- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Efficient Agrobacterium-mediated Transformation of the Elite–Indica Rice Variety Komboka
Published: Vol 10, Iss 17, Sep 5, 2020 DOI: 10.21769/BioProtoc.3739 Views: 9940
Reviewed by: Guotian LiDaniel F. CaddellAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Agrobacterium-mediated Transformation of Japonica Rice Using Mature Embryos and Regenerated Transgenic Plants
Ammar Elakhdar [...] Takahiko Kubo
Sep 20, 2021 6266 Views

Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Alka Srivastava [...] Praveen C. Verma
May 20, 2023 4428 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 2015 Views
Abstract
Genetic transformation is crucial for both investigating gene functions and for engineering of crops to introduce new traits. Rice (Oryza sativa L.) is an important model in plant research, since it is the staple food for more than half of the world’s population. As a result, numerous transformation methods have been developed for both indica and japonica rice. Since breeders continuously develop new rice varieties, transformation protocols have to be adapted for each new variety. Here we provide an optimized transformation protocol with detailed tips and tricks for a new African variety Komboka using immature embryos. In Komboka, we obtained an apparent transformation rate of up to 48% for GUS/GFP reporter gene constructs using this optimized protocol. This protocol is also applicable for use with other elite indica rice varieties.
Keywords: Agrobacterium-mediated transformationBackground
Various methods for genetic transformation of plants have been developed, e.g. PEG-mediated transfection of protoplasts (Shimamoto et al., 1989; Datta et al., 1992), biolistic transformation (Christou et al., 1991) and Agrobacterium-mediated transformation (Slamet-Loedin et al., 2014). Agrobacterium-mediated transformation is one of the most widely used methods to introduce DNA into plants (van Wordragen and Dons, 1992). This method has been used intensively for research and has become a key prerequisite for biotechnology. It has gained in importance since the development of new breeding technologies such as genome editing (Char et al., 2019). For crops, such as rice, genetic transformation could also be used to develop new genetic variations for plant breeding, for example, creating new disease- or insect-resistant lines (Cheng et al., 1998; Helliwell and Yang, 2013; Oliva et al., 2019). Also for rice, Agrobacterium-mediated transformation is the most popular method to transfer T-DNA into plant genomes (Hiei and Komari, 2008). Currently, there are multiple protocols for japonica and indica rice transformation using calli induced from mature seeds or immature embryos (Hiei and Komari, 2006 and 2008; Toki et al., 2006; Nishimura et al., 2006; Sahoo et al., 2011; Sahoo and Tuteja, 2012; Slamet-Loedin et al., 2014; Sundararajan et al., 2017). It is convenient to use mature seeds for transformation because they are available throughout the year and can be stored, although this method is predominantly used for japonica varieties. Methods that involve transformation of rice immature embryos generally yield higher transformation rates compared to mature seeds (Hiei and Komari, 2008; Slamet-Loedin et al., 2014). Overall, japonica varieties such as Kitaake and Nipponbare are apparently easier to transform, compared to indica rice such as IR64 or Ciherang-Sub1 (Oliva et al., 2019). For japonica varieties, highly efficient transformation can be obtained using calli induced from mature seeds with rates of 50-60% (Li et al., 2015). For indica varieties, despite some efforts to increase transformation rate using mature seeds, immature embryos-derived calli are still the tissue of choice for transformation. Notably, transformation efficiency is highly variety-dependent and it is necessary to optimize transformation protocols for each variety.
Komboka (IR 05N 221) is a new elite variety generated by IRRI and released in Tanzania by the National Rice Research Program-KATRIN Research Centre and IRRI-Tanzania (‘Komboka’ = ‘liberated’) (Kitilu et al., 2019). Komboka is high yielding (8.6 t ha-1), semi-aromatic with good grain quality, tolerant to blast and well adapted to upland and lowland areas. In the protocol reported here, we describe detailed steps for the stable transformation of Komboka immature embryos which produce transgenic plants within four months and with a high apparent transformation rate of up to 48%. This protocol was developed by combining and modifying published protocols from Slamet-Loedin et al., 2014 and Hiei and Komari, 2008 which were used to transform different indica rice varieties such as IR64, Ciherang-sub1 (Oliva et al., 2019), therefore, in principle this protocol can likely be adapted for transformation of other indica rice varieties. In this protocol, we highlighted all details, tips and tricks that are essential for setting up transformation protocols for other elite varieties.
Figure 1. Flow chart and timeline for Agrobacterium-mediated transformation of immature embryos of rice var. Komboka (indica) which takes about four months to generate transgenic lines. Briefly, the process starts with growing rice plants to the flowering stage under controlled greenhouse conditions at 30 °C ± 2 °C during the day and 25 °C ± 2 °C during the night, 50-70% relative humidity. Light conditions in the glass greenhouse are determined by natural daylight and additional lamplight (8/16 day/night photoperiod). Daylength varies in the course of the seasons due to the location of our greenhouse along latitude (latitudes and longitudes of Düsseldorf, Germany 51°11'31.2"N 6°47'40.1"E). Under these conditions, Komboka required 13-14 weeks till booting stage and additional 2-3 weeks to flower. 8-12 days post-pollination, rice panicles can be harvested for immature embryos isolation. Selecting the proper stage of seeds for immature embryos isolation is crucial. Immature embryos should be in the late milky stage (Video 1), with a size of 1.3-1.8 mm. After dehusking the seeds and isolating immature embryos (Videos 2 and 3; Figure 2), the isolated immature embryos were moved, as a whole, onto co-cultivation medium. Right after the isolation step immature embryos were co-cultivated with Agrobacteria harboring constructs-of-interest for seven days. Afterwards, germinated immature embryos were cleaned from Agrobacteria with sterile filter papers and shoots were excised. The immature embryos were then transferred onto resting/recovery medium without hygromycin B for five days. After resting, immature embryos were moved to two rounds of selection with 30 mg/L hygromycin B, each for 10 days. After the second selection step, microcalli appeared on brownish maternal immature embryos. Microcalli were carefully separated from the maternal tissues and transferred onto selection medium (30 mg/L hygromycin B) for the third selection round. In cases that the immature embryos did not produce microcalli after the first two rounds of selection, immature embryos were moved to an additional selection round (Supplementary Selection 2) of 10 days each. Resistant and multiplied microcalli after the third selection were transferred onto pre-regeneration medium (30 mg/L hygromycin B) for 1 or 2 rounds of growth for 10 days. Only greening calli were transferred onto regeneration medium (30 mg/L hygromycin B) for 14 days. When small rice plants (plantlets) were regenerated from the calli and reached about 5 cm in height, they were transferred onto rooting medium without hygromycin B for 14 days. Only well-developed plantlets with strong root systems were planted in pots. Pots were submerged in larger buckets (5 L).
Figure 1. Flow chart describing Agrobacterium-mediated transformation using immature embryos of rice variety Komboka
Figure 2. Steps in Agrobacterium-mediated transformation of immature embryos from rice variety Komboka. A. Immature embryo isolation. B. Agrobacterium infection. C. Co-cultivation. D. Resting phase. E. Selection phase. F. Pre-regeneration phase. G. Regeneration phase. H. Rooting phase.
Below, we provide detailed lists of chemicals, equipment, the transformation steps with important notes, as well as all templates for media preparation.
Materials and Reagents
Consumables
- Filtropur BT 100 bottle top filter (volume: 1000 ml; membrane: PES; diameter: 90 mm; pore size: 0.2 µm for sterile filtration) (Sarstedt, catalog number: 83.3942.101 ); use to filter stock solutions and for single use only
- Filtropur BT 25 bottle top filter (volume: 250 ml; membrane: PES; diameter: 90 mm; pore size: 0.2 µm for sterile filtration) (Sarstedt, catalog number: 83.3940.101 ); use to filter stock solutions and for single use only
- Filtropur BT 50 bottle top filter (volume: 500 ml; membrane: PES; diameter: 90 mm; pore size: 0.2 µm for sterile filtration) (Sarstedt, catalog number: 83.3941.101 ); use to filter stock solutions and for single use only
- Glass test tubes,14 x 16 mm; 21 ml (DURAN®, catalog number: 26 130 21 )
- Gene-Pulse Cuvettes, 0.2 cm gap (Bio-Rad, catalog number: 1652086 ), single use only
- Petri dishes, extra deep, disposable, Lab-tekTM (diameter: 100 mm; height: 26 mm; sterile) (Supplier: Thermo Scientific, VWR, catalog number: NUNC4031 ); use to pour Regeneration medium, single use only
- Petri dishes, deep, with 3 vents (diameter: 92.3 mm; height: 20 mm; sterile) (Supplier: Greiner Bio One, VWR, catalog number: 391-0493 ); use to pour Pre-regeneration medium, single use only
- Petri dishes, with cams (diameter: 92 mm; height: 16 mm; sterile) (Sarstedt, catalog number: 82.1473 ); use to pour YEP, Co-cultivation, Resting and Selection media, single use only
- Qualitative filter papers, standard grades, grade 1 and 1 V, Whatman® (diameter: 85 mm) (VWR, catalog number: 516-0593 )
- Reaction tubes, 1.5 ml (Sarstedt, catalog number: 72.690.001 )
- SafeSeal reaction tube, 2 ml (Sarstedt, catalog number: 72.695.500 )
- Scalpel blades (type 11 for scalpel handles using the BAYHA interlocking system; non-sterile) (Behr Labor-Technik, catalog number: 9409911 )
- Screw cap tube, 50 ml (Sarstedt, catalog number: 62.547.254 )
- Screw cap tube, 15 ml (Sarstedt, catalog number: 62.554.502 )
- Serological Pipette, 10 ml (Sarstedt, catalog number: 86.1254.001 ), single use only
- Serological Pipette, 25 ml (Sarstedt, catalog number: 86.1685.001 ), single use only
- Serological Pipette, 5 ml (Sarstedt, catalog number: 86.1253.001 ), single use only
- Stretch foil (Stretchplus; 300.0 m x 40.0 cm; foil thickness: 7 µm)
- Surgical tape (3MTM MicroporeTM; Width: 1.25 cm)
- Syringe (Injekt®; 20 ml; Luer lock) (Braun, catalog number: 4606205V ), single use only
- Syringe filtration unit Filtropur S 0.2 (membrane: PES; filtration surface: 5.3 cm2; pore size: 0.2 µm for sterile filtration) (Sarstedt, catalog number: 83.1826.001 ); use to filter phytohormones and antibiotic solutions, single use only
- Tissue culture vessel, MagentaTMGA-7 (Supplier: Sigma-Aldrich; 77 x 77 x 97 mm) (VWR, catalog number: SAFSV8505 ); use to pour ‘Roo’ medium, reusable
- Tooth pick made of wood (6.8 cm) (Pap Star, catalog number: 12736 )
Biological material
- Komboka seeds (IR05N221, L17WS.06#24)
Komboka seeds were kindly provided by the International Rice Research Institute (IRRI, Philippine) under a special material transfer agreement (SMTA-MLS).
Komboka seeds were sown directly into soil in round, 2 L pots (13.2 cm height and 16.7 cm diameter), one plant per pot. A soil mixture of profile porous ceramic (PPC) greens grade and ‘Arabidopsis soil’ with a ratio of 1:1 is used as a standard soil for rice cultivation. See Table S1 for the details of soil composition. As a slowly released fertilizer, 4 g of Osmocote Exact 3-4 M (16% N, 3.9% P, 10% K, 1.2% Mg, 0.45% Fe, 0.06% Mn, 0.02% B, 0.05% Cu, 0.02% Mo, 0.015% Zn) (ICL/SF UK) was added in 1 L soil. In addition, plants were fertilized weekly from the 2nd week and biweekly from the 6th week after germination using Peters Excel (14% N, 6% P, 14% K, 6.5% Ca, 2.5% Mg, 0.12% Fe, 0.06% Mn, 0.02% B, 0.015% Cu, 0.01% Mo, 0.015% Zn) (ICL/SF UK). Fertilization was terminated when plants reached the flowering stage. Rice plants in 2 L pots filled with soil were submerged into a 5 L buckets filled with water, so that the inner 2 L pot is under the waterline. Water can remain in the bucket till rice plants get seeds, no exchange needed. Alternatively, 2 L rice pots can also be placed in 60 x 40 x 6 cm trays and filled with water to the upper edge. In this case, water needs to be exchanged biweekly.
Rice plants were grown in the glass house with natural daylight and additional lamplight of 8/16 day/night photoperiod, however not strictly required, with day temperature of 30 °C ± 2 °C and night temperature of 25 °C ± 2 °C. The relative humidity was between 50-70% which was manually controlled by spraying water on the greenhouse floor. Plants were grown under a photosynthetic active radiation (PAR) or photosynthetic photon flux density (PPFD) of 200 µmol m-2s-1 with PPFD-blue of 40 µmol m-2s-1, PPFD-green of 70 µmol m-2s-1 and PPFD-red of 80 µmol m-2s-1.
Chemicals
Note: Chemical batches and brands are important factors that may affect transformation success. We found that the indistinctive use of alternative chemical brands often leads to failure when trying to transform Komboka for the first time. Therefore, we highly recommend to use the exact chemicals specified here (brands and catalog numbers), in particular when setting up a new transformation protocol. Make sure to store the chemicals in the required conditions and do not use products after the expiry dates. We declare no competing interest regarding chemicals or instrument choice.
