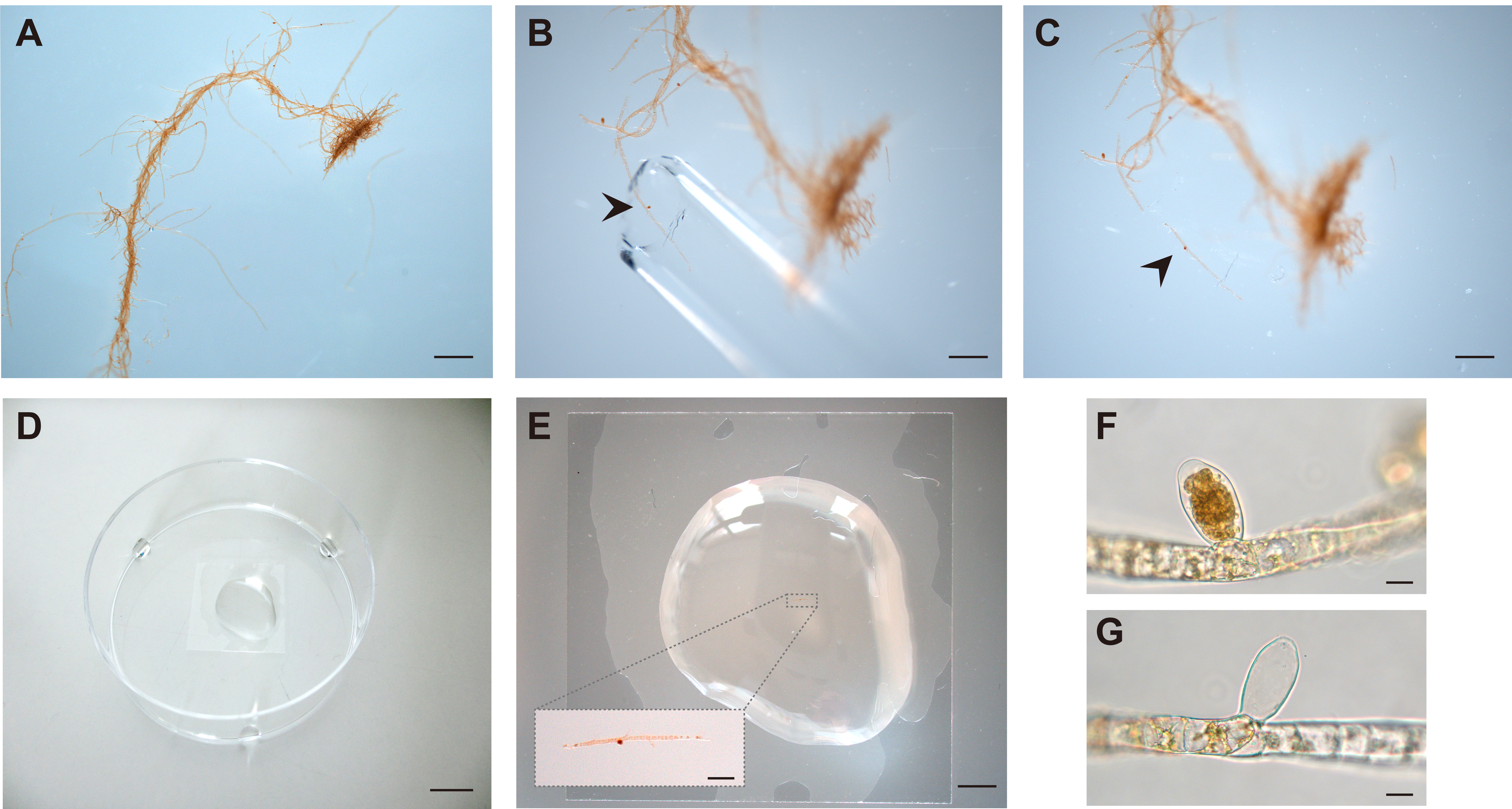
Figure 3. Dissection of unilocular sporangia and meio-spore release. A. Ectocarpus partheno-sporophyte bearing unilocular sporangia. B. Dissection of a filament fragment bearing a unilocular sporangium (arrowhead) using a sterile Pasteur pipette. C. Cut filament fragment with unilocular sporangium (arrowhead). D. Drop of PES on a coverslip in a 55-mm Petri dish humidified with drops of PES at the periphery. E. Filament fragment bearing unilocular sporangium transferred to the drop of PES. The inset shows the filament fragment in the drop of PES. F. Unilocular sporangium before meio-spore release. G. Unilocular sporangium after meio-spore release. Scale bars: 1 mm (A), 500 µm (B, C), 1 cm (D), 2.5 mm (E), 400 µm (E inset), 20 µm (F, G). - Wash the fragment twice in PES by transferring sequentially to two 55-mm Petri dishes, each containing 10 ml of PES (to ensure that there is no carry-over of possible contaminating mito-spores), then transfer into a drop of 300 µl of SCM (Figure 2) on a coverslip. The coverslip should be fixed to the bottom of the Petri dish by placing it on a drop of 10 µl of PES. Place three drops of PES around the edge of the Petri dish to keep the chamber moist and seal the Petri dish with parafilm. Note that the SCM can be replaced by any other seawater-based sample that you would like to test for diffusible factor activity.
