- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Long-term Devocalization of Zebra Finches
(*contributed equally to this work) Published: Vol 10, Iss 18, Sep 20, 2020 DOI: 10.21769/BioProtoc.3752 Views: 4449
Reviewed by: Trevor Martin SmithMohammed Mostafizur RahmanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Studying the Mechanisms of Developmental Vocal Learning and Adult Vocal Performance in Zebra Finches through Lentiviral Injection
Zhimin Shi [...] XiaoChing Li
Sep 5, 2018 6347 Views

Minimally Invasive Oral Surgery Induction of the FRICT-ION Chronic Neuropathic Pain Model
Marena A. Montera and Karin N. Westlund
Apr 20, 2020 5117 Views

Induction of Repeated Social Defeat Stress in Rats
Soumyabrata Munshi [...] J. Amiel Rosenkranz
Feb 5, 2022 3638 Views
Abstract
Songbirds, such as the zebra finch, are a popular animal model for studying the neural basis of vocal and complex skill learning. Adult male zebra finches produce courtship song toward females (referred to as ‘directed song’) and recording and analyzing sounds of directed song along with underlying neural activity is important for investigating behavioral and neural mechanisms of song production and learning. However, recording of directed song is easily contaminated by calls that are often as loud as directed songs and frequently produced by a female bird is presented in the same sound-recording chamber to elicit directed song. We developed a new surgical procedure to relatively easily and almost completely devocalize female zebra finches semi-permanently, without affecting other behaviors. This procedure enables researchers to record directed songs with almost no contamination by female calls. The procedure can also be used to devocalize male birds as well and, thus, has great potential for a variety of experimental purposes, such as long-term elimination of auditory feedback during singing in male birds.
Keywords: BirdsongBackground
Just as human infants learn to produce complex speech sounds from adults, young songbirds learn to produce complex vocalizations (i.e., song) from adult tutors during a critical period of development. Because general animal models in life science research, such as mice and monkeys, do not exhibit vocal learning, songbirds offer a great opportunity to study neural substrates of vocal learning, an essential element of human speech acquisition (Doupe and Kuhl, 1999; Bolhuis et al., 2010). Moreover, birdsong learning critically depends on a basal ganglia-thalamo-cortical circuit that is discrete and specialized for this single task. This circuitry contrasts sharply with homologous mammalian circuits. Thus, songbirds are considered a tractable model system for studying neural mechanisms underlying basal ganglia-dependent motor skill learning (Doupe et al., 2005).
In the zebra finch, the most popular songbird species used for neurobiological study of birdsong learning, adult male birds sing in two social contexts: singing toward females in a courtship context (directed singing) and singing alone in a solo context (undirected singing). A large body of evidence suggests that undirected singing is a state of vocal practice in which birds improve and optimize song structure, whereas directed singing is a state of motor performance in which birds exploit what they have already learned to impress a female (Jarvis et al., 1998; Kao et al., 2005 and 2008; Kojima and Doupe, 2011; Kojima et al., 2018). Thus, comparing song structure and neural activity between the two social contexts is a standard and important approach to examine behavioral and neural mechanisms of song learning. In contrast with recordings of undirected song, which a male bird produces in isolation, recordings of directed songs are often contaminated by vocalizations of a female bird presented to elicit directed song. Female birds often produce loud calls while the male is singing, and their calls often overlap with male songs. Such contamination with female calls can cause serious deterioration of data quality and in many cases, results in exclusion of song data from analyses. To avoid such contamination, we developed a relatively easy surgical procedure to devocalize female birds almost permanently.