- 1-Naphthaleneacetic acid, C12H10O2 (Sigma-Aldrich, catalog number: N0640 ; CAS number: 86-87-3), store at RT
- 2,4-Dichlorophenoxyacetic acid, Cl2C6H3OCH2CO2H (Sigma-Aldrich, catalog number: D7299 ; CAS number: 94-75-7), store at RT
- 3′,5′-Dimethoxy-4′-hydroxyacetophenone, acetosyringone, HOC6H2(OCH3)2COCH3 (Sigma-Aldrich, catalog number: D134406 ; CAS number: 2478-38-8), store at 4 °C
- 6-Benzylaminopurine, C12H11N5 (Sigma-Aldrich, catalog number: B3408 ; CAS number:1214-39-7), store at RT
- Agarose type I, low EEO (Sigma-Aldrich, catalog number: A6013 ; CAS number: 9012-36-6), store at RT
- Ammonium nitrate, NH4NO3 (Sigma-Aldrich, catalog number: A3795 ; CAS number: 6484-52-2), store at RT
- Ammonium sulfate, (NH4)2SO4 (Sigma-Aldrich, catalog number: A3920 ; CAS number: 7783-20-2), store at RT
- Bacto agar, dehydrated (Fischer Scientific, catalog number: 214050 ), store at RT
- Bacto beef extract, desiccated (Fischer Scientific, catalog number: 211520 ), store at RT
- Bacto peptone, dehydrated (Fischer Scientific, catalog number: 211677 ), store at RT
- Bacto yeast extract (Fischer Scientific, catalog number: 212750 ), store at RT
- Boric acid, H3BO3 (Sigma-Aldrich, catalog number: B6768 ; CAS number: 10043-35-3), store at RT
- Calcium chloride dihydrate, CaCl2·2H2O (Sigma-Aldrich, catalog number: C7902 ; CAS number: 10035-04-8), store at RT
- Carbenicillin disodium, C17H16N2Na2O6S (Duchefa, catalog number: C0109 ; CAS number: 4800-94-6), store at 4 °C
- Cefotaxime sodium, C16H16N5O7S2Na (Duchefa, catalog number: C0111 ; CAS number:64485-93-4), store at 4 °C
- Chloro cleaner, e.g., DanKlorix® original (2.8 g/100 ml sodium hypochlorite), store at RT
- Cobalt (II) chloride hexahydrate, CoCl2·6H2O (Sigma-Aldrich, catalog number: C2911 ; CAS number: 7791-13-1), store at RT
- Copper (II) sulfate pentahydrate, CuSO4·5H2O (Sigma-Aldrich, catalog number: C3036 ; CAS number: 7758-99-8), store at RT
- D-(+)-Glucose, C6H12O6 (Sigma-Aldrich, catalog number: G7021 ; CAS number: 50-99-7), store at RT
- D-Mannitol, C6H14O6 (Sigma-Aldrich, catalog number: M1902 ; CAS number: 69-65-8), store at RT
- D-Sorbitol, C6H14O6 (Carl Roth, catalog number: 6213.1 ; CAS number: 50-70-4), store at RT
- DifcoTM casamino acids, vitamin assay (Thermo Fischer, catalog number: 228820 ), store at RT
- Dimethyl sulfoxide (DMSO) (Fisher Scientific, catalog number: D/4121/PB15 ; CAS number: 67-68-5), store at RT
- Ethylenediaminetetraacetic acid disodium salt dihydrate, C10H14N2Na2O8·2H2O (Sigma-Aldrich; catalog number: E6635 ; CAS number: 6381-92-6), store at RT
- GELRITETM (Duchefa, catalog number: G1101 ; CAS number: 71010-52-1), store at RT
- Glycerol, SOLVAGREEN® ≥ 98 %, anhydrous, Ph.Eur., C3H8O3 (Carl Roth, catalog number: 7530.1 ; CAS number: 56-81-5), store at RT
- Glycine, NH2CH2COOH (Sigma-Aldrich, catalog number: G8790 ; CAS number: 56-40-6), store at RT
- Hygromycin B solution, CELLPURE® 50 mg/ml, sterile, C20H37N3O13 (Carl Roth, catalog number: CP12.2 ; CAS number: 31282-04-9), store at 4 °C
- Hydrogen chloride, HCl, (Sigma-Aldrich, catalog number: H1758-100ML ; CAS number: 7647-01-0), store at RT
- Iron (II) sulfate heptahydrate, FeSO4·7H2O (Sigma-Aldrich, catalog number: F8263 ; CAS number: 7782-63-0), store at RT
- Kanamycine sulphate monohydrate, C18H36N4O11·H2SO4·H2O (Duchefa, catalog number: K0126 ; CAS number: 25389-94-0), store at 4 °C
- Kinetin, C10H9N5O (Sigma-Aldrich, catalog number: K3378 ; CAS number: 525-79-1), store at 4 °C
- L-Arginine, H2NC(=NH)NH(CH2)3CH(NH2)CO2H (Sigma-Aldrich, catalog number: A8094 ; CAS number: 74-79-3), store at RT
- L-Aspartic acid, HO2CCH2CH(NH2)CO2H (Sigma-Aldrich, catalog number: A7219 ; CAS number: 56-84-8), store at RT
- L-Glutamine, H2NCOCH2CH2CH(NH2)CO2H (Sigma-Aldrich, catalog number: G8540 ; CAS number: 56-85-9), store at RT
- Liquid nitrogen
- L-Proline, C5H9NO2 (Sigma-Aldrich, catalog number: P5607 ; CAS number: 147-85-3), store at RT
- Magnesium chloride, MgCl2 (Sigma-Aldrich; catalog number: M8266 ; CAS number: 7786-30-3), store at RT
- Magnesium sulfate heptahydrate, MgSO4·7H2O (Sigma-Aldrich, catalog number: M2773 ; CAS number: 10034-99-8), store at RT
- Maltose monohydrate, C12H22O11·H2O (Duchefa, catalog number: M0811 ; CAS number: 6363-53-7), store at RT
- Manganese (II) sulfate monohydrate, MnSO4·H2O (Sigma-Aldrich, catalog number: M7899 ; CAS number: 10034-96-5), store at RT
- Myo-Inositol, C6H12O6 (Sigma-Aldrich, catalog number: I7508 ; CAS number: 87-89-8), store at RT
- Nicotinic acid, C6H5NO2 (Sigma-Aldrich, catalog number: N0761 ; CAS number: 59-67-6), store at RT
- Potassium chloride, KCl (Sigma-Aldrich, catalog number: P5405 ; CAS number: 7447-40-7), store at RT
- Potassium hydroxide, KOH (Fisher Scientific, catalog number: 10366240 ; CAS number: 1310-58-3), store at RT
- Potassium iodide, KI (Sigma-Aldrich, catalog number: P8166 ; CAS number: 7681-11-0), store at RT
- Potassium nitrate, KNO3 (Sigma-Aldrich, catalog number: P8291 ; CAS number: 7757-79-1), store at RT
- Potassium phosphate monobasic, KH2PO4 (Sigma-Aldrich, catalog number: P5655 ; CAS number: 7778-77-0), store at RT
- Pyridoxine hydrochloride, C8H11NO3·HCl (Sigma-Aldrich, catalog number: P6280 ; CAS number: 58-56-0), store at RT
- Rifampicin, C43H58N4O12 (Sigma-Aldrich, catalog number: R3501 ; CAS number: 13292-46-1), store at 4 °C
- Sodium chloride, NaCl (Sigma-Aldrich, catalog number: S7653 ; CAS number: 7647-14-5), store at RT
- Sodium hydroxide, NaOH (Sigma-Aldrich, catalog number: 30620-1KG-M ; CAS number: 1310-73-2), store at RT
- Sodium molybdate dihydrate, Na2MoO4·2H2O (Sigma-Aldrich, catalog number: 331058 ; CAS number: 10102-40-6), store at RT
- Sodium phosphate monobasic, NaH2PO4 (Sigma-Aldrich, catalog number: S5011 ; CAS number: 7558-80-7), store at RT
- Sucrose, C12H22O11 (Duchefa, catalog number: S0809 ; CAS number: 57-50-1), store at RT
- Thiamine hydrochloride, C12H17ClN4OS·HCl (Sigma-Aldrich, catalog number: T1270 ; CAS number: 67-03-8), store at RT
- Zinc sulfate heptahydrate, ZnSO4·7H2O (Sigma-Aldrich, catalog number: Z1001 ; CAS number: 7446-20-0), store at RT
- FastAmp® Plant Direct PCR Kit (Intactgenomics, catalog number: 4612 )
- WTWTM TEP 4 Model Buffer Solution pH 4 (WTWTM, catalog number: 108700 )
- WTWTM TEP 7 Model Buffer Solution pH 7 (WTWTM, catalog number: 108702 )
- WTWTM TPL 10 Trace Model Buffer Solution pH 10.1 (WTWTM, catalog number: 108805 )
- Rice soil composition (see Recipes)
- Stock solution composition (see Recipes)
- Phytohormones and antibiotics (see Recipes)
- Cultivation medium composition (see Recipes)
- Suspension medium (see Recipes)
- Co-cultivation medium (see Recipes)
- Resting medium (see Recipes)
- Selection medium (see Recipes)
- Pre-regeneration medium (see Recipes)
- Regeneration medium (see Recipes)
- Rooting medium (see Recipes)
- Transformation cheat sheet (see Recipes)
- GUS staining solution (see Recipes)
- X-Gluc solution (see Recipes)
Equipment
Note: Equipment can be adapted to different lab conditions, however, PETRI DISHES, MAGENTA BOXES are VERY IMPORTANT TOOLS which affect transformation efficiency. We highly recommend to use the same brands as we used in this protocol. SCALPELS and TWEEZERS have to be high quality due to the frequent sterilization. Exact type and brand may be adapted to personal choice.
- -80 °C Ultra low temperature freezer (Panasonic, model: MDF-U76V-PE )
- Analytical and Precision Balances (Precisa Gravimetrics AG, Series 321LS, model: LS 2200C and LS 120A )
- Autoclave (Systec, model: 3850 EL )
- Benchtop pH meter; WTWTM inolabTM 7110 (Supplier: WTWTM 1AA114; Fisher Scientific, catalog number: 11731381 )
- Biospectrometer, basic (Eppendorf, catalog number: 6135000009 )
- Buckets, 5 L (Auer, catalog number: ER 5,6-226+DK )
- Erlenmeyer flask, 250 ml (DURAN®, catalog number: 21 216 36 )
- Growth chamber 1 [Percival, model: CU-41L5 ; condition: 30 °C, continuous light (24/0 day/night photoperiod) with photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1]
- Growth chamber 2 [Percival, model: CU-41L5 ; condition: 27 °C, 16/8 day/night photoperiod with photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 30 µmol m-2 s-1, PPFD-green of 70 µmol m-2 s-1 and PPFD-red of 60 µmol m-2 s-1]
- Glass bead sterilizer (SkinMate Apus Quartz)
- High precision tweezers (110 mm; type 5.SA; extra fine tip; stainless steel) (Behr Labor-Technik, catalog number: 6.266 876 )
- Electroporator (Bio-Rad Gene PulserTM)
- Incubator/shaker 28 °C (Infors HT, Multitron Standard)
- Inoculating loops, PS 10UL PK/25 (Hach, catalog number: 2749125 )
- Laboratory bottles with screw cap and pouring ring, 100 ml (Duran®, catalog number: 218012458 )
- Laboratory bottles with screw cap and pouring ring, 250 ml (Duran®, catalog number: 218013651 )
- Laboratory bottles with screw cap and pouring ring, 500 ml (Duran®, catalog number: 218014459 )
- Laboratory bottles with screw cap and pouring ring, 1,000 ml (Duran®, catalog number: 218015455 )
- Lightmeter (Quantum Spectrometer, UPRtek, PAR 300)
- Magnetic stirring bars, octagonal, with pivot ring, blue, 38 mm (VWR, catalog number: 442-0438 )
- Pipette controller, Pipetboy acu 2 (Supplier: Integra (Biosciences); for glass and plastic pipettes from 0.1 to 100 ml) (VWR, catalog number: 613-4437 )
- Polycarbonate vacuum desiccator (Sanplatec, catalog number: PC-250KG )
- Pots SM-H Container 2.0 L (Meyer-shop, catalog number: 737203 )
- Scalpel handles (Supplier: BAYHA GMBH; length: 160 mm) (VWR, catalog number: 233-5202 )
- Stereomicroscope (Zeiss, Discovery.v8, brightfield images)
- Stereo zoom and fluorescence microscope (Zeiss, AxioZoom.V16) for GFP imaging
- Sterile bench (Thermo ScientificTM HeraguardTM ECO Clean Bench)
- Thermometer (Laserliner ThermoSpot)
- Thermal cycler (Bio-Rad, model: T100TM, catalog number: 1861096 )
- Vacuum pump (Vacuubrand, catalog number: MZ 2C )
Procedure
- Prepare stock solutions
- Prepare 17 stock solutions including N6 major 1, N6 major 2, N6 major 3, N6 major 4, B5 minor 1, B5 minor 2, B5 minor 3, B5 minor 4, B5 vitamins, AA macro salts, AA micro salts, Glycine, MS 1, MS 2, MS 3, MS 4 and MS vitamins with composition according to Table S2. Prepare phytohormone and antibiotic stock solutions according to Table S3.
- Filter sterilize all stock solutions and keep at 4 °C.
Notes:- Always filter sterilize the stock solutions (filter pore size: 0.2 µm, Consumables 1, 2, 3, 20 and 21 for filter types), do not autoclave!
- Stock solutions can be stored at 4 °C for a maximum of 3 months.
- Do not use stock solutions older than 3 months.
- Prepare cultivation medium
- Prepare cultivation medium according to Table S4-Table S11. Cultivation medium composition.
Notes:- It is very critical to adjust pH properly: always calibrate the pH meter before use (for pH 5.8, use calibration solutions pH 4 and pH 7).
- Always adjust the pH very precisely: for a medium which e.g., pH 5.8 is needed, accepted pH value is 5.80-5.81.
- Record the pH values before and after pH adjustment.
- Always measure the pH at the same medium temperature and record the medium temperature while measuring pH (Figure 3).
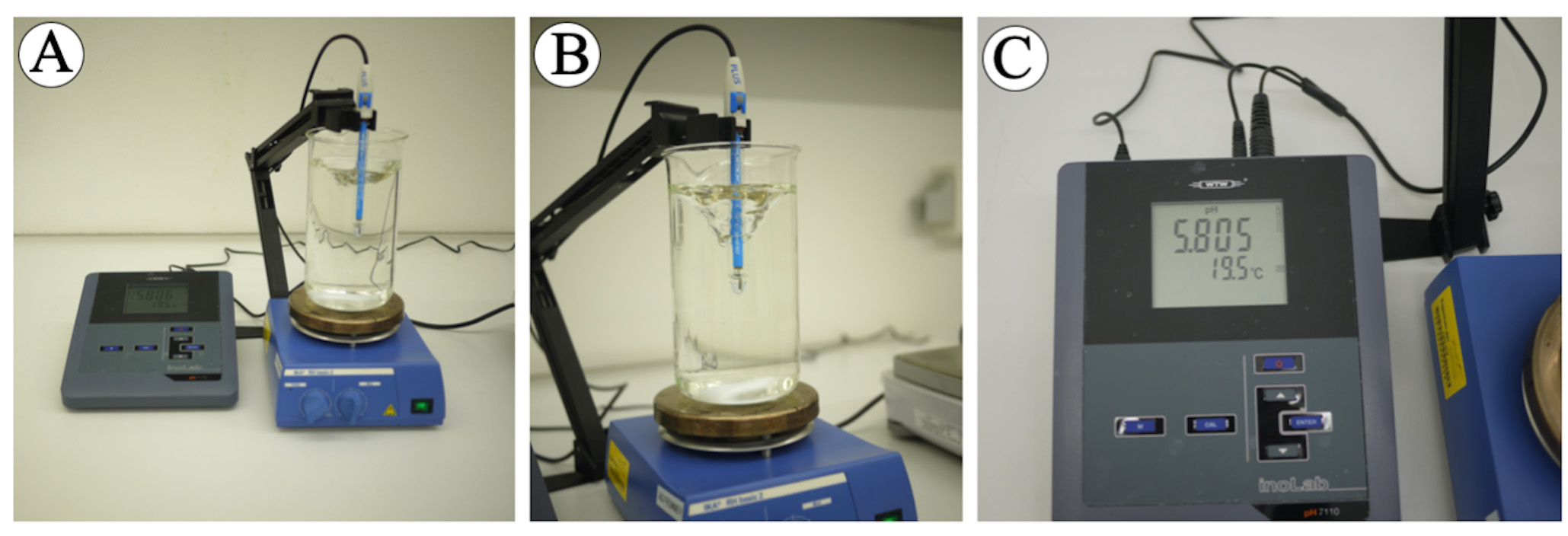
Figure 3. pH measuring. A. Recording pH and temperature while preparing medium. B. Detail of the right position of the electrode while recording the pH. C. Temperature and pH recording at the display. - pH is measured after all components are added, except for agarose, phytohormones and antibiotics (see Table S4-Table S11 for more details), before autoclaving the medium.