- In parallel, transfer unilocular sporangia to drops of PES, using the same set-up, as negative controls (i.e., incubation in seawater that does not contain any active factors).
- Place the unilocular sporangia in strong light conditions (30 µM photons/m2/s) overnight to induce release of the meio-spores (100-200 meio-spores per unilocular sporangium) directly into the 300 µl drop of test or control solution. Release of the meio-spores from the unilocular sporangia should take less than 48 h. Discard any plates in which release has not occurred within this time frame.
- After meio-spore release, remove the piece of upright filament with forceps.
- After 72 h, gently add 10 ml of PES to the Petri dish. The meio-spore-derived germlings will be weakly attached to the coverslip at this stage and the added medium assures that they grow under optimal conditions.
- After an additional three or four days examine the Petri dish under an inverted microscope to score the numbers of gametophyte and sporophyte individuals (Figure 4). Sporophytes can be distinguished from gametophytes based on a symmetrical pattern of initial cell division and the presence of thick walled round cells rather than wavy rhizoid cells. Note that, if meio-spores are released within 48 h but then germinate slowly, the germination process can be accelerated by adding an additional 300 µl of the test medium to the 300 µl drop. This will allow the germlings to grow and attach to the coverslip before the plate is flooded with the 10 ml of PES.
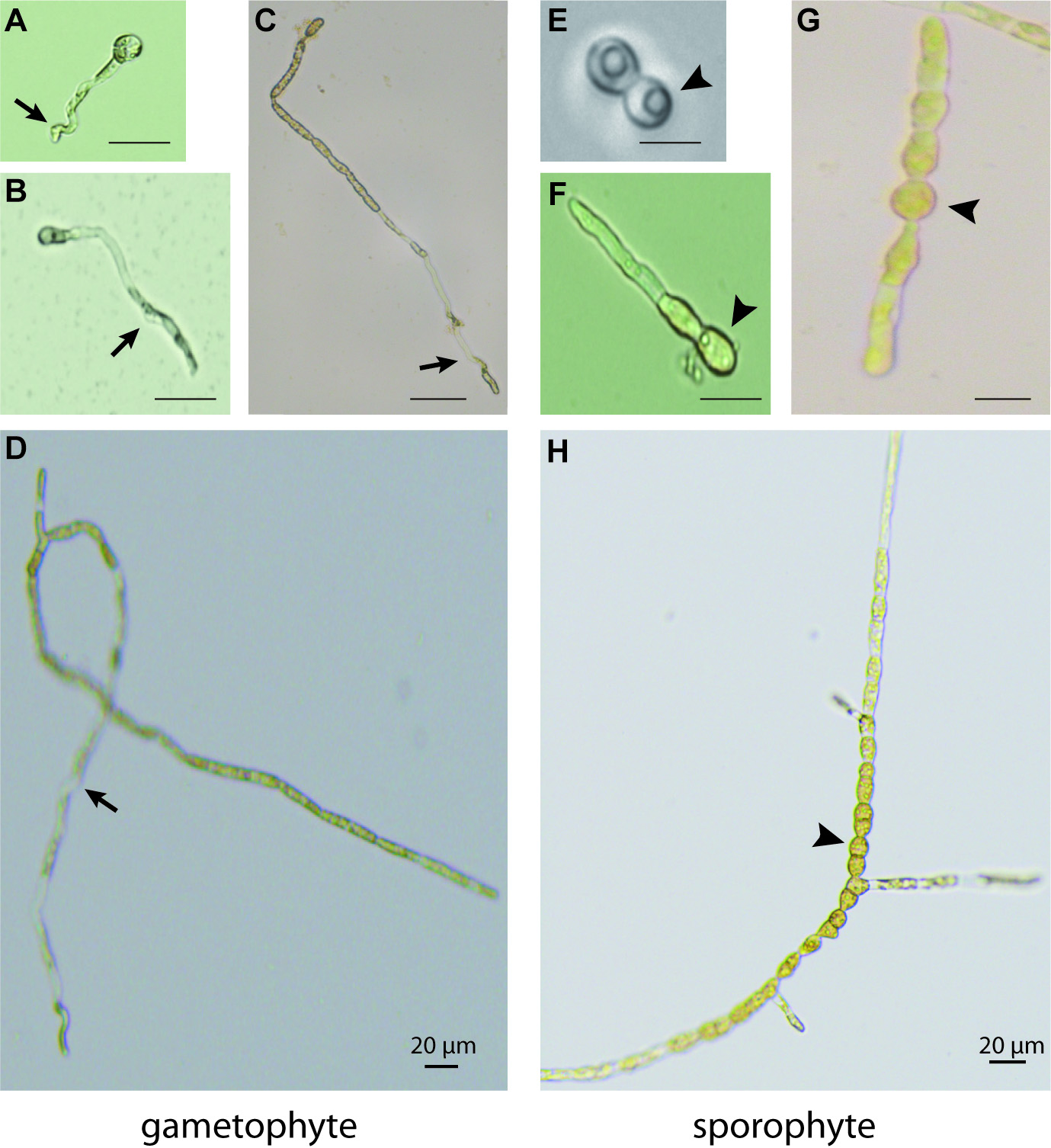
Figure 4. Morphological differences between gametophytes (normal development, left) and sporophytes (modified development, right) derived from SCM-treated meio-spores. Meio-spores were incubated in SCM for three days and then grown in PES. A. and E. two-cell stages of the gametophyte (asymmetrical cell division) and the sporophyte (symmetrical cell division), respectively. B. and F. gametophyte and sporophyte germlings, respectively, after three days in SCM. C. and G. gametophyte