Several previous studies have reported surgical procedures to devocalize birds, but they are either technically difficult (Cooper and Goller, 2004) or unsuitable for long-term devocalization (Pytte and Suthers, 1999). The current protocol describes a novel method for devocalizing female zebra finches using tubing inserted into the bronchi to prevent adduction of the lateral labia into the expiratory air stream that is responsible for phonation (Goller and Larsen, 1997; Larsen and Goller, 2002). This method enables us to devocalize birds almost permanently without great technical difficulties. Because this devocalization protocol can be applied to males as well as females, it can be used in both sexes for investigating the role of vocalizations in social communication, including courtship behavior and social bonding.
Materials and Reagents
- Surgical tape (3M, Durapore, catalog number: 1538-0 )
- Kim wipe (Yuhan-Kimberly, catalog number: 41112 )
- Toothpick (Young Star)
- Polyimide tubing (Wilco, I.D. 0.6 mm, O.D. 0.72 mm, catalog number: PITU-0610 )
- Absorbable suture (AILEE, Surgifit, catalog number: AV818 )
- Tissue adhesive (3M, Vetbond, catalog number: 1469SB )
- Syringe with needle (Feel Tech Bio Co., catalog number: 30G-0.5-5/16 )
- Syringe filter (ADVANTEC, catalog number: DISMIC-13CP )
- 15 ml conical tube (Hyundai Micro, catalog number: H20015 )
- Lidocaine HCl, 2% (Dai Han Pharm. Co., Ltd.)
- Ketoprofen (Unibiotech Co. Ltd., UNI KETOPRO inj.)
- Pentobarbital solution for anesthesia (see Recipes)
Entobar (100 mg/2 ml Pentobarbital Sodium, Hanlim Pharm. Co., Ltd.)
Propylene glycol (Sigma-Aldrich, catalog number: P4347 )
100% EtOH (Sigma-Aldrich, catalog number: E7023-500ML )
Equipment
- Sharp forceps (Fine Science Tools, catalog number: 11254-20 )
- Spring scissors (Fine Science Tools, catalog number: 15018-10 )
- Needle holder (Fine Science Tools, catalog number: 12565-14 )
- Scalpel blade (Fine Science Tools, catalog number: 10011-00 )
- Blade holder (Fine Science Tools, catalog number: 91003-12 )
- Body holder made of clay (Bostik, Blu Tack Original; dimensions W55 x D40 x H35 mm; Figures 1A-1D) attached on a retraction system (Fine Science Tool, Magnetic Fixator Retraction System, catalog number: 18200-03; Figure 1E )
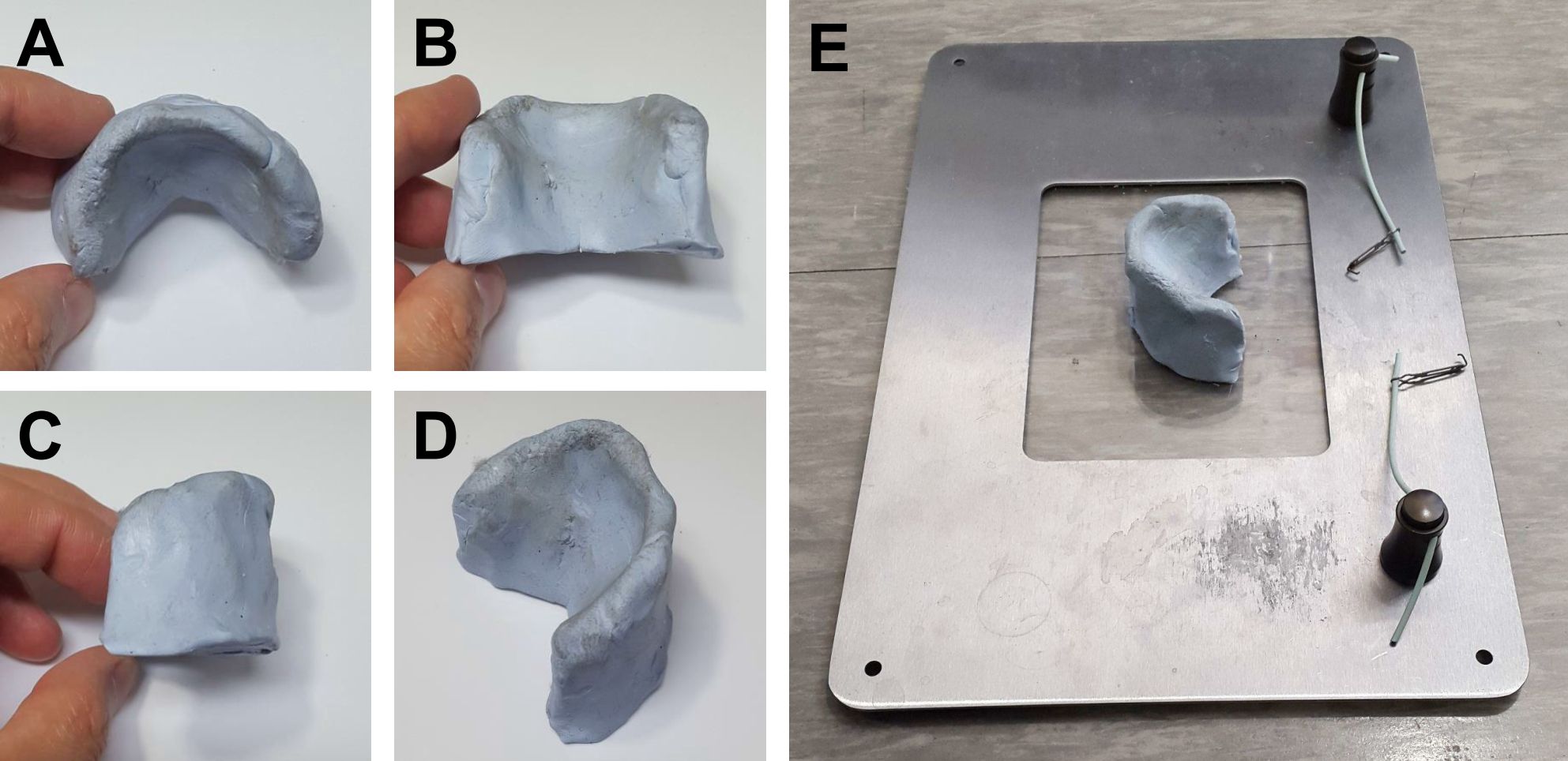
Figure 1. Body holder and retraction system. A-D. Top (A), front (B), side (C), and diagonally upward (D) views of a body holder. E. A body holder attached on a retraction system. - Cauterizer (Fine Science Tool, catalog number: 18010-00 ); the tip of the cauterizer is tapered using a longnose plier (Figure 2)

Figure 2. Tapered tip of cauterizer (blue arrow) - Surgical microscope (Leica, model number: M320 )
- Heating lamp (Forsens Lights Co., Ltd., model number: SJ-10 )
- UV sterilizer (Bluezone, model number: CM-1-1 )
- Bird cage (Quality Cage Crafters, model number: CC-1 , dimensions: 8"w x 8"d x 8"h)
- Pipette (Nichiryo, Benchimate 1000)
- Crimp-top vial and Aluminum Seal with Septa for Pentobarbital solution (LK Lab Korea, catalog numbers: V01-214-228 and V01-214-603 , respectively)
- Vortex shaker (IKA, Vortex 1)
Procedure
The devocalization protocol consists of two major components, (A) tubing making and (B) surgical insertion of tubing into the bronchi to prevents adduction of the lateral labia.
- Tubing making
- Cut ~2.5-30 mm long polyimide tubing.
- Cut out the middle part and the edges as shown in Figures 3A and 3B using a blade or a spring scissors. Make sure that the cut ends are smooth.
- Fold it in two (Figure 3C) using forceps as shown in Figure 3D.
- Sterilize tubing with a UV sterilizer.
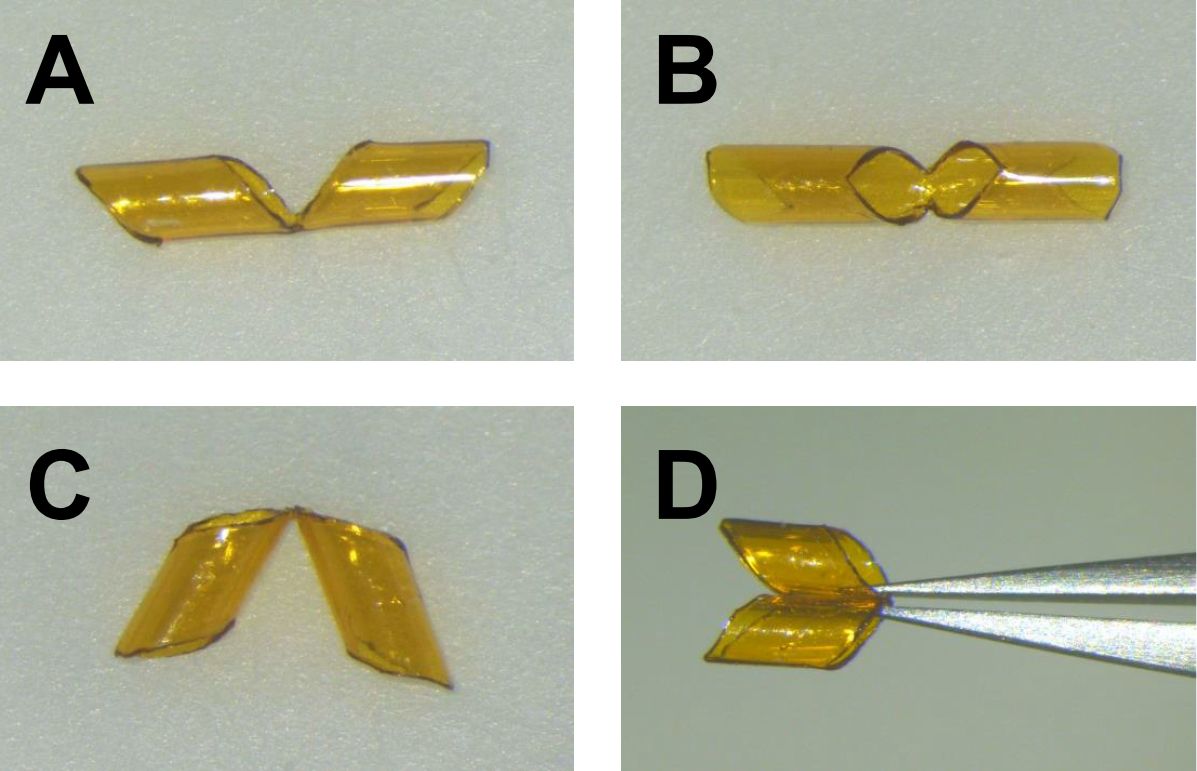
Figure 3. Polyimide tubing to be inserted into bronchi. A-B. Side view (A) and top view (B) of tubing after cutting out the middle part and the edges. C. Side view of tubing after being folded. D. Tubing folded by forceps.
- Surgery
- Hold a bird supine by pinning the bird’s neck with a forefinger and bird’s legs with a little finger (Figures 4A-B). Put a drop of 70% EtOH on the chest and expose the skin above the left pectoral muscle by putting the feathers aside using a finger (Figure 4C). Inject Pentobarbital solution (40 µl for a 12-g bird [50 mg/kg]; see Recipes) into the left pectoral muscle using a 30-gauge needle (Figure 4D). The bird typically becomes immobile within 10 min.