- It is critical to use short autoclave cycles for autoclaving media: apply a sterilization time of 5 min (!) at 121 °C (101 kPa) and transfer media to RT as soon as the autoclave has cooled down to 95 °C, do not let media stay longer in the autoclave (Figure 4). Sterilization time varies depending on the amount of medium because the autoclaving process includes additional time for the heating up and cooling down. In this protocol, we prepared medium in 0.5 L bottles, and applied a sterilization time of 5 min at 121 °C (101 kPa), the whole autoclave cycle took 1.5 h.

Figure 4. Comparison of media autoclaved for 5 min (A) and 20 min (B) at 121 °C (101 kPa). The caramel color is a good indication of over-autoclaved media. Media color should remain almost colorless after autoclaving like show on the left picture. - Add phytohormones and antibiotics after autoclaving only when media have cooled to 40-45 °C (always use the Laserliner thermometer to check temperature!).
- Pour plates under sterile bench (biosafety cabinet), see Table S4 for types of Petri dishes and amount to pour for each medium. Types of Petri dishes are different for different media and it is critical to follow our recommendation.
- Close the lids only when plates have completely dried and no water condensation is visible on the side of Petri dishes (Figure 5). This is critical because calli prefer to stay dry on the medium, wet calli do not regenerate. But also, do not overdry. Overdried media shrink and form cracks, and due to reduced water content, solute concentrations increase substantially, affecting the sensitive calli and the regeneration process.
- Plates are wrapped with stretch foil and stored at room temperature and in the dark (Figure 5). Do not refrigerate media.
- Discard unused plates with solid media after 2 weeks.
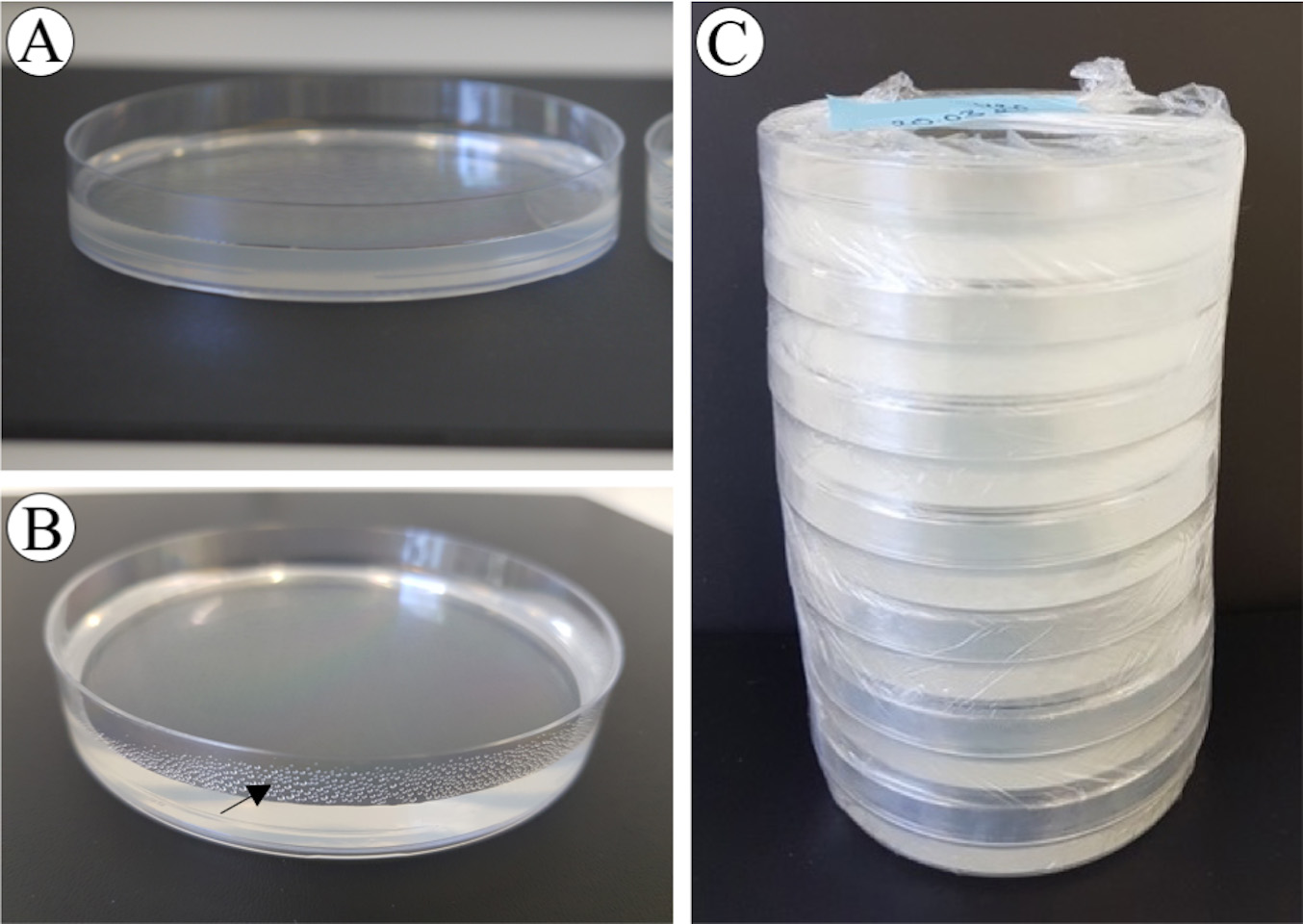
Figure 5. Medium drying and storage. A. Completely dried Petri dish. B. Wet Petri dish, with condensed water; the black arrow indicates condense water. C. Plates are wrapped and stored at RT and in the dark.
- Prepare cultivation medium according to Table S4-Table S11. Cultivation medium composition.
- Competent Agrobacteria preparation and transformation
- Prepare Agrobacterium (LBA4404 or EHA105) competent cells.
- Streak out Agrobacterium cells from 10% glycerol stock (stored at -80 °C) on a plate with solid YEP medium containing 20 mg/L rifampicin and incubate for 2 days in the dark at 28 °C.
- Pick a single colony with a sterile tooth pick or pipette tip and culture in a glass test tube with 5 ml YEP liquid medium containing 20 mg/L rifampicin overnight (12 to 16 h) in the dark and at 28 °C and shake at 200 rpm.
- Sub-culture 1 ml overnight culture into 35 ml liquid YEP medium plus 20 mg/L rifampicin in a 250 ml flask, incubate at 28 °C, dark and shake at 200 rpm for about 8 h until OD600 reaches 0.5-0.8.
- Chill Agrobacteria on ice for 5 min.
- Centrifuge the bacteria in a 50 ml Falcon® tube at 3,000 x g, 4 °C for 5 min.
- Resuspend the pellet in 1 ml 20 mM CaCl2 in a 50 ml Falcon® tube (for 50 ml YEP, add 1 ml 1 M CaCl2).
- Aliquot (50 μl) in a 2 ml safe lock microcentrifuge tube, flash freeze with liquid nitrogen and store at -80 °C.
- Transformation of competent Agrobacterium cells (LBA4404 or EHA105) with GUS/GFP reporter constructs.
- Take an aliquot (50 μl) of competent A. tumefaciens LBA4404 or EHA105 from 10% glycerol stock (stored at -80 °C) and thaw on ice for 10 min.
- Mix cells with 1 μl (~100 ng) plasmid DNA in a 1.5 ml microcentrifuge tube.
- Fill mixture into a sterile ice-cold electroporation cuvette (0.2 cm gap).
- Perform electroporation using an electroporator at 1.8 kV. Pressing until ‘buzzing’ sound can be heard (about 5 ms), leave all other settings as default: 200 Ω, capacitance extender 250 μFD, capacitance 25 μFD.
- Add 1 ml YEP medium without antibiotics immediately after pulse, mix cells by pipetting up and down using 1 ml pipet tip.
- Transfer suspension into an autoclaved 1.5 ml microcentrifuge tube and incubate for 30 min at 28 °C without shaking.
- Spin cells down for 1 min at 210 x g.
- Carefully remove supernatant, but leave about 100 μl medium in the 1.5 ml microcentrifuge tube.
- Re-suspend the cells in the remaining medium in a 1.5 ml microcentrifuge tube and place 100 μl on a YEP agar plate containing 50 mg/L kanamycin and 20 mg/L rifampicin.
- Incubate the plates for 2 days at 28 °C in the dark.
- Conduct colony PCR to select for transformed colonies by checking for the presence of the selection marker gene. In our case, to confirm the presence of the Hpt gene (hypoxanthine phosphoribosyl transferase encoding gene), we used the following primers: Hpt_F: 5′-AGCCTGACCTATTGCATCT-3′; Hpt_R: 5′-CATATGAAATCACGCCATGT-3′, amplicon size 200 bp, Tm 55 °C.
- Select 1-3 positive colonies and inoculate in a glass test tube with 3 ml liquid YEP medium containing 50 mg/L kanamycin and 20 mg/L rifampicin. Grow bacteria at 28 °C in the dark with shaking speed at 200 rpm overnight.
- Prepare glycerol stock of Hpt positive Agrobacterium cultures by mixing 0.6 ml culture with 0.3 ml 10% glycerol (autoclaved) in 2 ml safe lock microcentrifuge tubes, freeze immediately in liquid N2 and store at -80 °C.
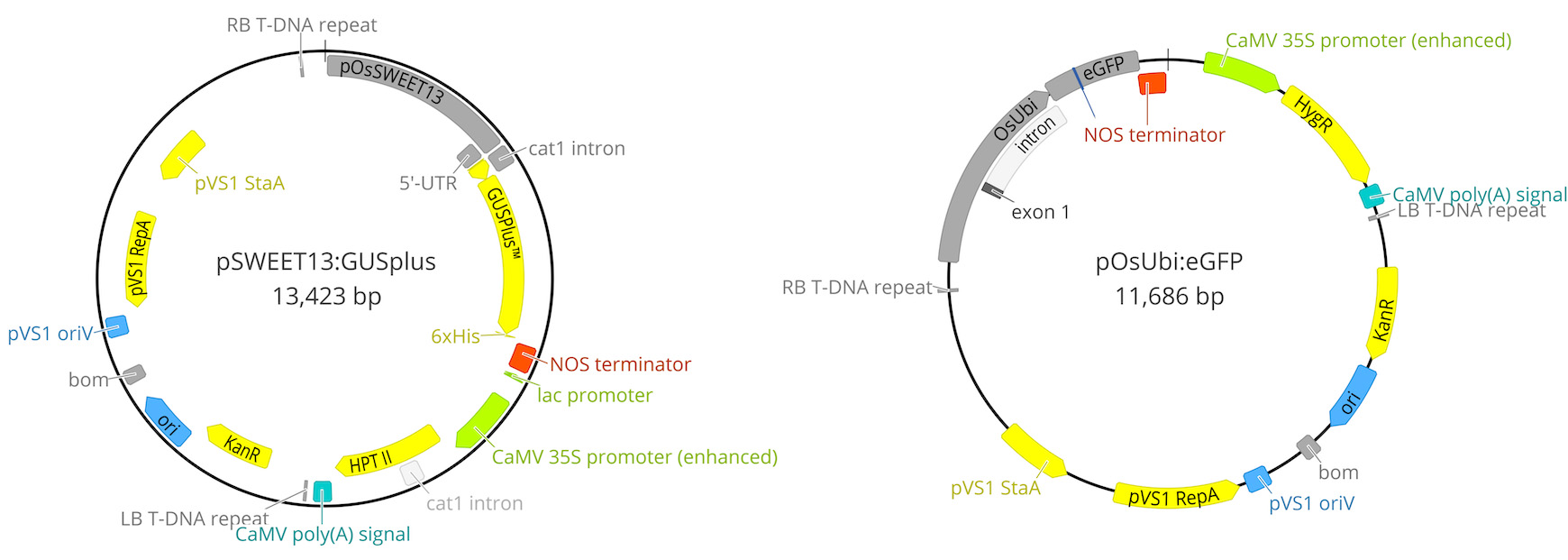
Figure 6. Maps of the two plasmids carrying GUS and GFP reporter genes used in this study
- Prepare Agrobacterium (LBA4404 or EHA105) competent cells.
- Isolation of the immature embryos
- Harvest rice immature seeds (8-12 days post-pollination) and place 300-400 seeds in a 50 ml Falcon® tube. It is important to get immature seeds at the right developmental stage. To check for the stage of immature embryos, squeeze immature seeds gently and feel their hardness. Immature seeds should be at the late milky stage at which the endosperm is not completely cellularized yet, see Video 1 for more details. This harvesting step can be done outside of a sterile hood, but all steps below including dehusking, sterilization, isolation of immature embryos are done under sterile condition.Video 1. Selection of immature seeds
- Under sterile condition, wash seeds with 40 ml ethanol 70% for 30 s with hand shaking in a 50 ml Falcon® tube; discard ethanol. Then wash seeds with 40 ml sterile Milli-Q water three times, discard the water.