and sporophyte germlings, respectively, after five days in SCM. D. and H. gametophyte and sporophyte germlings, respectively, after 14 days in SCM. Arrows indicate wavy rhizoid cells typical of the gametophyte germling, arrowheads indicate round cells typical of the sporophyte germling. Scale bars: 20 µm. - When working with active preparations of SCM, expect between 2% and 30% of the meio-spores to be switched from gametophyte to sporophyte identity but note that the percentage of switching can be highly variable between assays. It is therefore preferable to carry out at least three assays for each test to obtain statistically robust estimations of diffusible factor activity.
- Under a binocular microscope, dissect a piece of sporophyte upright filament that bears one or more unilocular sporangia using a sterile glass Pasteur pipette that has been broken to create a sharp, cutting point (Figure 3).
- Production of unilocular sporangia containing meio-spores
Data analysis
Use the Wilcoxon rank sum test with Holm-Bonferroni P-value adjustment to determine whether test samples produce significantly more sporophyte individuals than the PES control treatments (Figure 5).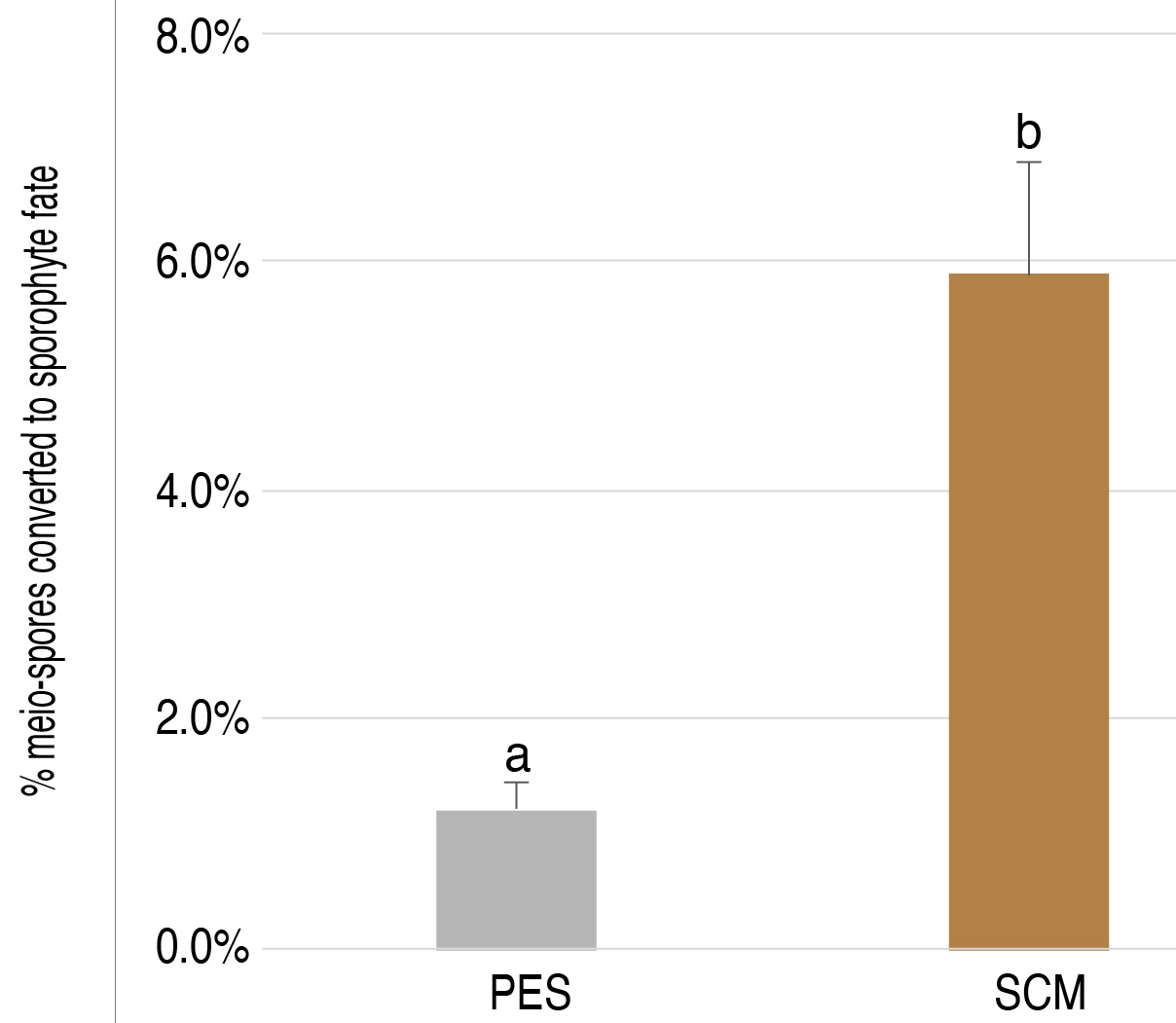
Figure 5. Example of data obtained using the diffusible factor bioassay. Percent of germlings that exhibited sporophyte morphology following the release of meio-spores from unilocular sporangia treated with either Provasoli-enriched natural seawater (PES, 69 unilocular sporangia, 7040 meio-spores) or sporophyte-condition medium (SCM, 103 unilocular sporangia, 9106 meio-spores). Error bars indicate standard error of the mean, letters above bars indicate significant differences (P-value < 0.05).
Notes
One difficulty with this procedure is that the percentage of meio-spores that switch from the gametophyte to the sporophyte developmental program can be very variable (between 2% and 30%). This appears to due to two factors, variation in the quantity of diffusible factor in different batches of SCM and variation in the capacity of meio-spores to respond to the diffusible factor. The exact cause of this variation has not been determined but it is important that the operator be prepared to carry out additional replicate tests if the effect of the SCM is weak.