Note: Other anesthetics injections can be used as well, but inhalation anesthesia (e.g., isoflurane) is not suitable for this surgery because the surgical procedure includes incisions in the respiratory system, through which anesthetic gas can easily leak out of the body.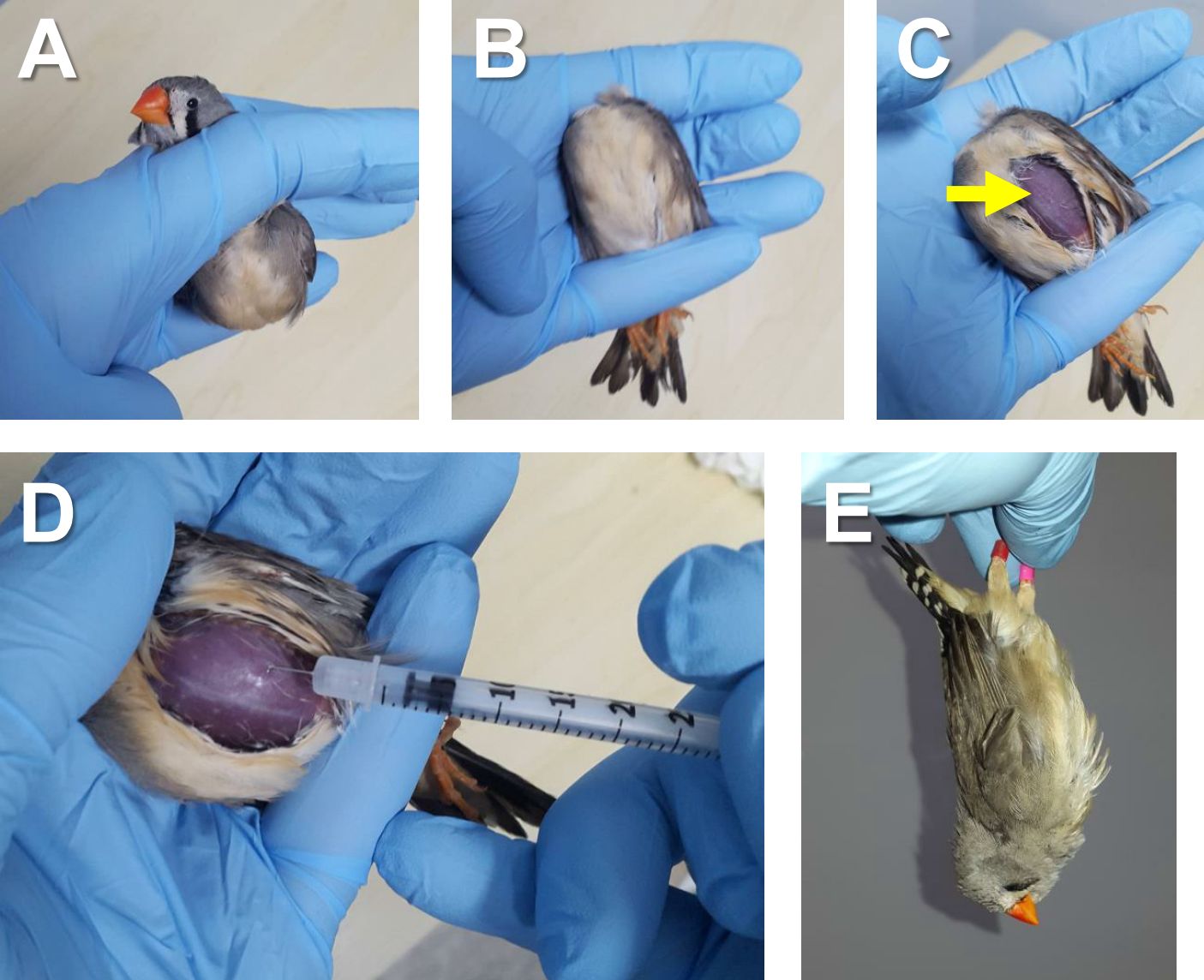
Figure 4. Administration of anesthesia. A-B. Holding a bird supine by pinning bird’s neck with a forefinger (A) and bird’s legs with a little finger (B). C. Exposed skin above the left pectoral muscle (yellow arrow). D. Injection of Pentobarbital solution into the left pectoral muscle using a 30-gauge needle. E. Checking leg reflex by holding the legs and hanging the bird’s body. - Check for leg reflex by holding legs and hanging the bird’s body (Figure 4E) to assure full anesthesia. If the bird remains immobile in the hanging position, proceed to the next step. If not, inject 10 µl of Pentobarbital solution and wait for 10 more min. Repeat this procedure until the bird is immobile in the hanging position.
- Pluck feathers around the upper chest and lower neck areas by pinching and pulling out a few feathers at a time with fingers.
- Put the bird on the body holder (Figure 5A) and tape the legs and head on the base plate with surgical tape as shown in Figure 5B.
Note: Make sure that the neck is well-stretched and that the head is pulled back well enough. Otherwise the syrinx will not be fully exposed in the following steps.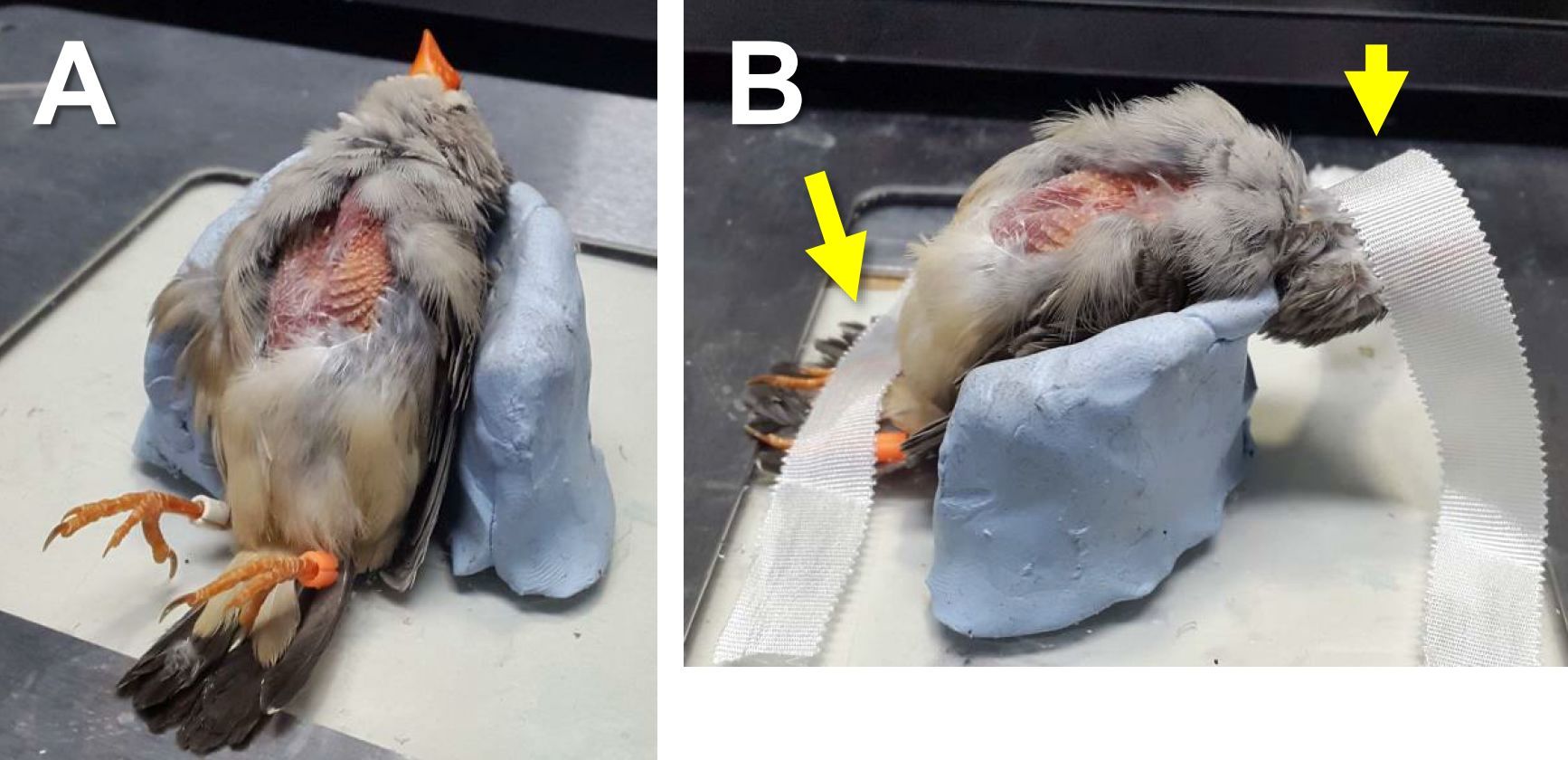
Figure 5. A bird laid on the body holder (A) and taped on the base plate (B). The yellow arrows indicate surgical tape. - Wipe the skin around the lower neck and upper chest areas with 70% EtOH.
