- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Karyopherin-β2 Inhibits and Reverses Aggregation and Liquid-liquid Phase Separation of the ALS/FTD-Associated Protein FUS
Published: Vol 10, Iss 16, Aug 20, 2020 DOI: 10.21769/BioProtoc.3725 Views: 5240
Reviewed by: Steven BoeynaemsIndranil MalikDonya Pakravan

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Step-By-Step Protocol for Correlative Light and Electron Microscopy Imaging of Proteinaceous Deposits in Cultured Cells and Human Brain Tissues
Peizhou Jiang and Dennis W. Dickson
Aug 5, 2025 2337 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2626 Views

Colocalizing Telomeres With PML or γH2AX Foci by IF-FISH in Mouse Brain Neurons
Anna Konopka
Nov 5, 2025 1506 Views
Abstract
The study of RNA-binding proteins (RBP) offers insight into the mechanisms of pathologic protein aggregation in neurodegenerative diseases. We developed a protocol for purifying an RBP FUS and a nuclear import receptor (NIR) Kapβ2 and testing the ability of Kapβ2 to mitigate FUS aggregation and liquid-liquid phase separation.
Keywords: FUSBackground
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are neurodegenerative diseases characterized by the mislocalization and aggregation of several RNA-binding proteins (RBP) (Mackenzie et al., 2010; Da Cruz and Cleveland, 2011; King et al., 2012). One of these proteins is FUS (Fused in Sarcoma), a nuclear protein mislocalizes in the cytoplasm of neurons in ALS/FTD patients, where it undergoes liquid-liquid phase transition followed by aberrant phase transition to form insoluble aggregates (Altmeyer et al., 2015; Burke et al., 2015; Lin et al., 2015; Molliex et al., 2015; Murakami et al., 2015; Patel et al., 2015) (Figure 1). Karyopherin-β2 (Kapβ2), a nuclear import receptor (NIR), has been established as a chaperone and disaggregase of proteins with PY-nuclear localization sequences (NLS), such as FUS (Guo et al., 2018; Hofweber et al., 2018; Yoshizawa, et al., 2018). The following protocol was developed to test the ability of Kapβ2 to inhibit and reverse FUS aggregation and phase separation ex vivo (Guo et al., 2018).
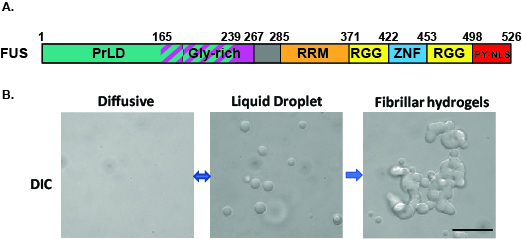
Figure 1. Aberrant phase transition of FUS (10 μM) leads to the formation of fibrillar hydrogel. A. Domain architecture of FUS. B. DIC images show that FUS undergoes reversible liquid-liquid phase transition to form liquid droplets followed by aberrant phase transition to form hydrogels. Scale bar: 10 μm.
Materials and Reagents
- 1.5 ml tube holder
- 15 ml tube holder
- 50 ml tube holder
- Glass beads (MP Biomedicals, catalog number: MP07DP1070 )
- Nitrile gloves (FisherBrand, catalog number: 19-130-1597 )
- Ethanol spray bottle
- MilliQ water spray bottle
- Bleach spray bottle
- Ice bucket
- Foam liquid nitrogen container
- 1.5 ml Semi-Micro Plastic Cuvettes (Fisherbrand, catalog number: 14955127 )