- Place immature seeds into a Petri dish layered with moistened sterile filter papers and remove the seed coat (lemma and palea) very carefully under a stereo microscope and in sterile conditions (under laminar flow chamber) with scalpel and forceps (Video 2, Figures 7A-7B). Scalpel and forceps should be sterilized by using for example a glass bead sterilizer. Tools need to be cooled down to avoid heat damage to immature embryos.Video 2. Dehusking of immature seeds
- Place the immature seeds in sterile conditions in a 50 ml Falcon® tube (once lemma and palea have been removed) to start the sterilization procedure (Figure 7C).
- Add 40 ml ethanol 70%, under sterile conditions, to the Falcon® tube and shake by hand for 30 s. Discard ethanol. Wash immature seeds with 40 ml sterile Milli-Q water. Discard the water.
- In a sterile 50 ml Falcon® tube, prepare a mixture of 5 ml commercial bleach Klorix® with 35 ml sterile Milli-Q water [final concentration of NaClO is 28 g/L (0.376 M)].
- Add the mixture to the 50 ml Falcon® tube containing the immature seeds and shake by hand for 5 min.
- Discard the Klorix® mixture and rinse the seeds with 40 ml sterile Milli-Q water, repeat the procedure 15 times until all remains of Klorix® are completely washed out.
Note: It is very important that Klorix® is washed out completely, because Klorix® can affect the germination of immature embryos. We recommend to rinse the immature embryos in a 50 ml Falcon® tube 15 times with 40 ml sterile Milli-Q water each time. - Place immature seeds into a Petri dish with sterile filter paper and carefully isolate immature embryos (IEs) using scalpel and fine forceps under the stereo microscope (Video 3, Figure 7D) in a sterile hood. Be careful and gentle to avoid damaging or wounding the immature embryos!Video 3. Immature embryo isolation
- Only select undamaged immature embryos with sizes between 1.3-1.8 mm. The immature embryos should be opaque, off-white colored. Transparent immature embryos will not germinate. Place about 50 immature embryos (scutellum face up) on co-cultivation medium (Figure 7E).
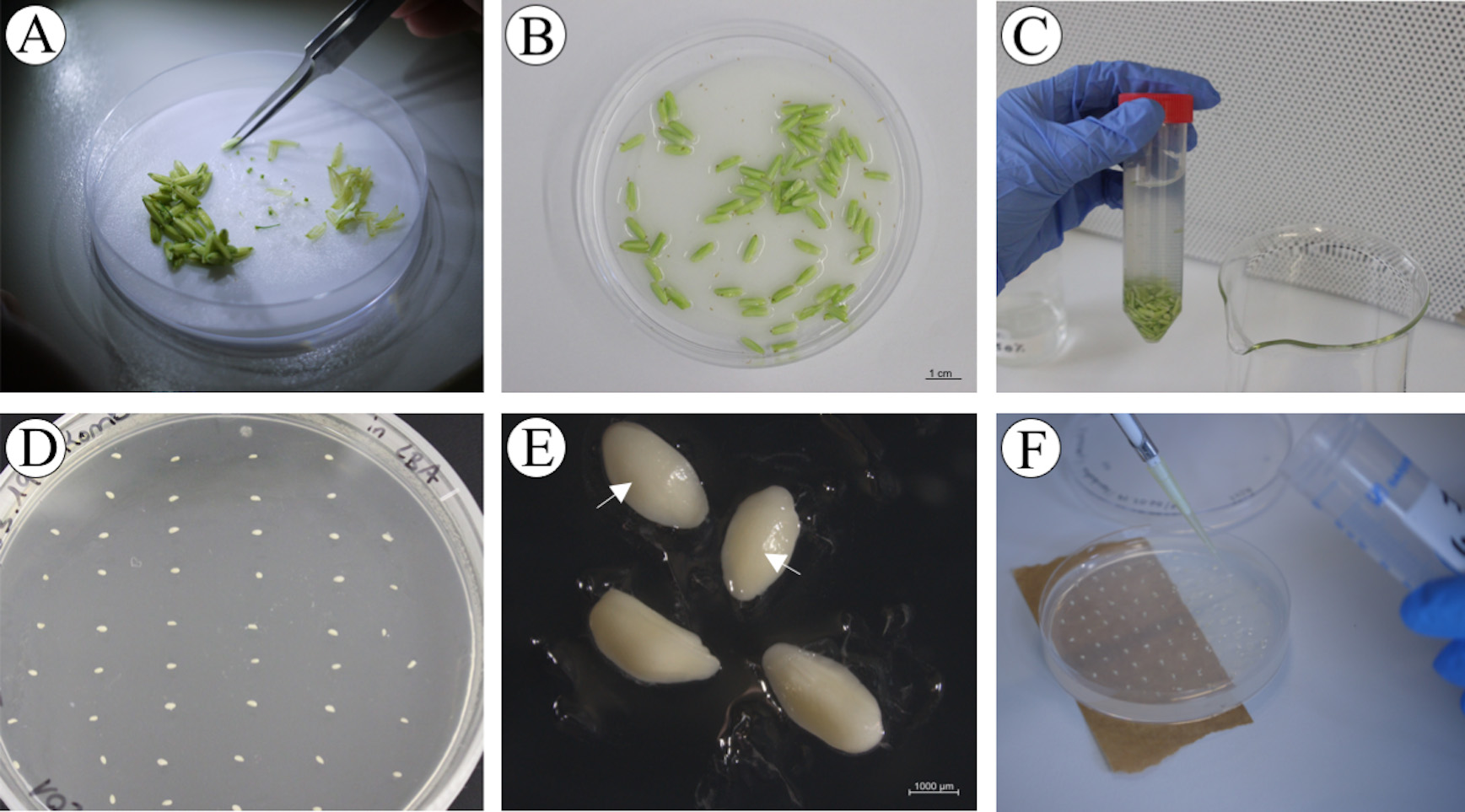
Figure 7. Immature embryo isolation and inoculation with Agrobacteria. A. Removal of palea and lemma under a stereo microscope. B. Dehusked immature seeds in Petri dish with wet Whatman® paper. Scale bar: 1 cm. C. Seed sterilization with ethanol and Klorix®. D. Isolated immature embryos in a plate. E. Immature embryos with scutellum up (white arrows) and immature embryos lying on the side. Scale bar: 1 mm. F. Inoculation with Agrobacteria.
- Harvest rice immature seeds (8-12 days post-pollination) and place 300-400 seeds in a 50 ml Falcon® tube. It is important to get immature seeds at the right developmental stage. To check for the stage of immature embryos, squeeze immature seeds gently and feel their hardness. Immature seeds should be at the late milky stage at which the endosperm is not completely cellularized yet, see Video 1 for more details. This harvesting step can be done outside of a sterile hood, but all steps below including dehusking, sterilization, isolation of immature embryos are done under sterile condition.
- Transformation and co-cultivation
Notes:- Generally, we recommend to start with 100 immature embryos for one transformation. Depending on the transformation efficiency, the number of immature embryos as starting materials can be reduced or increased.
- We recommend to use 12 immature embryos as controls without Agrobacterium inoculation. In the first selection step, 6 control immature embryos will be moved onto selection medium without hygromycin B to check for the regeneration ability. The other 6 control immature embryos will be moved onto selection medium with 30 mg/L hygromycin B to control the effectiveness of hygromycin B selection. These are very important controls, especially when trying to adapt this transformation protocol to other varieties. Hygromycin B concentration, regeneration medium and inoculation time may have to be adapted for other varieties.
- We also recommend to record all data for each transformation, e.g., date, number of immature embryo as well as any notes (see Table S12 for the template).
- Strike Agrobacteria (LBA4404 or EHA105) from frozen -80 °C stocks on solid YEP plates containing antibiotics (50 mg/L kanamycin and 20 mg/L rifampicin) two days before infection. Then incubate for two days at 28 °C in the dark.
- On the day of the transformation, take a 3 mm-size loop of Agrobacterium culture from the YEP plate and suspend in suspension medium (Table S5) in a 50 ml Falcon® tube.
- Vortex bacterial suspension and adjust to OD600 0.3. Incubate in dark at 25 °C for 1 h, no shaking needed.
Notes:- Add acetosyringone just before use and prepare freshly!
- Dissolve acetosyringone: for 50 ml suspension culture, dissolve 10 mg acetosyringone in 100 μl DMSO and then take 9.81 μl solution to obtain 0.981 mg acetosyringone; no filter sterilization needed.
- Always adjust OD600 very precisely: recommended values, depending on the spectrometer, are between 0.30 and 0.31.
- Drop 5 μl Agrobacterium suspension on top of each immature embryo, with scutellum side-up (Figure 7F).
- Incubate plates with embryos in the presence of Agrobacteria for 7 days at 25 °C in the dark (Figure 8).
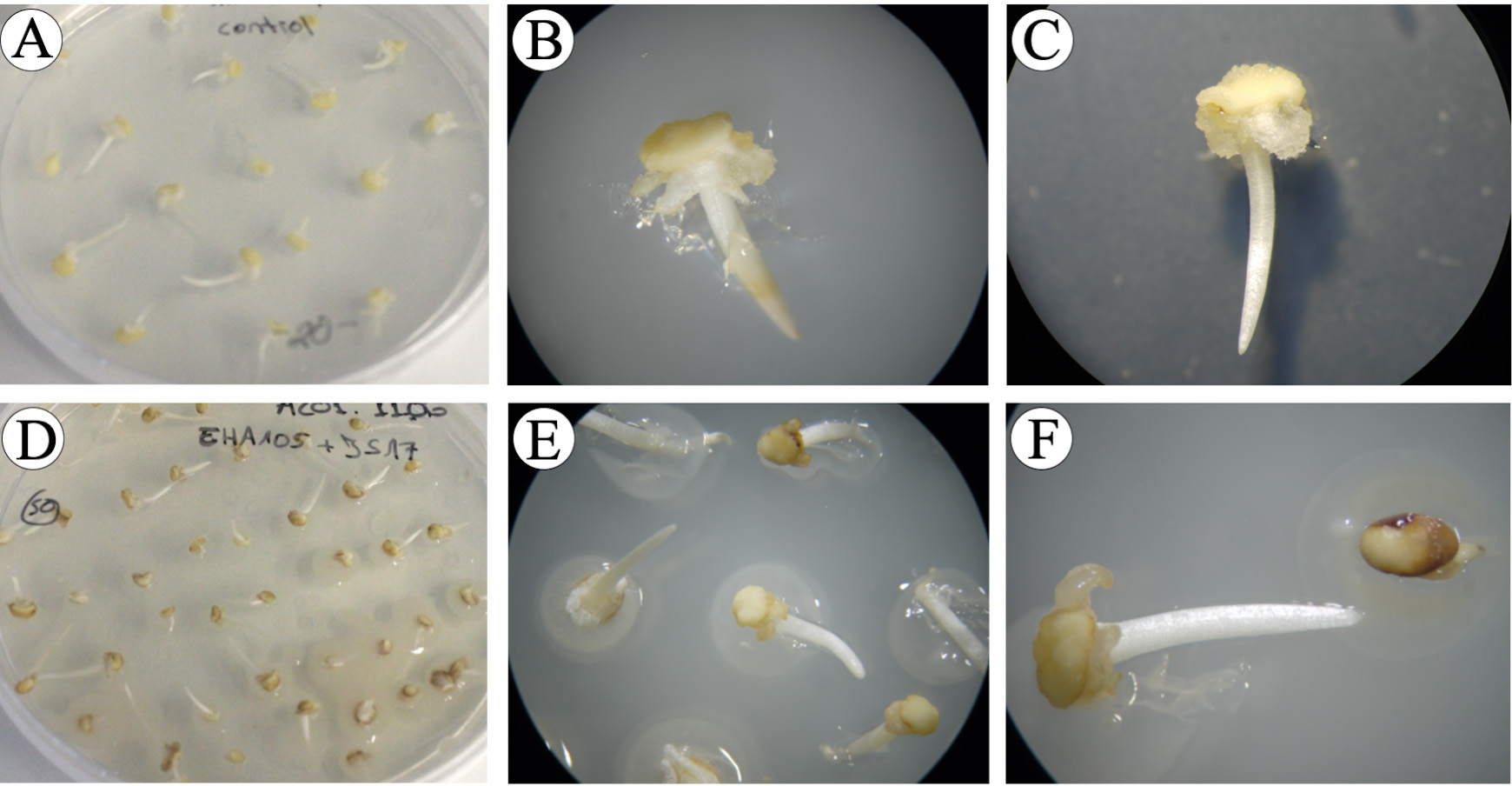
Figure 8. Immature embryos at the end of co-cultivation phase. A-C. Control calli without Agrobacterium infection. D-F. Agrobacterium-infected calli.
- Resting
- Check immature embryos for possible contamination (Figure 9). If immature embryos are contaminated, discard them. If possible, uncontaminated immature embryos in the contaminated plate can be rescued and placed on a separate plate. Otherwise discard the whole plate. Contamination can happen if dehusking or immature embryos isolation was not done carefully enough and embryos were damaged with forceps or scalpel during the process, facilitating contamination with other microorganisms (Figure 9).