- Lift the skin around the lower neck area using sharp forceps and inject lidocaine HCl, 2% (approx. 50 μl) subcutaneously.
- Make an incision (10-15 mm long) in the skin along the midline from the lower neck to the upper chest (Figure 6).
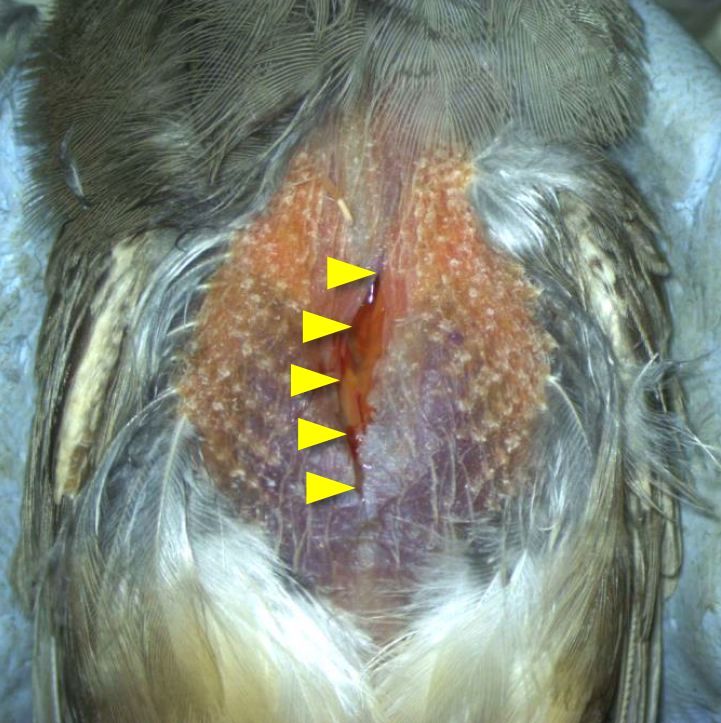
Figure 6. An incision in the skin along the midline from the lower neck to upper chest (yellow triangles) - Separate adipose tissue from pectoral muscles using scissors and forceps, and push the adipose tissue toward the beak. This action will expose the interclavicular air sac (Figures 7A-7B).
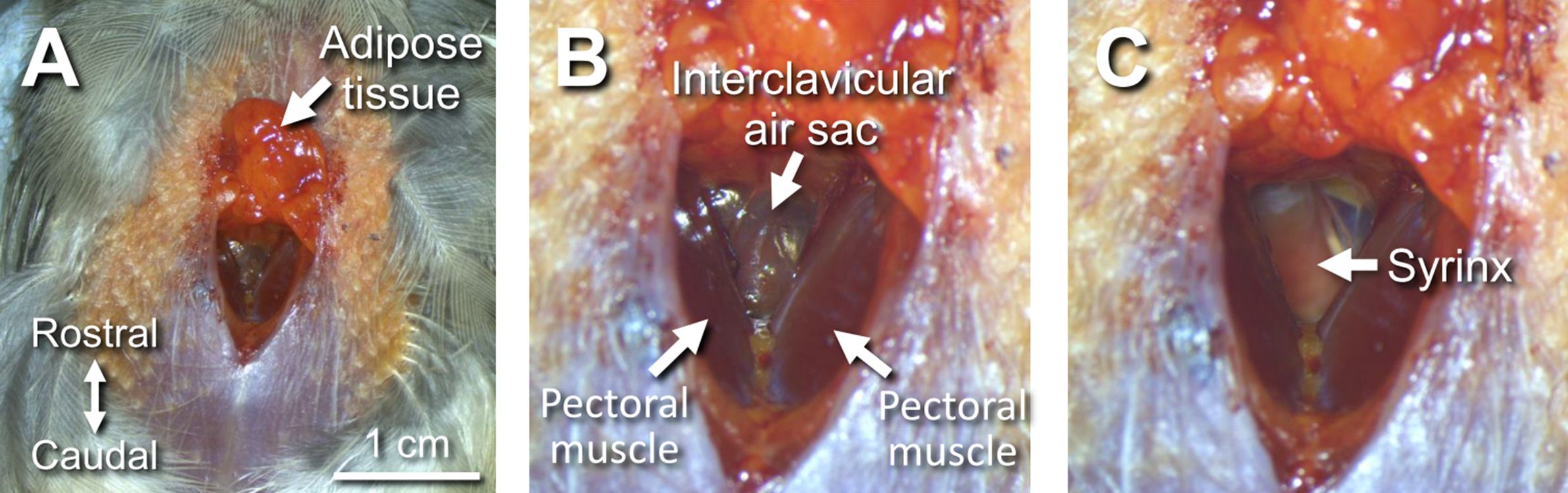
Figure 7. Exposing and cutting open the interclavicular air sac. A. Distant view of the chest area after pushing the adipose tissue toward the beak. B. Magnified view of A, showing the interclavicular air sac between the right and left pectoral muscles. C. After cutting open the air sac, the syrinx is seen in the body cavity. - Cut open the interclavicular air sac with spring scissors, revealing the syrinx in the body cavity (Figure 7C).
- Attach retractors to the edges of the pectoral muscles and pull them to the sides (Figure 8). Adjust body and head positions so that the entire syrinx is clearly visible.