- 96-well plate (Corning, catalog number: 08-757-230 )
- Lens paper (FisherBrand, catalog number: 11-995 )
- Glass microscope slides (Fisherbrand, catalog number: 12548B )
- 1.5 ml autoclaved Eppendorf tubes (Eppendorf, catalog number: 05-408-129 )
- 1.5 ml Protein LoBind Tubes (Eppendorf, catalog number: 22431081 )
- 15 ml conical tubes (Thermo Scientific, catalog number: 12-565-268 )
- 50 ml conical tubes (Thermo Scientific catalog number: 339652 )
- 50 ml plastic round-bottom centrifugal tubes (Thermo Scientific, catalog number: 05-529C )
- Pipette tips
0.1-10 µl (USA Scientific, catalog number: 1121-3810 )
2-20 µl (Fisherbrand, catalog number: 02-707-432 )
20-200 µl (Fisherbrand, catalog number: 02-707-430 )
200-1,000 µl (Fisherbrand, catalog number: 02-707-40 ) - Serological pipettor
- 5 ml, 10 ml, 25 ml, and 50 ml serological pipettes (Fisherbrand, model: 13-678-11 )
- BL21(DE3) Ril cells (Agilent, catalog number: 230245 )
- FUS expression construct in pDUET vector (Addgene Plasmid #29629) (Figure 2A)
- Kapβ2 expression construct in pGEX-Tev vector (Figure 2B)
- LB liquid media (Fisher Chemical, catalog number: BP9723-2 )
- Ampicillin (Gold Bio, catalog number: A-301-25 )
- LB + ampicillin agar plates
- Lysozyme (Gold Bio, catalog number: L-040-25 )
- Ice
- Liquid nitrogen
- Bradford Reagent (Thermo Scientific, catalog number: PI23236 )
- Microscope immersion oil (Carl Zeiss, catalog number: 12-070-397 )
- Bio-spin Chromatography Columns (Bio-Rad, catalog number: 7326008 )
- HiTrap-Q anion exchange chromatography column (GE Healthcare, catalog number: 17115301 )
- IPTG (GoldBio, catalog number: 367-93-1 )
- Phosphate-Buffered Saline tablets (Fisher Scientific, catalog number: BP2944100 )
- D-(+)-trehalose (Fisher Scientific, catalog number: BP2687100 )
- Glutathione Sepharose 4 Fast Flow beads (GE Healthcare, catalog number: 17-5132-01 )
- NaCl (Fisher Scientific, catalog number: S271-3 )
- EDTA (Fisher Scientific, catalog number: BP2482500 )
- Glycerol (Fisher Scientific, catalog number: BP2294 )
- DTT (Gold Bio, catalog number: DTT10 )
- Pepstatin (Gold Bio, catalog number: P-020-25
- EGTA (Millipore, catalog number: 324626-25GM )
- MgCl2 (Fisher BioReagents, catalog number: BP214-500 )
- Protease inhibitor tablets (Thermo Scientific, catalog number: A32955 )
- Imidazole (Fisher Scientific, catalog number: O3196-500 )
- Reduced glutathione (Fisher Scientific, catalog number: AC120000250 )
- FUS Wash Buffer (see Recipes)
- FUS Elution Buffer (see Recipes)
- Kapβ2 Resuspension Buffer (see Recipes)
- Kapβ2 ATP Buffer (see Recipes)
- Kapβ2 Buffer A (see Recipes)
- Kapβ2 Elution Buffer (see Recipes)
- TEV Buffer (see Recipes)
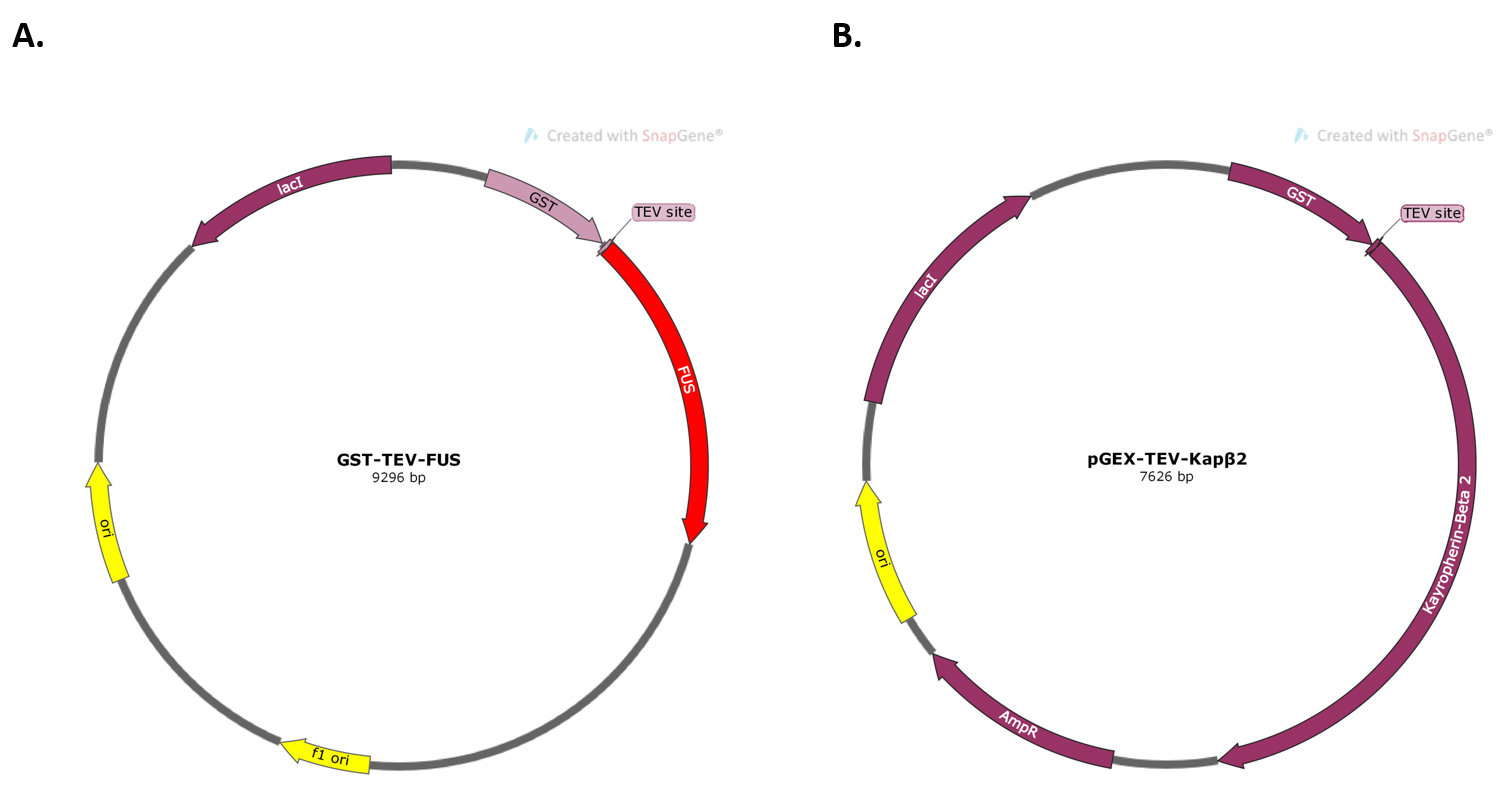
Figure 2. Plasmid maps for the constructs used in the experiment. A. Plasmid map of FUS expression construct in pDUET vector. B. Plasmid map of Kapβ2 expression construct in pGEX-Tev vector.