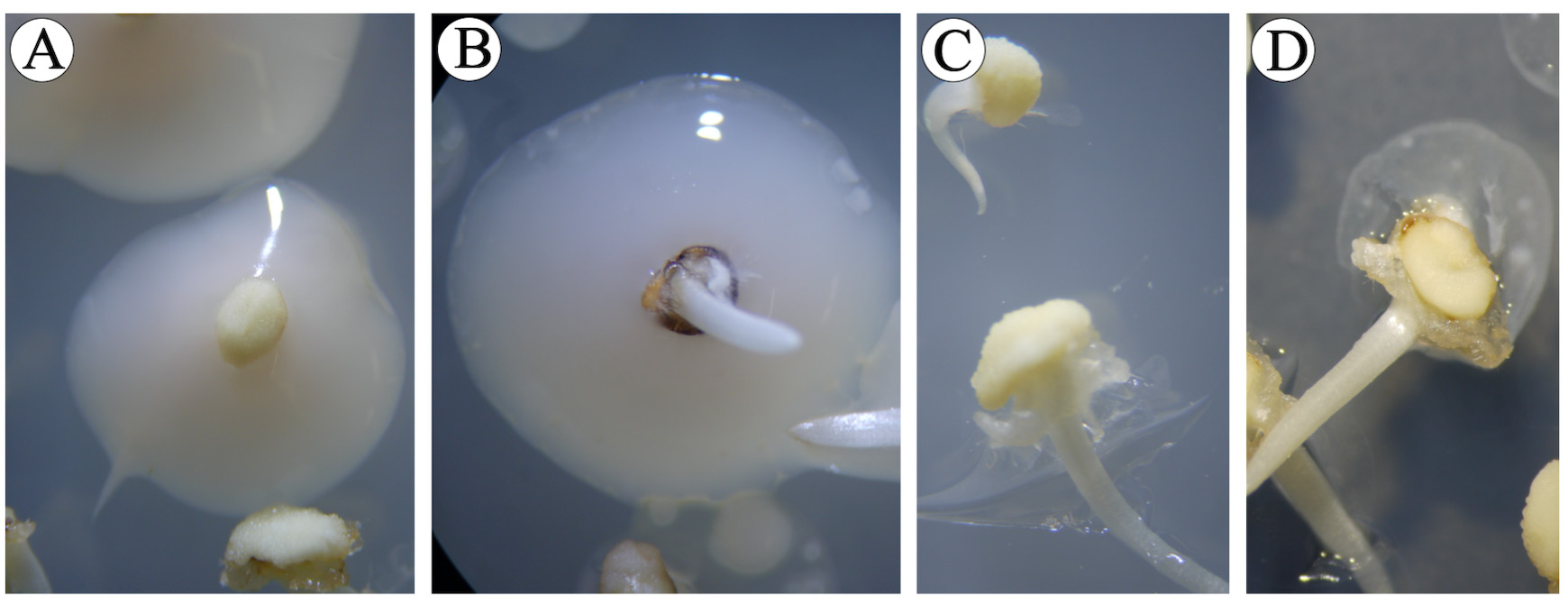
Figure 9. Contamination of immature embryos. A. Control immature embryos (no Agrobacterium inoculation) showing bacterial contamination. B. Agrobacterium-inoculated immature embryos showing contamination with other bacteria. C. Control non-infected immature embryos without contamination. D. Agrobacterium-inoculated immature embryos without contamination. - Place immature embryos without contamination on sterile filter paper and remove shoots with scalpel and forceps. Performing this steps under a stereo-microscope to guarantee complete excision of shoots.
- No parts of the shoot and rim should be left over, remove everything (Figures 10A and 10B).
- Remove the brown tissue from calli if there is excess, but be careful not to damage the immature embryos.
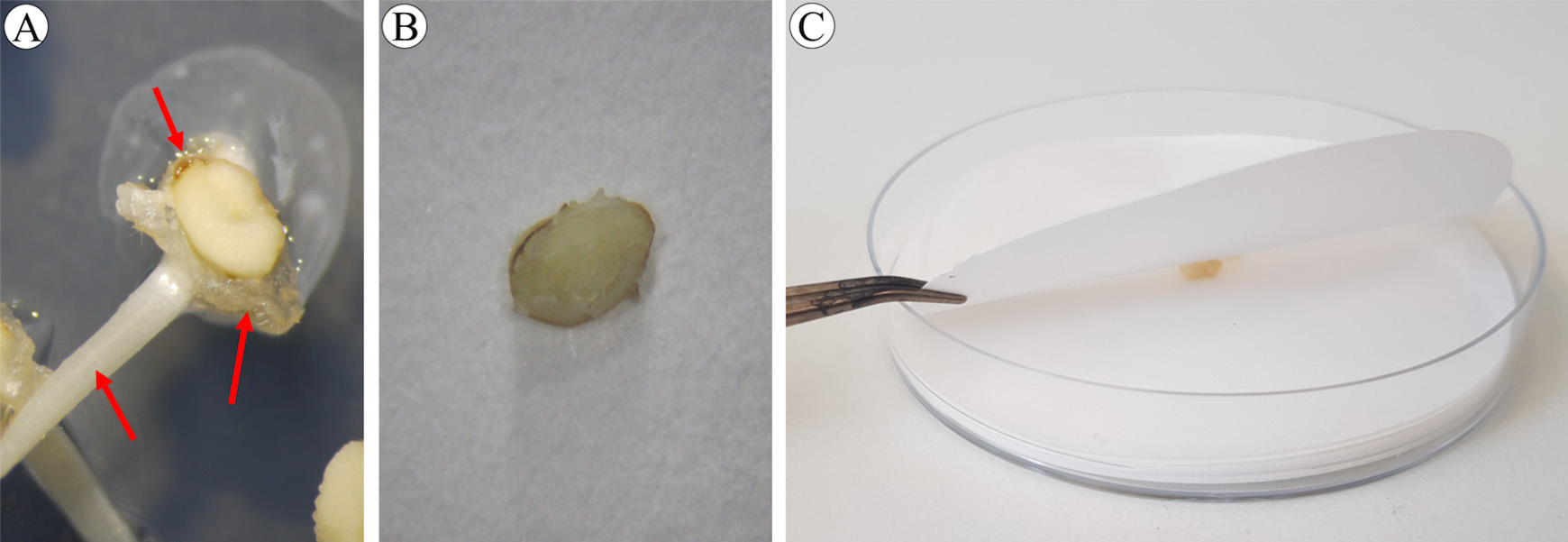
Figure 10. Immature embryos before (A) and after (B) removing shoots. Arrows indicate parts to be removed: shoot (bottom left), rim (bottom right) and brown tissue (upper). C. Cleaning immature embryo using two layers of sterile filter paper.
- Clean the immature embryos by placing them between two layers of sterile filter paper (Figure 10C) and carefully dab off the Agrobacteria. Repeat this procedure at least two times to remove the surplus Agrobacteria.
- Transfer immature embryos scutellum side-up onto resting medium (16 immature embryos/plate) and incubate for 5 days in growth chamber 1 [30 °C, continuous light, 24/0 day/night photoperiod, with photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (Figure 11).
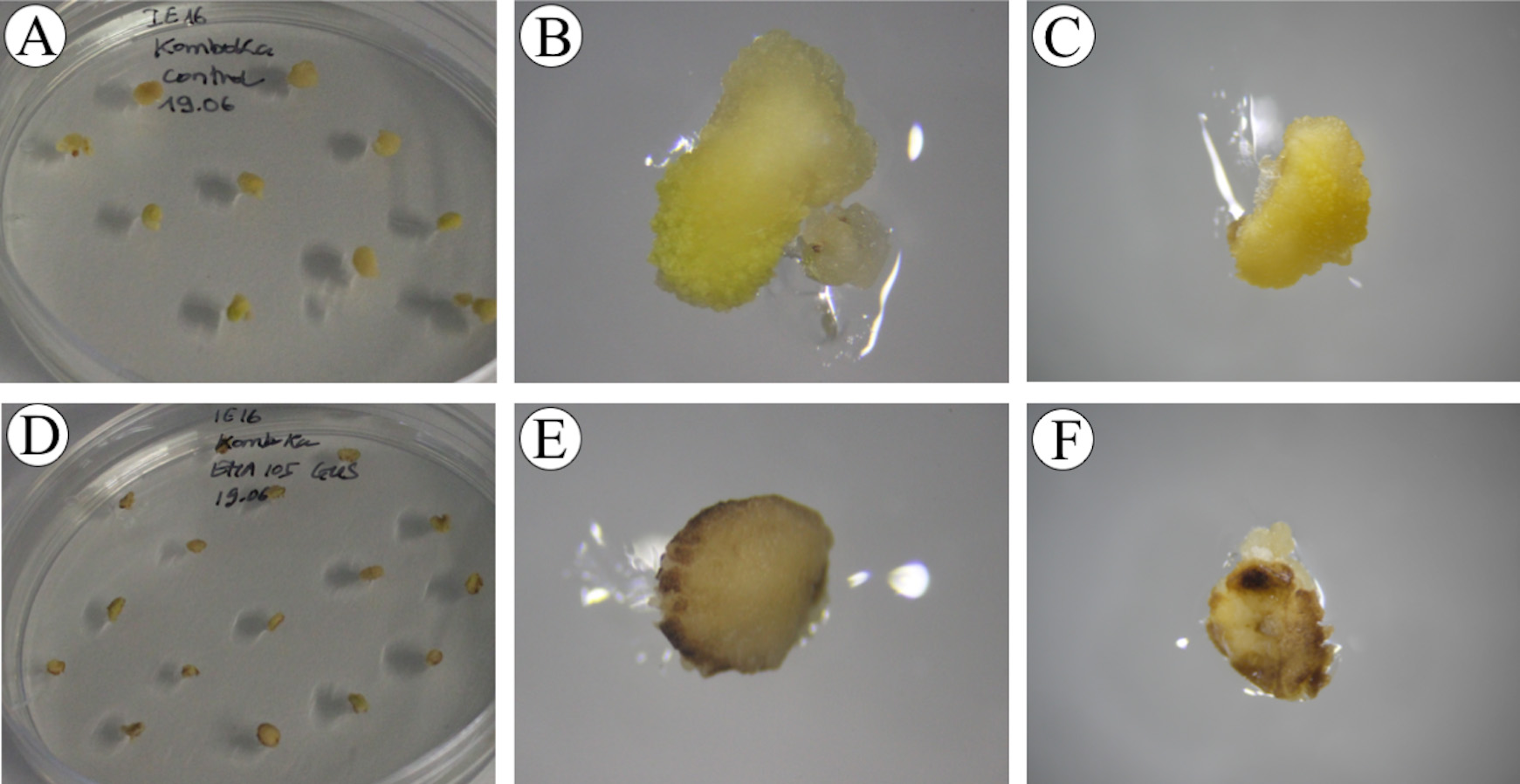
Figure 11. Immature embryos at the end of the resting phase. A-C. Control immature embryos. D-F. Agrobacterium-inoculated immature embryos.
Notes:- Use the stereo microscope to check for the state of the immature embryos (e.g., if there is any contamination), to clean brown tissues and take immature embryos more gently.
- Do not push immature embryos into the medium, let them stay loosely on top of the medium.
- Keep immature embryos on the same position when transferring between media (scutellum up-side to the medium).
- Seal plates with two layers of Micropore 3M tape.
- Check immature embryos for possible contamination (Figure 9). If immature embryos are contaminated, discard them. If possible, uncontaminated immature embryos in the contaminated plate can be rescued and placed on a separate plate. Otherwise discard the whole plate. Contamination can happen if dehusking or immature embryos isolation was not done carefully enough and embryos were damaged with forceps or scalpel during the process, facilitating contamination with other microorganisms (Figure 9).
- Selection
- After 5 days of resting, remove the brown tissue completely with forceps and a scalpel by scratching or cutting brown tissue off the surface of the immature embryo. Transfer the immature embryos onto the selection medium, containing 30 mg/L hygromycin B (16 calli/plate). Incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (1st selection) (Figure 12).
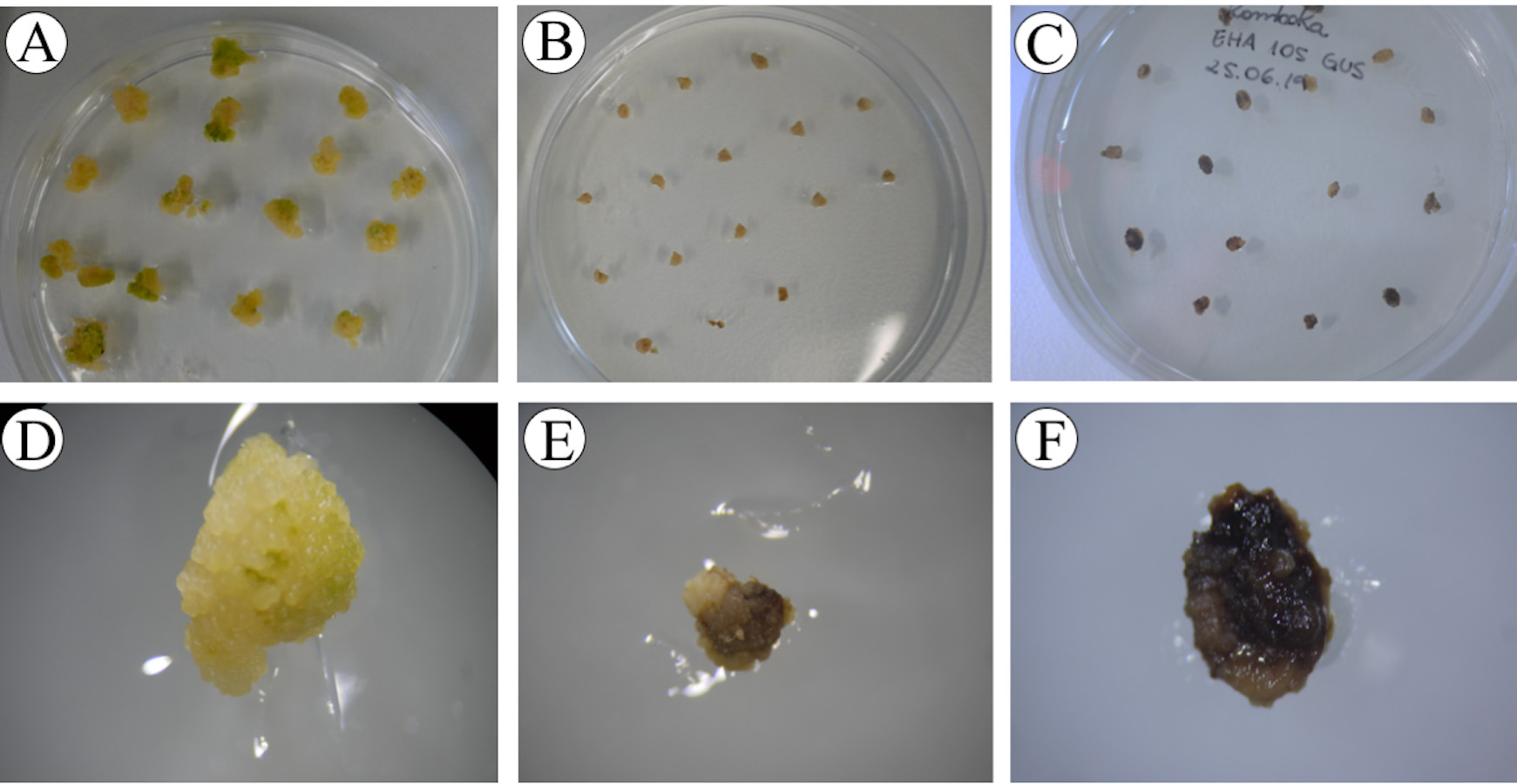
Figure 12. Immature embryos at the end of the first selection phase. A, D. Control immature embryos without hygromycin B, immature embryos produced compact and embryogenic calli and started turning green. B, E. Control immature embryos on selection medium with 30 mg/L hygromycin B. Immature embryos do not turn brown completely but are also not growing either. C, F. Agrobacterium-inoculated immature embryos may turn brown partially or completely. - After 10 days of 1st selection, transfer the calli to a freshly prepared plate with selection medium (16 immature embryos/plate). Remove brown tissue as much as possible from the immature embryos and incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (2nd selection) (Figure 13). Some immature embryos turn brown completely after the 1st selection. In this case, do not remove the brown tissue and keep the whole immature embryos.