Recipes
- Provasoli solution
Solution 1
1.9 g H3BO3
0.05 g FeCl3
0.273 g MnSO4·H2O
0.0367 g ZnSO4·7H2O
0.008 g CoSO4·7H2O
11.4 ml EDTA (0.5 M, pH 8)
ad 1,000 ml ddH2O
Solution 2
3.35 mg Vitamin B12 (cyanocobalamine)
165 mg Thiamine hydrochloride (vitamin B1)
1.65 mg Biotin C10H16N2O3S
166.5 g TRIS (Trisma base) C4H11NO3
ad 500 ml ddH2O
Solution 3
1.17 g (NH4)2Fe(SO4)2·6H2O
6.8 mL EDTA (0.5 M, pH 8)
ad 1,000 ml ddH2O
Solution 4
23 g NaNO3
ad 1,000 ml ddH2O
Solution 5
3.33 g C3H7Na2O6P·5H2O “glycerophosphate”
ad 1,000 ml ddH2O
Prepare each stock solution separately, autoclave, and store in glass bottles at 4 °C. Use a dark bottle for Solution 2. For 1 L of Provasoli solution, add 100 ml of each of Solutions 1, 3, 4, and 5 plus 10 ml of Solution 2 to ddH2O (the starting pH should be between 9.6 and 9.8). Adjust to pH 7.8 with concentrated HCl (37%) and adjust the volume to 1 L with ddH2O. Aliquot into small glass bottles (20, 50, 100, or 200 ml), autoclave, and store at 4 °C. - Provasoli-enriched natural seawater (PES)
If possible, collect natural seawater offshore.- Prepare 1 L of natural seawater by filtering through a 5 µm mesh and autoclaving in a plastic bottle
- Add 20 ml of Provasoli solution
- Autoclaved natural seawater can be stored at 13 °C before addition of the Provasoli solution and PES can be stored at 4 °C
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, the European Research Council (grant agreement 638240) and Sorbonne University. Support was also provided by the French National Research Agency via the investment expenditure programmes IDEALG (ANR-10-BTBR-04-02) and Epicycle (ANR-19-CE20-0028-01). HY was supported by a grant from the Chinese Scholarship Council (grant number 201608310119). Previous versions of this method were reported in Arun et al. (2013 and 2019).