Note: When pulling the retractors to the sides, make sure that they do not prevent the chest movements for breathing; the breathing cycle should be always regular and > 1 Hz.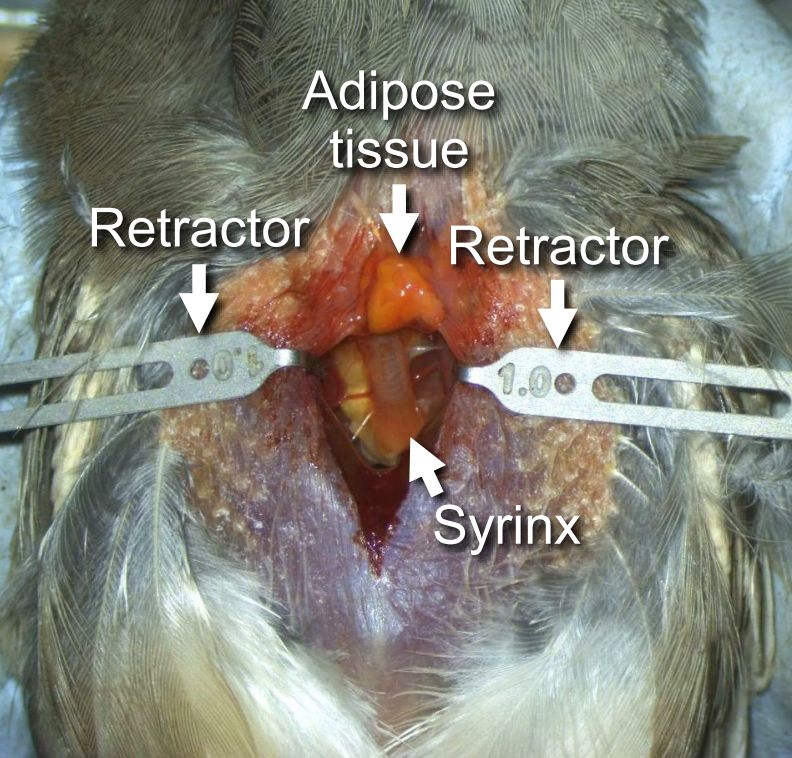
Figure 8. The syrinx exposed - Completely cauterize blood vessels connecting to the syrinx (oesophagotracheobronchial arteries) (blue arrows in Figure 9). This step is critical for reducing bleeding from an incision in the syrinx that will be made in Step B12.
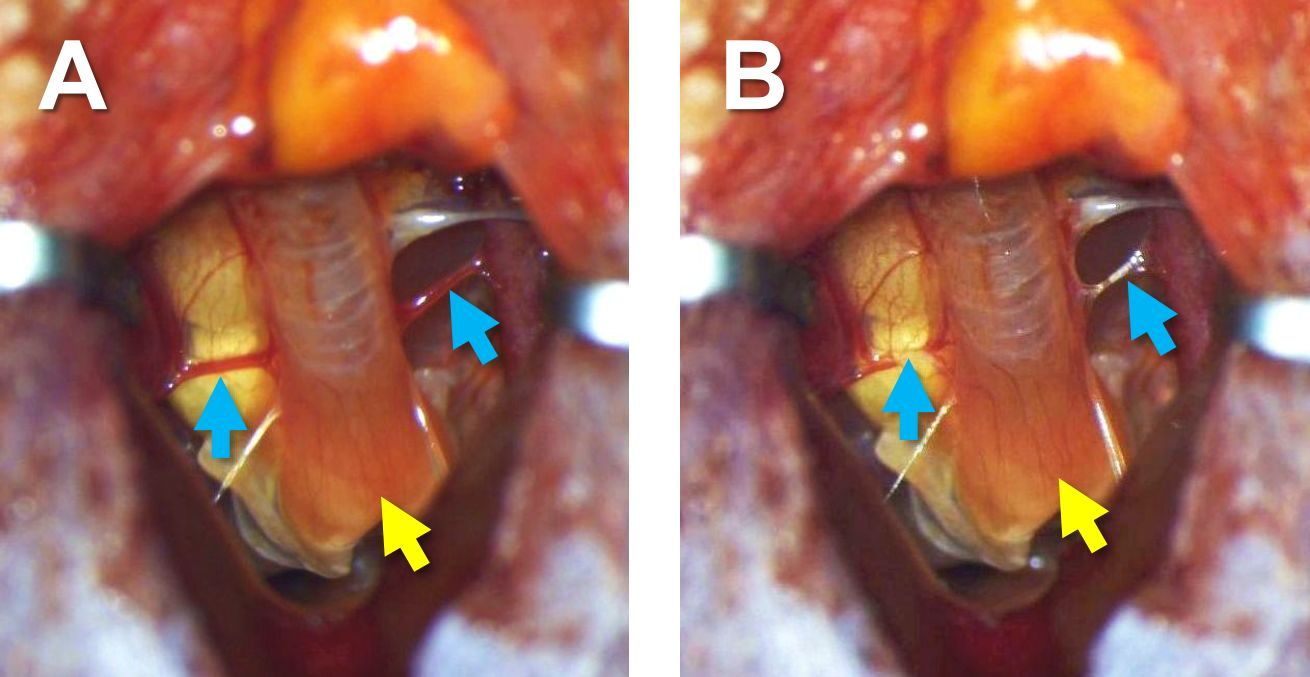
Figure 9. The blood vessels before (A) and after (B) cauterization. Blue arrows indicate sites of cauterization on blood vessels, and yellow arrows indicate the syrinx. - Make a horizontal incision on the very bottom and ventral part of the trachea just above the syrinx using spring scissors (Figure 10A), and then make a vertical incision in the ventral side of the syrinx along the midline (Figure 10B).
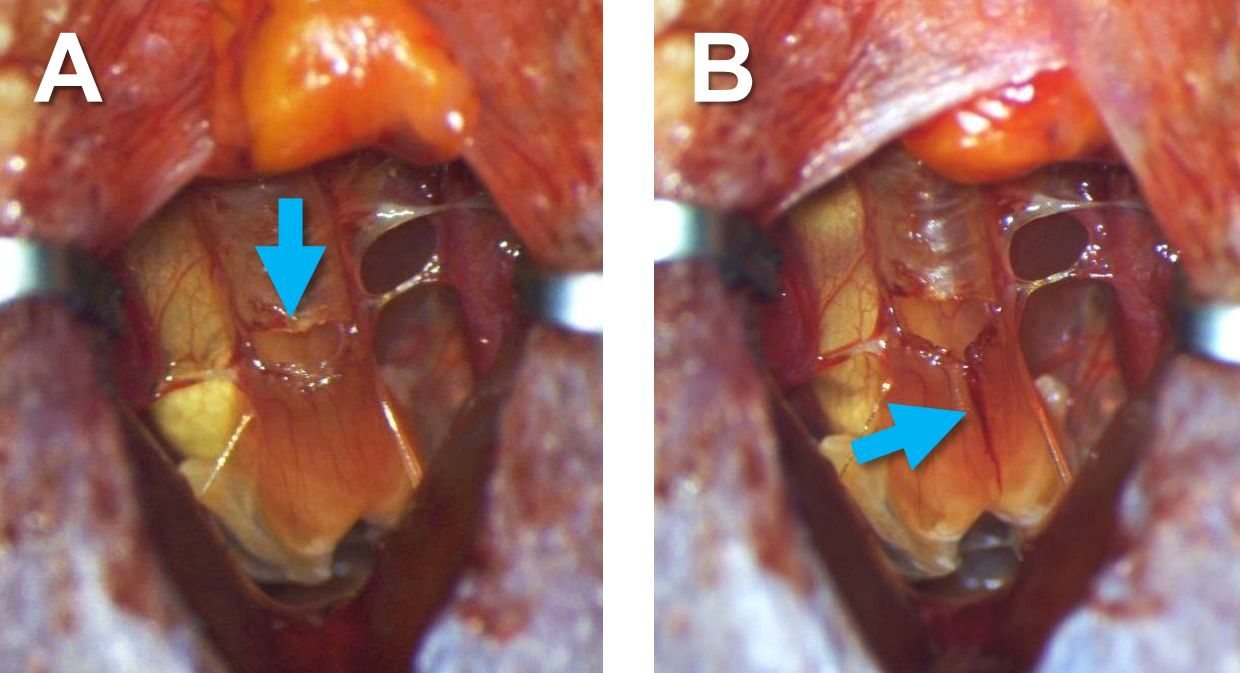
Figure 10. A horizontal incision made on the trachea (arrow in A) and a vertical incision made in the syrinx (arrow in B) - Gently open the syrinx using forceps and make sure that the openings of the bronchi inside the syrinx are visible (Figure 11A). If bleeding from the incision occurs, put a piece of twisted Kimwipe on the incision and leave it for a while to absorb the blood. Make sure no blood is present inside the syrinx, or the bird will have difficulty breathing after surgery.