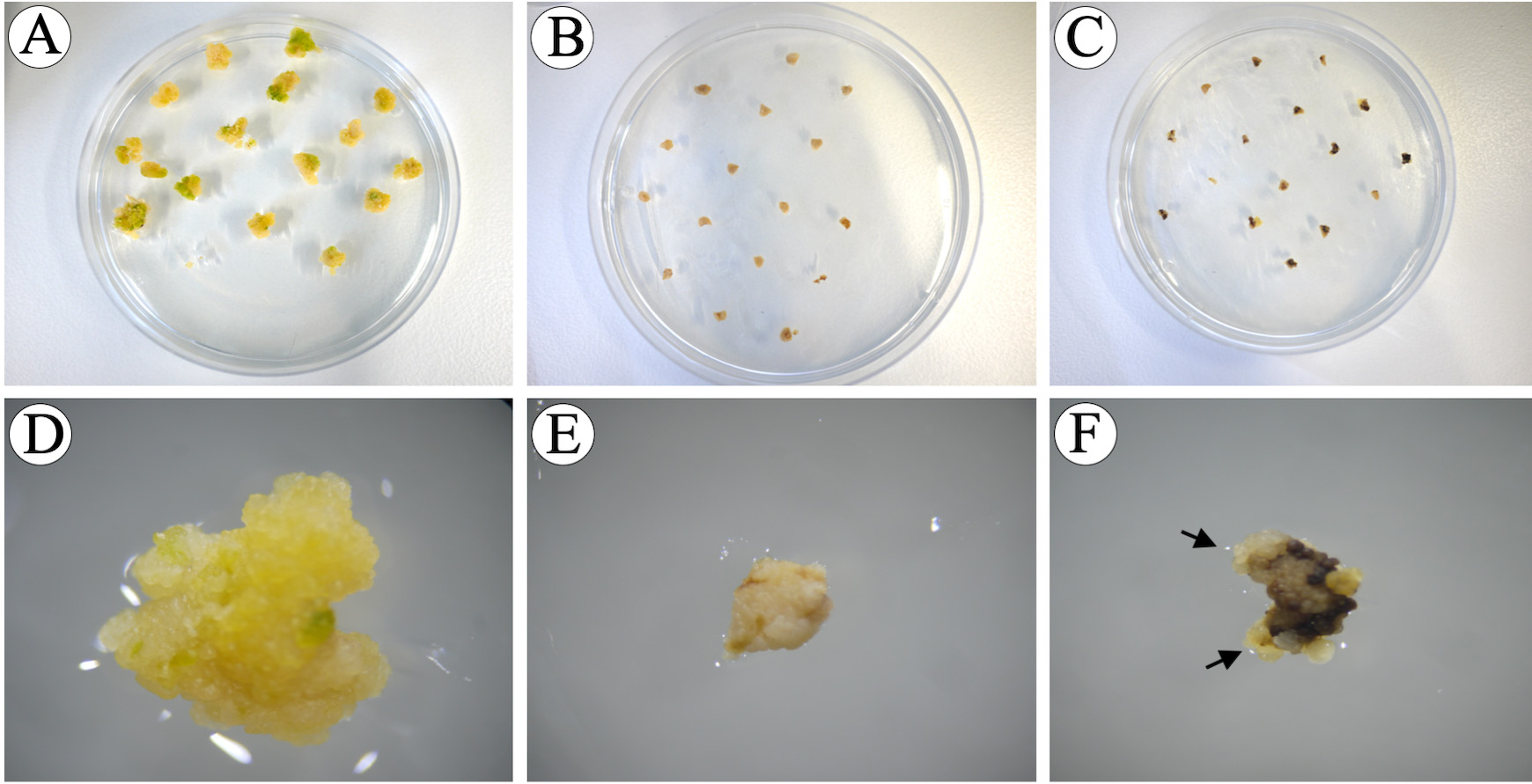
Figure 13. Immature embryos after the 2nd selection phase. A, D. Control immature embryos without hygromycin B produced proliferating embryogenic calli. B, E. Control immature embryos with 30 mg/L hygromycin B, immature embryos remain their size (2-4 mm) without producing any callus. C, F. Agrobacterium-inoculated immature embryos on selection medium produced small, compact and light yellow embryogenic microcalli (black arrows). - After 10 days of 2nd selection, take only the embryogenic microcalli from the black immature embryos and place onto fresh selection medium (16 immature embryos/plate), incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (3rd selection) (Figure 14). The embryogenic calli are small, compact clusters of cells, usually light yellow in color (Figure 13 F). Non-embryogenic calli are usually larger, soft, semi-transparent and yellow or gray loosely- held clusters of cells. The “mother immature embryo” can be kept on the selection medium as a back-up in case the freshly-isolated microcalli do not grow, or more microcalli are needed. For this step, microcalli from different immature embryos are kept separately by dividing the Petri dish into small areas (Figure14 C). The separated microcalli will be considered as independent events.
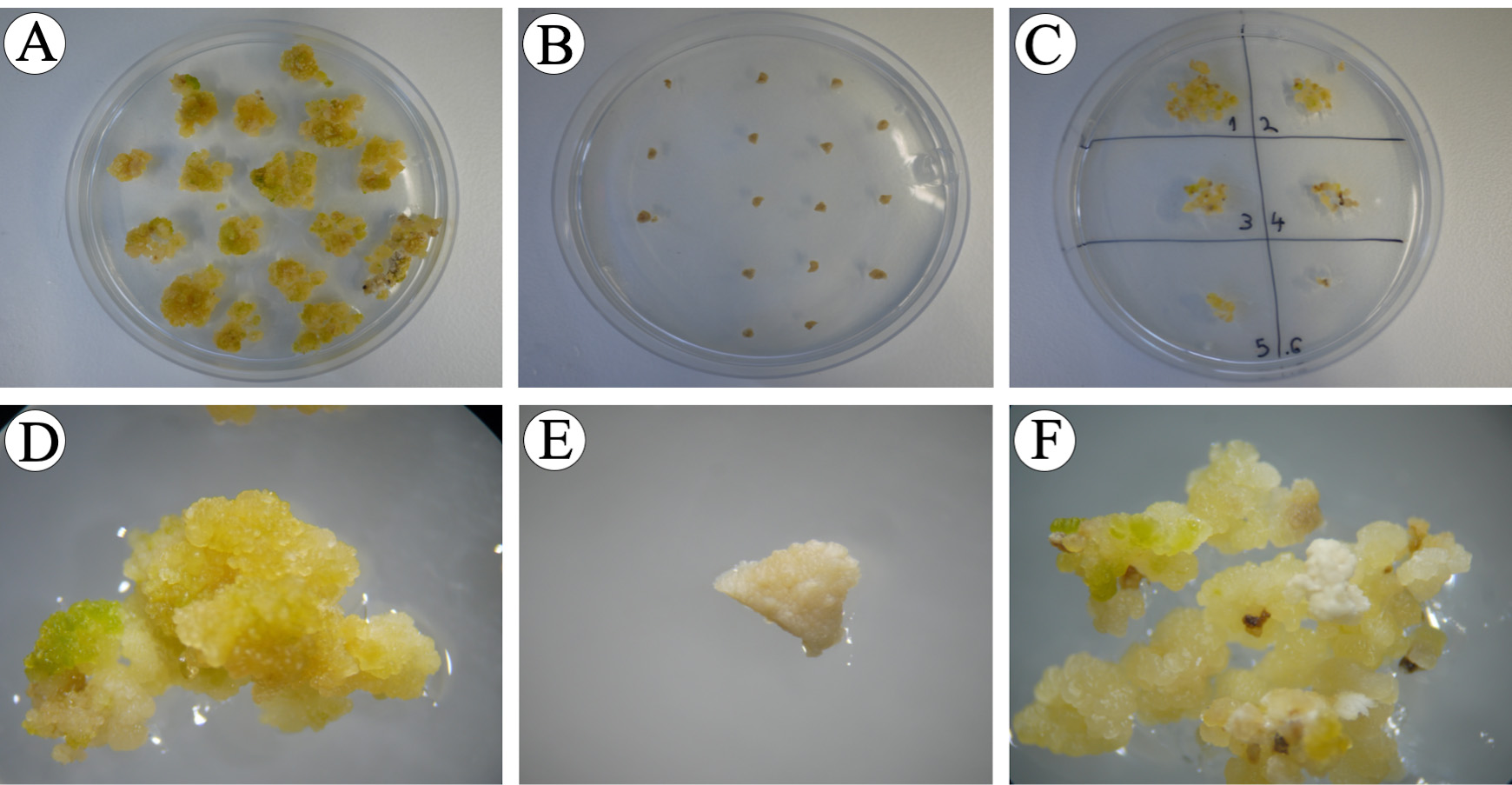
Figure 14. Microcalli at the end of the 3rd selection phase. A, D. Control calli without hygromycin B are compact, embryogenic and turned green partly. B, E. Control immature embryos with 30 mg/L hygromycin B, immature embryos remain the same size and do not grow. C, F. Agrobacterium-inoculated microcalli proliferating on selection medium.
Notes:- Use the stereo microscope to check for the state of the immature embryos/calli, to remove the brown tissue and to select the embryogenic calli more precisely.
- Don’t push immature embryos/calli into to the medium, let them stay loosely on top of the medium.
- Seal plates with two layers of Micropore 3M tape.
- If no microcalli is generated after 2nd selection, refresh the selection medium and culture for another 10 days. If after 2-3 additional rounds of selection, no microcalli are produced, discard the immature embryos.
- After 5 days of resting, remove the brown tissue completely with forceps and a scalpel by scratching or cutting brown tissue off the surface of the immature embryo. Transfer the immature embryos onto the selection medium, containing 30 mg/L hygromycin B (16 calli/plate). Incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (1st selection) (Figure 12).
- Plant regeneration
- Transfer resistant calli to pre-regeneration medium containing 30 mg/L hygromycin B (6-9 calli/plate) and incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (Figure 15).
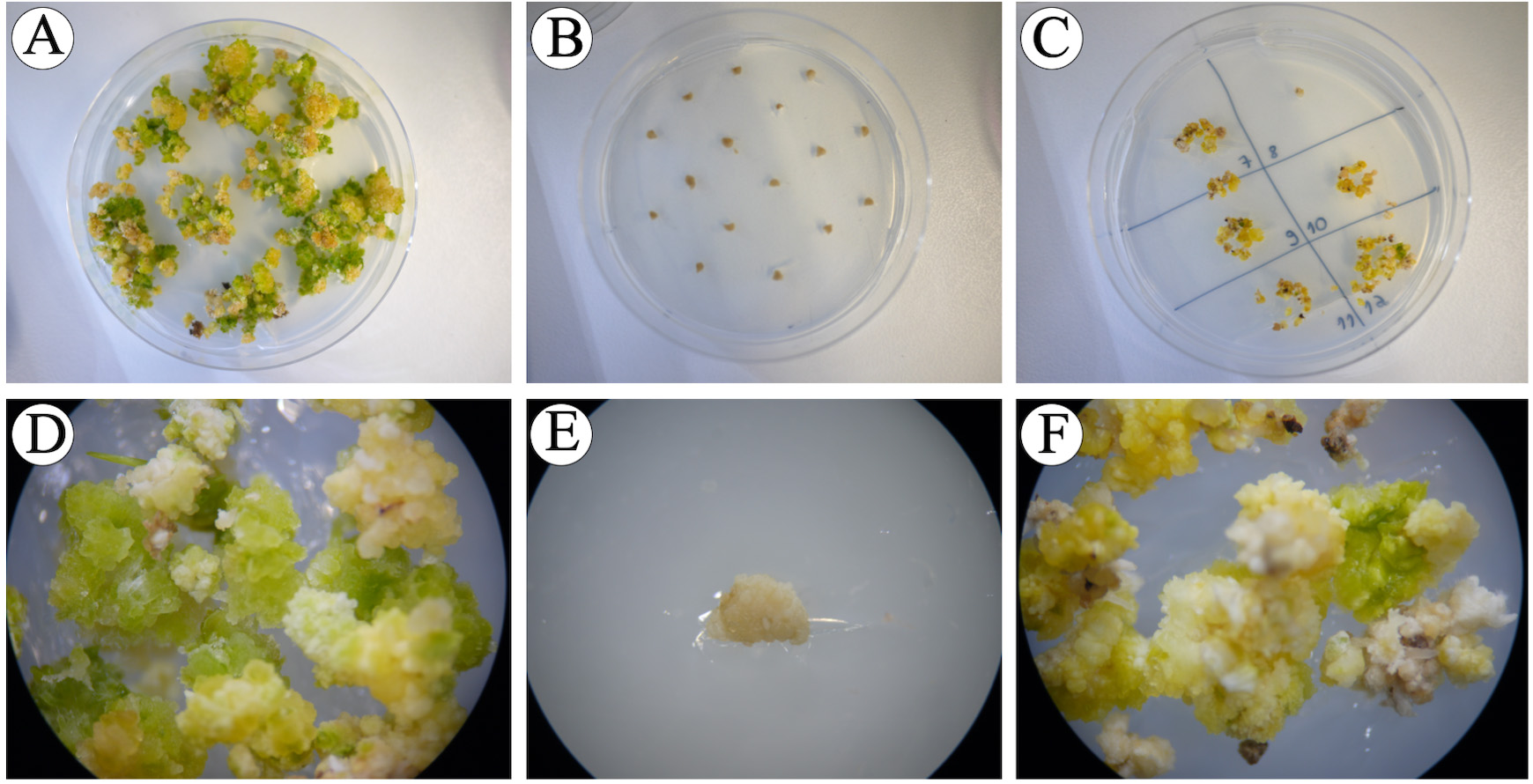
Figure 15. Calli at the end of pre-regeneration phase. A, D. Control calli without hygromycin B are mostly green. B, E. Control immature embryos with 30 mg/L hygromycin B; immature embryos remain their sizes and did not grow. C, F. Agrobacterium-inoculated proliferated calli with greening spots. - Select proliferating calli with green spots that covered approximately 2/3 of the calli (Figure 15F) and transfer to regeneration medium containing 30 mg/L hygromycin B (6 calli/plate), for shoot development and incubate at 30 °C for 14 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (Figure 16).