Competing interests
The authors have no competing interests.
References
- Arun, A., Coelho, S. M., Pétèrs, A. F., Bourdareau, S., Peres, L., Scornet, D., Strittmatter, M., Lipinska, A. P., Yao, H., Godfroy, O., Montecinos, G. J., Avia, K., Macaisne, N., Troadec, C., Bendahmane, A. and Cock, J. M. (2019). Convergent recruitment of TALE homeodomain life cycle regulators to direct sporophyte development in land plants and brown algae. Elife 8: e43101.
- Arun, A., Peters, N. T., Scornet, D., Peters, A. F., Cock, J. M. and Coelho, S. M. (2013). Non-cell autonomous regulation of life cycle transitions in the model brown alga Ectocarpus. New Phytol 197(2): 503-510.
- Bothwell, J. H., Marie, D., Peters, A. F., Cock, J. M. and Coelho, S. M. (2010). Role of endoreduplication and apomeiosis during parthenogenetic reproduction in the model brown alga Ectocarpus. New Phytol 188(1): 111-121.
- Cock, J. M., Godfroy, O., Macaisne, N., Peters, A. F. and Coelho, S. M. (2014). Evolution and regulation of complex life cycles: a brown algal perspective. Curr Opin Plant Biol 17: 1-6.
- Coelho, S. M., Godfroy, O., Arun, A., Le Corguille, G., Peters, A. F. and Cock, J. M. (2011). OUROBOROS is a master regulator of the gametophyte to sporophyte life cycle transition in the brown alga Ectocarpus. Proc Natl Acad Sci U S A 108(28): 11518-11523.
- Coelho, S. M., Scornet, D., Rousvoal, S., Peters, N. T., Dartevelle, L., Peters, A. F. and Cock, J. M. (2012a). Ectocarpus: a model organism for the brown algae. Cold Spring Harb Protoc 2012(2): 193-198.
- Coelho, S. M., Scornet, D., Rousvoal, S., Peters, N. T., Dartevelle, L., Peters, A. F. and Cock, J. M. (2012b). How to cultivate Ectocarpus. Cold Spring Harb Protoc 2012(2): 258-261.
- Müller, D. G. (1967). Generationswechsel, Kernphasenwechsel und Sexualität der Braunalge Ectocarpus siliculosus im Kulturversuch. Planta 75(1): 39-54.
- Peters, A. F., Marie, D., Scornet, D., Kloareg, B. and Mark Cock, J. (2004). Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J Phycol 40(6): 1079-1088.
- Peters, A. F., Scornet, D., Ratin, M., Charrier, B., Monnier, A., Merrien, Y., Corre, E., Coelho, S. M. and Cock, J. M. (2008). Life-cycle-generation-specific developmental processes are modified in the immediate upright mutant of the brown alga Ectocarpus siliculosus. Development 135(8): 1503-1512.
- Starr, R. C. and Zeikus, J. A. (1993). UTEX-The culture collection of algae at the University of Texas at Austin 1993 list of cultures. J Phycol 29: 1-106.
Article Information
Copyright
Yao et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yao, H., Scornet, D., Badis, Y., Peters, A. F., Jam, M., Hervé, C., Potin, P., Coelho, S. M. and Cock, J. M. (2020). Production and Bioassay of a Diffusible Factor that Induces Gametophyte-to-Sporophyte Developmental Reprogramming in the Brown Alga Ectocarpus. Bio-protocol 10(18): e3753. DOI: 10.21769/BioProtoc.3753.
- Arun, A., Coelho, S. M., Pétèrs, A. F., Bourdareau, S., Peres, L., Scornet, D., Strittmatter, M., Lipinska, A. P., Yao, H., Godfroy, O., Montecinos, G. J., Avia, K., Macaisne, N., Troadec, C., Bendahmane, A. and Cock, J. M. (2019). Convergent recruitment of TALE homeodomain life cycle regulators to direct sporophyte development in land plants and brown algae. Elife 8: e43101.
Category
Developmental Biology > Cell signaling > Fate determination
Plant Science > Phycology > Physiology
Cell Biology > Cell signaling > Development
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