- Hold the tubing to keep it folded with forceps (as shown in Figure 3D), open the vertical incision of the syrinx with other forceps (Figure 11A), and insert the tubing into the openings of the bronchi inside the syrinx (Figure 11B).
Note: Make sure both ends of the tubing are properly inserted into the bronchi so that it prevents adduction of the lateral labia into the expiratory air stream. The position of the tubing can be checked by looking inside the tubing from the top; if the tubing is properly positioned, one can see the inner wall of the bronchi continuing deep inside through the tubing. - Leave the incision of the syrinx as it is (Figure 11C) and remove the retractors.
Note: Closing the incision using surgical adhesive is not recommended because it could interfere with air flow inside the syrinx and decrease the success rate of the surgery.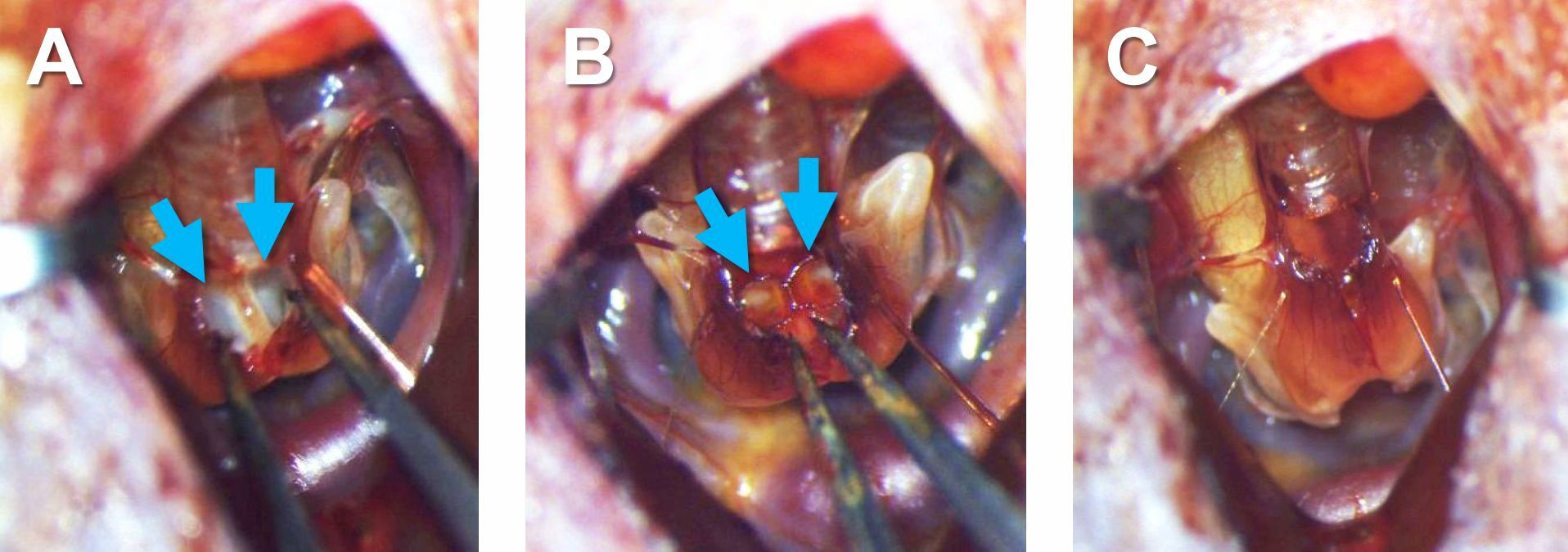
Figure 11. Insertion of tubing into bronchi. A. The inside of the syrinx before inserting tubing. Arrows indicate the openings of the bronchi. B. The inside of the syrinx after inserting tubing into the bronchi. Arrows indicate the right and left parts of the tubing. C. The syrinx after inserting the tubing. - Put back the adipose tissue to the original position (between the right and left pectoral muscles), and glue it to the muscles with tissue adhesive using toothpick and forceps (Figures 12).
Note: When gluing adipose tissue, it is critical to glue the entire area of the adipose tissue touching to the muscle. Otherwise, air may enter or exit the body cavity through the opening between the adipose tissue and the muscle. Such abnormal breathing will prevent healing of the incision in the syrinx.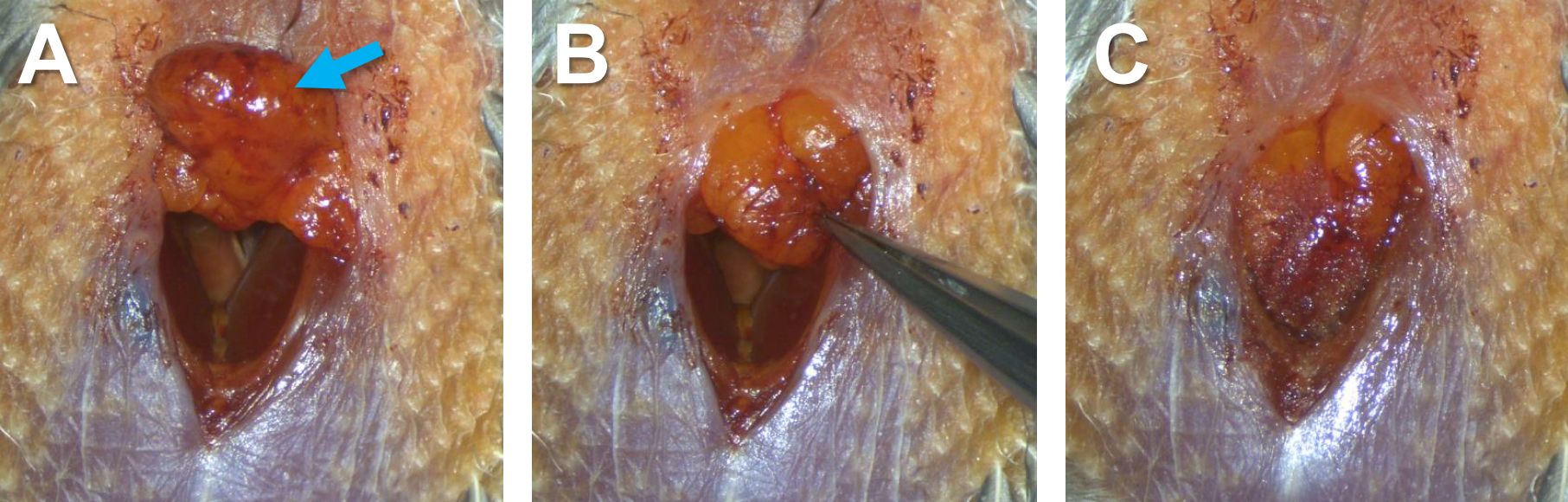
Figure 12. Gluing dispose tissue to pectoral muscles. Adipose tissue before being glued (A, blue arrow), partly glued to the left pectoral muscle (B), and (C) completely glued to the left and right pectoral muscles. - Close the incision by suturing the skin from the top to the bottom of the wound (Figure 13). Start with puncturing the left and right sides of the skin with a suture needle (Figures 13A-13C), knot a tie there to fix the thread in place, continue to suture toward the bottom of the wound (Figures 13D-13E), and end the suture with another tie to prevent loosening (Figure 13F).