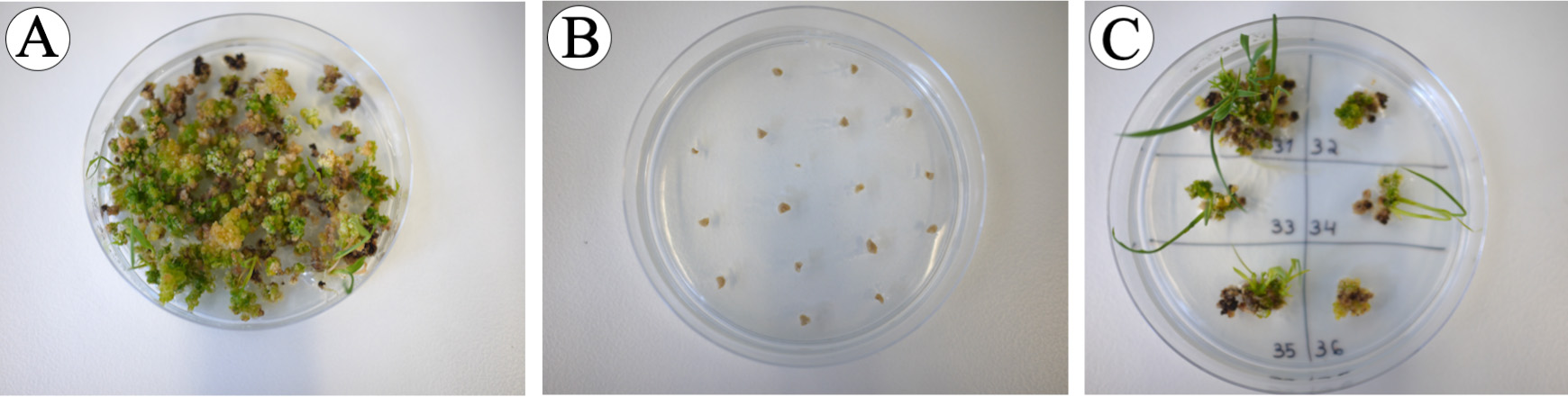
Figure 16. Calli at the end of the Regeneration phase. A. Regenerated plants from a control calli without hygromycin B. B. Control immature embryos with 30 mg/L hygromycin B, immature embryos did not grow. C. Regenerated plants from Agrobacterium-inoculated calli are putative transformants. - If calli have not produced any shoot or only produce small shoots, transfer the calli to freshly prepared plates of regeneration medium (6 calli/plate) every 14 days and incubate at 30 °C in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1], until small plantlets develop (small rice plants regenerated from a callus with at least two leaves and small root system, Figures 16-17).
- Select 1-3 plantlets from each callus and transfer into a MagentaTM GA-7 vessel containing rooting medium without hygromycin B. Incubate them for 7 days at 27 °C [16/8 day/night photoperiod with photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 30 µmol m-2 s-1, PPFD-green of 70 µmol m-2 s-1 and PPFD-red of 60 µmol m-2 s-1]. At this stage, plantlets regenerated from different calli are considered independent transformation events, therefore taking more than one plantlet as a backup to ensure that all putative events grow vigorously in the rooting step.
- After 7 days, when plantlets reach the top of the MagentaTM box, place another MagentaTM box on top to create more space for the plants to grow (Figure 17A). Place ‘double’ MagentaTM box in the growth chamber with 27 °C [16/8 day/night photoperiod with photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 30 µmol m-2 s-1, PPFD-green of 70 µmol m-2 s-1 and PPFD-red of 60 µmol m-2 s-1] for another 7 days.
- Transfer plantlets of approx. 15 cm height into soil by gently washing away excess agarose attached to roots. Grow plantlets in a pot which is submerged in a larger bucket to maintain high humidity condition in the glass house with natural daylight and additional lamplight of 8/16 day/night photoperiod with a day temperature of 30 °C ± 2 °C and a night temperature of 25 °C ± 2 °C. The relative humidity was between 50-70%. Plants were grown under a photosynthetic active radiation (PAR) or photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 70 µmol m-2 s-1 and PPFD-red of 80 µmol m-2 s-1.

Figure 17. Rooting and planting. A, B. Two-week-old regenerated plants, before transfer to soil. C. Regenerated plant right after planting. D. Regenerated plants, two weeks after planting.
Notes:- Use the stereo microscope to check for the state of the calli/plantlets, to select the calli and separate plantlets more gently.
- Don’t push the calli into to the medium, let them stay loosely on top of the medium.
- Seal Petri dishes or MagentaTM GA-7 vessels with two layers of Micropore 3M tape.
- Since the plantlets were regenerated under continuous light (24/0 day/night photoperiod), we recommend to grow the plantlet for the rooting step under long-day conditions (16/8 day/night photoperiod) to slowly acclimate them to the upcoming short-day conditions in the glasshouse (8/16 day/night photoperiod).
- Transfer resistant calli to pre-regeneration medium containing 30 mg/L hygromycin B (6-9 calli/plate) and incubate at 30 °C for 10 days in continuous light [24/0 day/night photoperiod, photosynthetic photon flux density (PPFD) of 200 µmol m-2 s-1 with PPFD-blue of 40 µmol m-2 s-1, PPFD-green of 80 µmol m-2 s-1 and PPFD-red of 70 µmol m-2 s-1] (Figure 15).
- Screening for transformed plants
Note: In this protocol, we did not unambiguously determine transformation events. We used plants that had been regenerated in parallel, but without infection by Agrobacteria as controls. In these control plants, we did not observe GUS staining or GFP fluorescence. All transformation events counted here were derived from independent immature embryos (one plant per one transformed immature embryo).- Transformation of 100 Komboka immature embryos with GUS-intron reporter construct using two different Agrobacterium strains (EHA105 and LBA4404) resulted in 14 and 31 putative transformants from independent immature embryos, respectively (Table 1). All regenerated plants were tested for GUS activity. All leaves of regenerated plants showed GUS activity (Figures 18A-18B), except control plants which also underwent the whole protocol but without Agrobacterium infection (Figure 18C). Our results might indicate that the LBA4404 strain is more efficient in transforming Komboka when compared to EHA105, however the relative efficiency of different Agrobacterium strains can not be judged without careful quantitative analyses across many independent transformations.
Table 1. Apparent transformation efficiency of Komboka using different Agrobacterium strain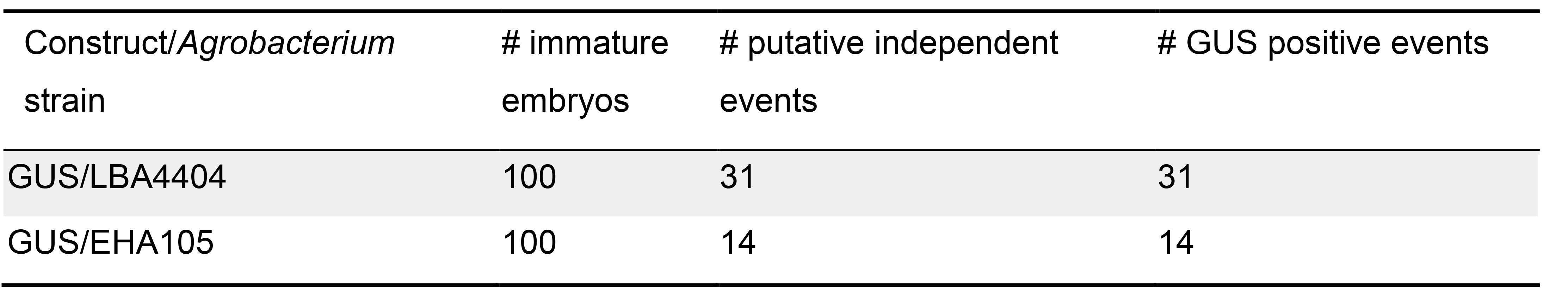
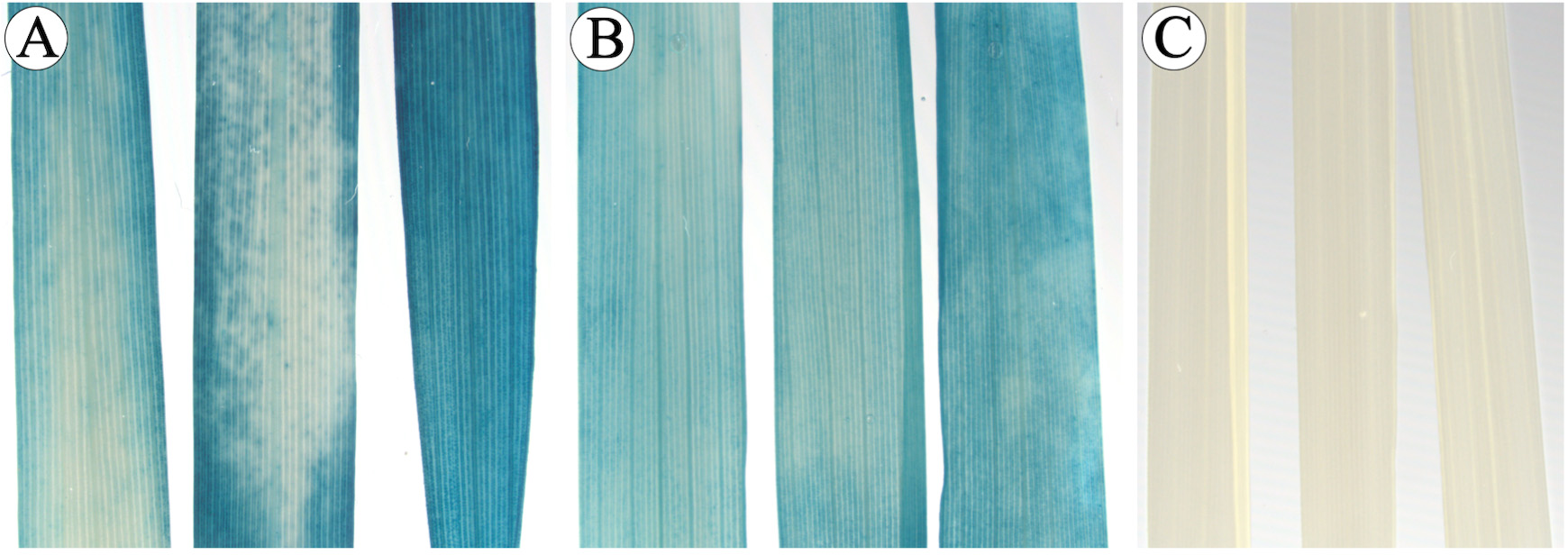
Figure 18. GUS histochemistry of transformed Komboka leaves. A, B. GUS-stained leaves of two plants which are regenerated from two independent immature embryos. C. GUS-stained leaves from one control plant regenerated without Agrobacterium infection, no GUS activity detectable. - Transformation of Komboka with a GFP reporter construct using the Agrobacterium strain LBA4404 resulted in 54 putative independent events (Table 2). Roots of all these 54 plants were GFP positive (Figures 19A-19E). No GFP fluorescence was observed in uninfected plants (Figure 19F). From 54 GFP positive events, 200 bp GFP gene fragment was amplified by PCR from 48 plants (Figure 20). Full T-DNA insertion, inheritance and copy number remain to be validated.
Table 2. Apparent transformation efficiency of Komboka with GFP reporter construct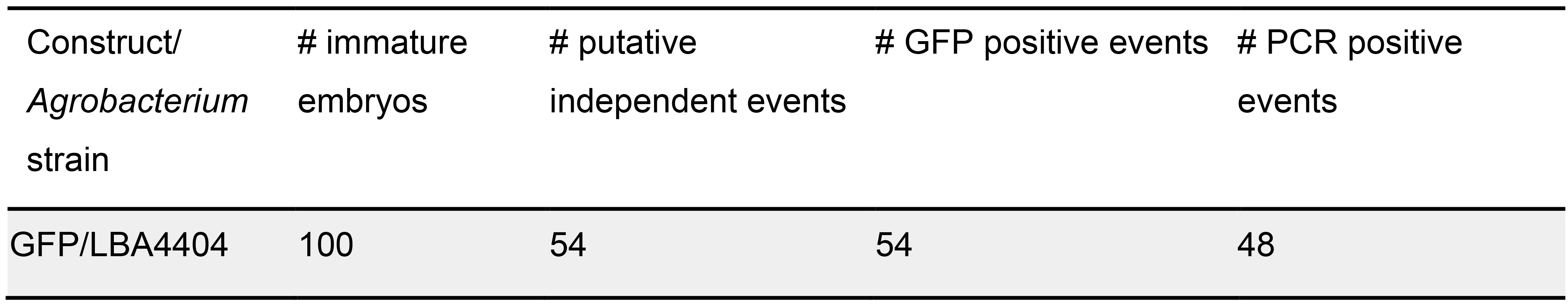
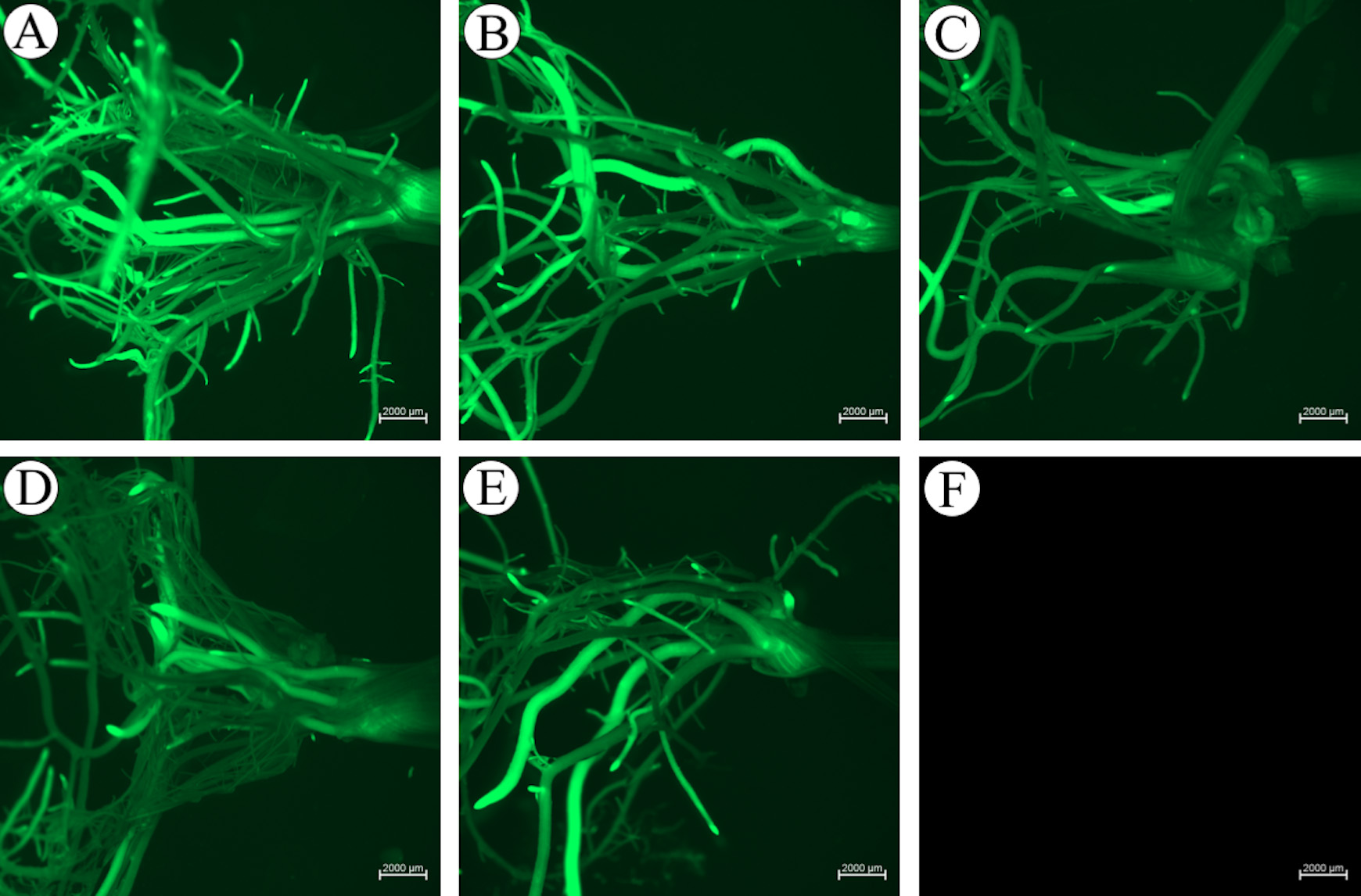
Figure 19. GFP fluorescence of transformed Komboka. A-E. Root of five independent transformants under blue light. F. Root of a non-infected plant regenerated from tissue culture (control). Scale bar: 2000 μm. All photos are taken and displayed at the same setting.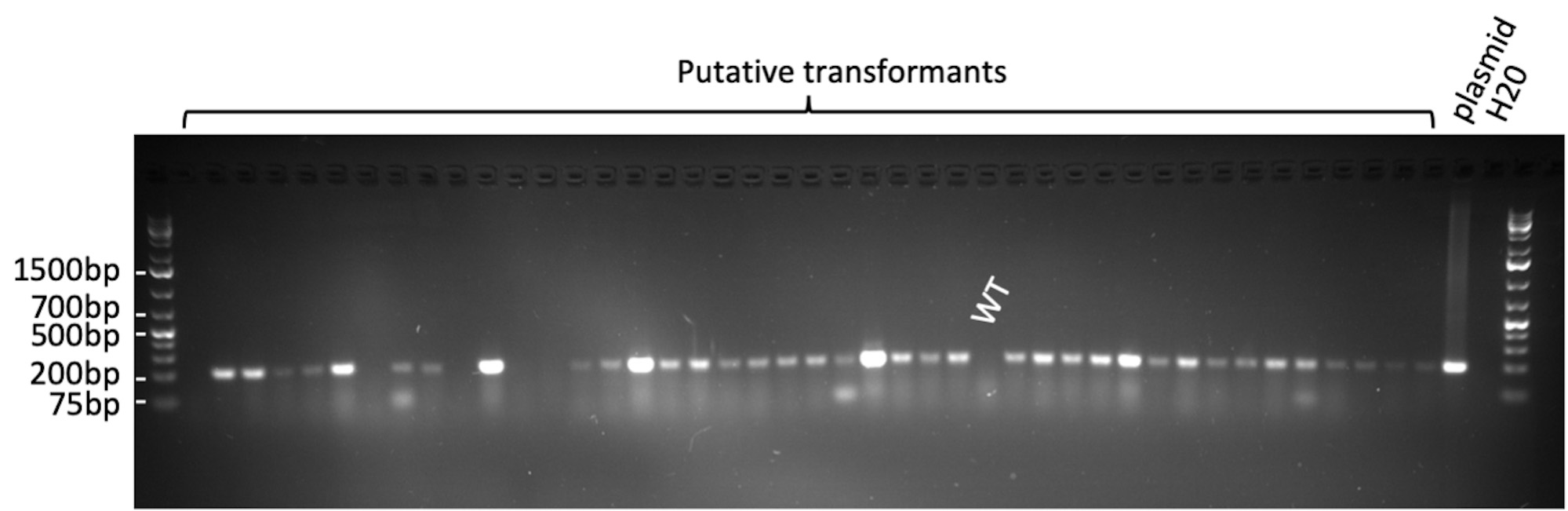
Figure 20. Representative gel picture for confirmation of a 200 bp GFP gene fragment in transformed plants by PCR. WT: non-infected plant regenerated from tissue culture.
- Transformation of 100 Komboka immature embryos with GUS-intron reporter construct using two different Agrobacterium strains (EHA105 and LBA4404) resulted in 14 and 31 putative transformants from independent immature embryos, respectively (Table 1). All regenerated plants were tested for GUS activity. All leaves of regenerated plants showed GUS activity (Figures 18A-18B), except control plants which also underwent the whole protocol but without Agrobacterium infection (Figure 18C). Our results might indicate that the LBA4404 strain is more efficient in transforming Komboka when compared to EHA105, however the relative efficiency of different Agrobacterium strains can not be judged without careful quantitative analyses across many independent transformations.
- Adjusting the protocol for transformation of other rice varieties
To adapt this protocol for the transformation of other varieties, we recommend to evaluate the efficiency after each step using GUS or GFP intron reporter constructs (Vancanneyt et al., 1990). For example, after co-cultivation, use five calli for GUS staining or GPF screening to test whether the co-cultivation step was successful or whether co-cultivation time needs to be adjusted.
We also recommend to use non-infected immature embryos as controls to check for the effectiveness of hygromycin B selection as well as the regeneration ability on media without hygromycin B (see Note 2, Section E), because hygromycin B sensitivity and regeneration ability may differ among varieties. Hygromycin B concentrations can be adjusted between 5-50 mg/L. Criteria for adjustment include: control immature embryos cannot grow or produce microcalli on hygromycin B containing media (Figures 13, 14, 15).
Another relevant parameter is the stage of immature embryo development, which may differ between varieties; immature seeds should be in the late milky stage (Video 1), typically with a size of 1.3-1.8 mm at about 8-12 days post pollination. Agrobacterium strains (AGL1, LBA4404, EHA105) can be compared. Also, the number of rounds of selection and rounds of pre-generation steps can be adjusted between 1-3 until microcalli or green spots are produced.
Data analysis
- GUS staining
- Harvest 3 cm leaves and place in 15 ml Falcon® tubes.
- Add 5 ml staining solution (Tables S13 and S14).
- Vacuum the samples for 10 min at RT.
- Close the tubes and incubate at 37 °C overnight in the dark.
- Remove staining solution.
- Add 70% ethanol for destaining (chlorophyll removal) and incubate samples at 65 °C. The duration of incubation affects destaining, the longer the tissue will be incubated, the higher contrast of blue dye to background can be achieved, usually 2-3 days are fine. During this incubation time, exchange 70% ethanol for 1 or 2 times
- As long as the tissue stays in ethanol, it can be stored at RT for long time (at least several months without losing the color). It is recommended to store the samples in a dark place.
- GFP imaging
Roots of GFP-transformed Komboka were observed under blue light with a Zeiss AxioZoom.V16 stereo microscope, filter excitation wavelength: 450-490 nm, filter emission wavelength: 500-550 nm, excitation wavelength: 488 nm, emission wavelength: 509 nm, exposure time 4.59 s, zoom: 0.7, total optical magnification: 7x. All photos are taken and displayed at the same setting: black: 2000, gramma: 1.0, white: 22897. - PCR for GFP encoding gene
To check for the presence of the GFP gene in regenerated plants, FastAmp® Plant Direct PCR Kit (Intactgenomics) was used to amplify a 200 bp fragment of the GFP coding region using the following primers: VL_GFP_F1 5′-GCAAGCTGACCCTGAAGTTC-3′, VL_GFP_R1 5′-GTCTTGTAGTTGCCGTCGTC-3′. PCR conditions: initial denaturation step at 95 °C for 5 min, followed by 35 cycles each at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 20 s, last extension at 72 °C for 10 min and the reaction was kept at 10 °C.
Recipes
Note: See Supplementary file (Tables S1-S14) for Recipes.
- Rice soil composition (Table S1)
- Stock solution composition (Table S2)
- Phytohormones and antibiotics (Table S3)
- Cultivation medium composition (Table S4)
- Suspension medium (Table S5)
- Co-cultivation medium (Table S6)
- Resting medium (Table S7)
- Selection medium (Table S8)
- Pre-regeneration medium (Table S9)
- Regeneration medium (Table S10)
- Rooting medium (Table S11)
- Transformation cheat sheet (Table S12)
- GUS staining solution (Table S13)
- X-Gluc solution (Table S14)
Acknowledgments
We gratefully acknowledge funding from the Bill and Melinda Gates Foundation, Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy – EXC-2048/1 – project ID 390686111, and the Alexander von Humboldt Professorship to WBF. We thank IRRI for providing the Komboka seeds, Bing Yang (University of Missouri, USA) and Joon S. Eom (Heinrich Heine University, Düsseldorf, Kyung Hee University, South Korea) for providing the GFP and GUS reporter constructs. We thank Marina Manzanilla for her training effort at IRRI genetic transformation lab. We thank Monika Streubel, Joon S. Eom for their valuable advices in plant transformation.
Competing interests
The Institute for Molecular Physiology, Heinrich Heine University of Düsseldorf (HHU), Germany, The Center for Tropical Agriculture (CIAT), Cali, Colombia and the International Rice Research Institute (IRRI), Philippine contributed equally towards the development of the protocol. The authors declare no competing interests.
References
- Char, S. N., Li, R. and Yang, B. (2019). CRISPR/Cas9 for Mutagenesis in Rice. Methods Mol Biol 1864: 279-293.
- Cheng, X., Sardana, R., Kaplan, H. and Altosaar, I. (1998). Agrobacterium-transformed rice plants expressing synthetic cryIA(b) and cryIA(c) genes are highly toxic to striped stem borer and yellow stem borer. Proc Natl Acad Sci U S A 95(6): 2767-2772.
- Christou, P., Ford, T. L. and Kofron, M. (1991). Production of transgenic rice (Oryza Sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/Technology 9(10): 957-962.
- Datta, S. K., Datta, K., Soltanifar, N., Donn, G. and Potrykus, I. (1992). Herbicide-resistant Indica rice plants from IRRI breeding line IR72 after PEG-mediated transformation of protoplasts. Plant Mol Biol 20(4): 619-629.
- Helliwell, E. E. and Yang, Y. (2013). Molecular strategies to improve rice disease resistance. Methods Mol Biol 956: 285-309.
- Hiei, Y. and Komari, T. (2008). Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3(5): 824-834.
- Hiei, Y. and Komari, T. (2006). Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell, Tissue and Organ Culture 85(3): 271.
- Kitilu, M., Nyomora, A. and Charles, J. (2019). Growth and yield performance of selected upland and lowland rainfed rice varieties grown in farmers and researchers managed fields at Ifakara, Tanzania. Afr J Agricult Res 14: 197-208.
- Li, D., Xu, H., Sun, X., Cui, Z., Zhang, Y., Bai, Y., Wang, X., Chen, W. (2015). Differential transformation efficiency of Japonicarice varieties developed in northern China. Crop Breed Appl Biotechnol 15(3): 162-168.
- Nishimura, A., Aichi, I. and Matsuoka, M. (2006). A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1(6): 2796-2802.
- Oliva, R., Ji, C., Atienza-Grande, G., Huguet-Tapia, J. C., Pérez-Quintero, A., Li, T., Eom, J. S., Li, C., Nguyen, H., Liu, B., Auguy, F., Sciallano, C., Luu, V. T., Dossa, G. S., Cunnac, S., Schmidt, S. M., Slamet-Loedin, I. H., Vera Cruz, C., Szurek, B., Frommer, W. B., White, F. F. and Yang, B. (2019). Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol 37(11): 1344-1350.
- Sahoo, K. K., Tripathi, A. K., Pareek, A., Sopory, S. K. and Singla-Pareek, S. L. (2011). An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods 7(1): 49.
- Sahoo, R. K. and Tuteja, N. (2012). Development of Agrobacterium-mediated transformation technology for mature seed-derived callus tissues of indica rice cultivar IR64. GM Crops Food 3(2): 123-128.
- Shimamoto, K., Terada, R., Izawa, T. and Fujimoto, H. (1989). Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338(6212): 274-276.
- Slamet-Loedin, I. H., Chadha-Mohanty, P. and Torrizo, L. (2014). Agrobacterium-mediated transformation: rice transformation. Methods Mol Biol 1099: 261-271.
- Sundararajan, S., Sivaraman, B., Rajendran, V. and Ramalingam, S. (2017). Tissue culture and Agrobacterium-mediated genetic transformation studies in four commercially important indica rice cultivars. J Crop Sci Biotechnol 20(3): 175-183.
- Toki, S., Hara, N., Ono, K., Onodera, H., Tagiri, A., Oka, S. and Tanaka, H. (2006). Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47(6): 969-976.
- van Wordragen, M. F. and Dons, H. J. M. (1992). Agrobacterium tumefaciens-mediated transformation of recalcitrant crops. Plant Mol Biol Rep 10(1): 12-36.
- Vancanneyt, G., Schmidt, R., O'Connor-Sanchez, A., Willmitzer, L. and Rocha-Sosa, M. (1990). Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220(2): 245-250.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Luu, V. T., Stiebner, M., Maldonado, P. E., Valdés, S., Marín, D., Delgado, G., Laluz, V., Wu, L., Chavarriaga, P., Tohme, J., Slamet-Loedin, I. H. and Frommer, W. B. (2020). Efficient Agrobacterium-mediated Transformation of the Elite–Indica Rice Variety Komboka. Bio-protocol 10(17): e3739. DOI: 10.21769/BioProtoc.3739.
Category
Plant Science > Plant transformation > Agrobacterium
Microbiology > Microbial genetics > Transformation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