Note: Make sure that no air goes in and out through the incision; if air leaks, a part of the incision periodically opens along with a breathing cycle.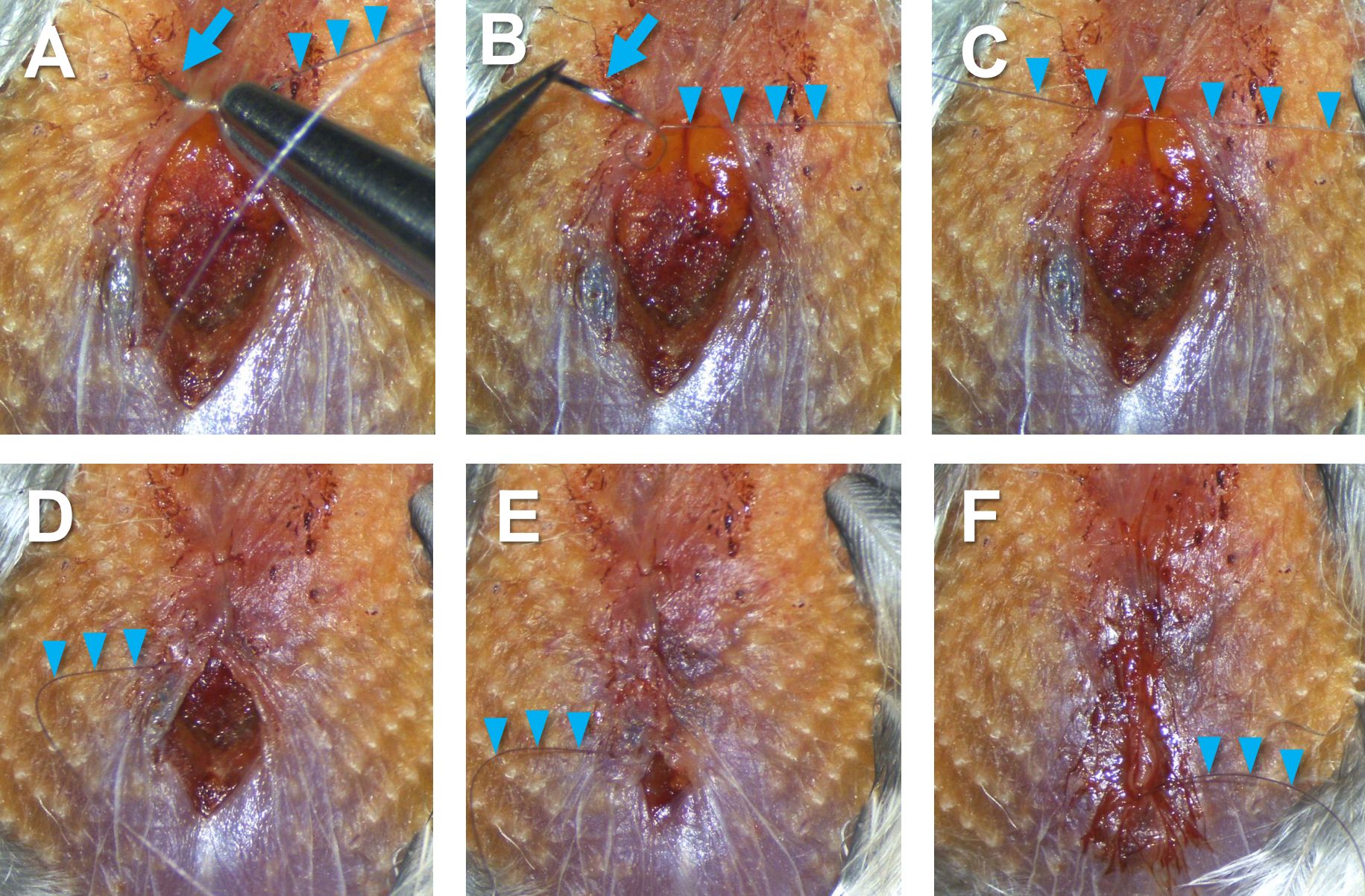
Figure 13. Closure of the incision by suturing the skin. Arrows indicate a suture needle; triangles indicate a suture thread. - Inject Ketoprofen (5 mg/kg) into the pectoral muscle using a 30-gauge needle (as shown in Figure 4D) to alleviate potential pain after the anesthetic wears off.
- Put the bird in a small cage and keep a heating lamp on until the bird fully recovers. Birds normally recover within 12 h; signs of full recovery are frequent hopping and flying in the cage without puffiness of body feathers.
Note: If a bird does not show signs of full recovery in one day after surgery, euthanize it immediately. If a bird produces wheezing sounds after full recovery, it is likely that tubing inserted into the bronchi is partially clogged with blood or mucus. This condition can be resolved by redoing the surgery and cleaning or replacing the tubing.
- Hold a bird supine by pinning the bird’s neck with a forefinger and bird’s legs with a little finger (Figures 4A-B). Put a drop of 70% EtOH on the chest and expose the skin above the left pectoral muscle by putting the feathers aside using a finger (Figure 4C). Inject Pentobarbital solution (40 µl for a 12-g bird [50 mg/kg]; see Recipes) into the left pectoral muscle using a 30-gauge needle (Figure 4D). The bird typically becomes immobile within 10 min.
Data analysis
Birds that underwent devocalization surgery begin behaving normally immediately after full recovery and can be used for other experiments, including induction of directed singing of male birds. Devocalized females do not usually show motor disabilities or difficulty in breathing (see Video 2). In contrast with intact females, which produce loud calls (Figure 14A and Video 1), devocalized females are almost completely mute; they produce only very quiet, whispered sounds of short duration (Figures 14B-14C and Video 2), which hardly contaminate directed song recordings.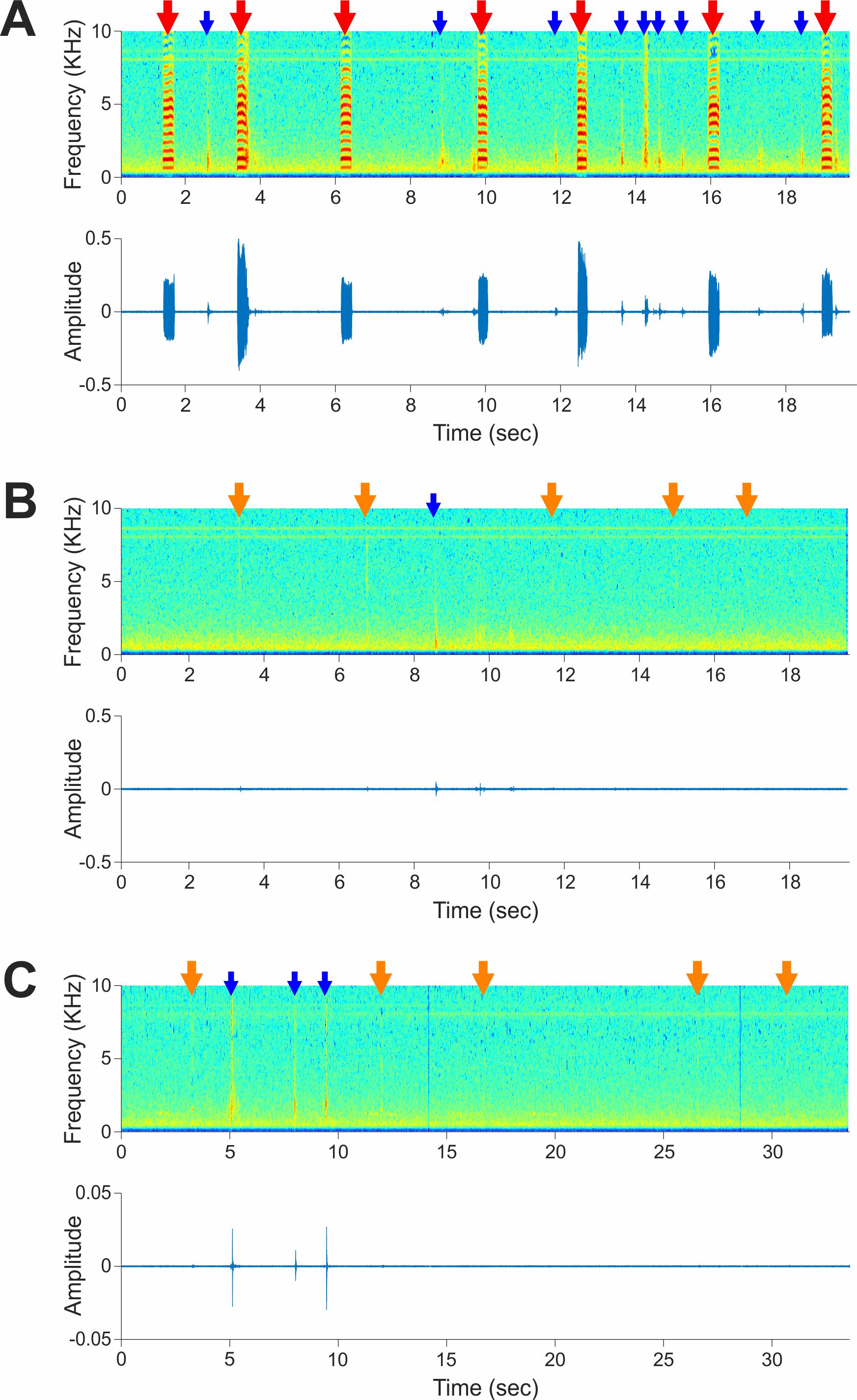
Figure 14. Devocalization surgery mostly eliminates female vocalizations. A. Vocalization of a female bird one day before devocalization surgery, shown as a spectrogram (top) and sound waveform (bottom). Red arrows indicate vocalizations (calls) and blue arrows indicate motion noises. See also Video 1, which was recorded simultaneously with this sound recording. B. Sound recording from the same bird one day after devocalization surgery. Orange arrows indicate the time points where the bird produced whispered sounds that were identified by visual inspection of the video simultaneously recorded (Video 2). C. Sound recording from the same bird 34 days after devocalization surgery. Conventions are as in B. See also Video 3, which was recorded simultaneously.
Notes
- Check breathing of the bird throughout the surgery. If it becomes irregular or abnormally slow (approx. < 1 Hz), adjust retractors so they do not interfere with breathing. If breathing is still irregular or slow, interrupt the surgery and keep the bird under a heat lamp until regular breathing recovers.
- During surgery, minor bleeding often occurs from incisions in the skin, dispose tissue, or syrinx. Such bleeding can be stopped by putting a piece of twisted Kimwipe on the incision and leaving it for a while, or by cauterizing the bleeding site. More serious bleeding will occur if a major blood vessel in the body cavity is accidentally nicked. Control of bleeding is difficult in these cases, and the bird should be euthanized immediately.
Recipes
- Pentobarbital solution for anesthesia
Entobar (100 mg/2 ml Pentobarbital Sodium)
Propylene glycol
100% EtOH
ddH2O
Mix Entobar (2 ml), propylene glycol (4.2 ml), EtOH (1 ml), and ddH2O (2.8 ml) in 15 ml conical tube using a voltex shaker. The mixture (10.0 ml) contains 10 mg/ml of Pentobarbital
Filter the solution using a syringe filter and store it in a crimp-top vial with a septum at room temperature
Acknowledgments
This work was supported by grants from the KBRI basic research program (Grant 18-BR-01-06 to S.K.). The original research paper where this protocol was used is (Kojima et al., 2013 and 2018). We thank M. H. Kao and J. Ahn at Tufts University for discussion and comments on this manuscript.
Competing interests
The authors declare no competing interests.
Ethics
Care and treatment of experimental animals were reviewed and approved by the Animal Care and Use Committee at the Korea Brain Research Institute (ID: IACUC-18-00011; validity period: 4/1/2018-3/31/2021).
References
- Bolhuis, J. J., Okanoya, K. and Scharff, C. (2010). Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci 11(11): 747-759.
- Cooper, B. G. and Goller, F. (2004). Partial muting leads to age-dependent modification of motor patterns underlying crystallized zebra finch song. J Neurobiol 61(3): 317-332.
- Doupe, A. J. and Kuhl, P. K. (1999). Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567-631.
- Doupe, A. J., Perkel, D. J., Reiner, A. and Stern, E. A. (2005). Birdbrains could teach basal ganglia research a new song. Trends Neurosci 28(7): 353-363.
- Goller, F. and Larsen, O. N. (1997). A new mechanism of sound generation in songbirds. Proc Natl Acad Sci U S A 94(26): 14787-14791.
- Jarvis, E. D., Scharff, C., Grossman, M. R., Ramos, J. A. and Nottebohm, F. (1998). For whom the bird sings: context-dependent gene expression. Neuron 21(4): 775-788.
- Kao, M. H., Doupe, A. J. and Brainard, M. S. (2005). Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433(7026): 638-643.
- Kojima, S., Kao, M. H., Doupe, A. J. and Brainard, M. S. (2018). The avian basal ganglia are a source of rapid behavioral variation that enables vocal motor exploration. J Neurosci 38(45): 9635-9647.
- Kao, M. H., Wright, B. D. and Doupe, A. J. (2008). Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28(49): 13232-13247.
- Kojima, S. and Doupe, A. J. (2011). Social performance reveals unexpected vocal competency in young songbirds. PNAS 108(4): 1687-1692.
- Kojima, S., Kao, M. H. and Doupe, A. J. (2013). Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci U S A 110(12): 4756-4761.
- Larsen, O. N. and Goller, F. (2002). Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol 205(1): 25-35.
- Pytte, C. L. and Suthers, R. A. (1999). A bird's own song contributes to conspecific song perception. Neuroreport 10(8): 1773-1778.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kim, Y., Mizuguchi, D. and Kojima, S. (2020). Long-term Devocalization of Zebra Finches. Bio-protocol 10(18): e3752. DOI: 10.21769/BioProtoc.3752.
- Kojima, S., Kao, M. H., Doupe, A. J. and Brainard, M. S. (2018). The avian basal ganglia are a source of rapid behavioral variation that enables vocal motor exploration. J Neurosci 38(45): 9635-9647.
Category
Neuroscience > Sensory and motor systems > Animal model
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link