Equipment
- 50 ml beakers (Pyrex, catalog number: CLS100050 )
- 100 ml beakers (Pyrex, catalog number: CLS100050 )
- 250 ml Erlenmeyer flask (Pyrex, catalog number: 4980250 )
- 2800 ml Erlenmeyer flasks (Pyrex, catalog number: CLS44202XL )
- Plastic nalgene 1 L centrifugal jars (Thermo Scientific, catalog number: 11-825B )
- 0.1-10 µl, 2-20 µl, 20-200 µl, and 200-1,000 µl pipettes
- Centrifuge 5424 R (Eppendorf, catalog number: 2231000655 )
- Centrifuge 5810 R (Eppendorf, catalog number: 0 22625004 )
- Sorvall Lynx 4000 Superspeed Centrifuge (Thermo Scientific, catalog number: 75-006-580 )
- Sorvall BP 8 Centrifuge (Thermo Scientific, catalog number: 75007681 )
- ThermomixerTM C with SmartBlock (Eppendorf, catalog number: 2231000667 )
- MaxQTM 4450 Benchtop Orbital Shaker (Thermo Scientific, catalog number: SHKE4450 )
- Innova® 44 Series Shaking Incubator (New Brunswick, catalog number: M1282 )
- HerathermTM General Protocol Microbiological Incubator (Thermo Scientific, catalog number: 51028063 )
- Tube Revolver/Rotator (Thermo Scientific, catalog number: 88881001 )
- Multi-Purpose Tube Rotator (Fisherbrand, catalog number: 88-861-049 )
- Digital Vortex Mixer (Fisherbrand, catalog number: 02-215-418 )
- Cimarec+TM Stirring Hotplate S (Thermo Scientific, catalog number: P88857100 )
- EntrisTM Precision Balance (SartoriusTM, catalog number: ENTRIS423i-1S )
- Accumet® AB150 pH Benchtop Meter (Fisherbrand, catalog number: 13-636-AB150 )
- Model 705 Sonic Dismembrator (Fisherbrand, catalog number: FB705110 )
- 160 UV-Vis Spectrophotometer (Biomate, catalog number: 840-301000 )
- TecanTM Spark® Multimode Microplate Reader
- HP Z230 Tower Workstation
- LEICATM DMi8 S Platform Inverted Microscope
- Dell Intel Core i7
- Cold room
- 4 °C Refrigerator
- -20 °C freezer
- -80 °C freezer
- AKTA pure FPLC
Software
- Excel (Microsoft Office)
- Tecan SparkControl
- Leica LAS X Hardware
- AKTA Unicorn
Procedure
- FUS Purification
- Generate a FUS expression construct in a pDuet vector with a TEV-cleavable site, resulting in a GST-TEV-FUS construct. Express and purify GST-TEV-FUS protein from E. coli BL21-CodonPlus(DE3)-RIL cells (Agilent) as follow.
- Grow FUS-expressing cells in 6 L of Lennox Broth (LB) at 37 °C with shaking at 250 rpm until the OD600 is between 0.4-0.6. Induce cells with 1 ml of 1 M IPTG (final conc. 1 mM IPTG), then grow overnight at 15 °C with shaking at 250 rpm.
- Spin cells down at 4,000 rpm for 20 min at 4 °C. To lyse cells, resuspend pellet in FUS Wash Buffer (Recipe 1), add 20 µg of lysozyme per 1 ml resuspended cells, and incubate on ice for 30 min. Sonicate the cells for 4 min at amplitude 35 for 30 s intervals on/off. And then spin lysed cells down at 16,000 rpm for 20 min at 4 °C.
- To bind and separate GST-tagged proteins, 5 ml Glutathione Sepharose 4 Fast Flow beads (GE Healthcare) is added to lysate supernatant and the mixture was incubated for 90 min at 4 °C. Centrifuge beads at 1,792 x g for 3 min at 4 °C and discard the supernatant. Wash beads three times with Wash Buffer, centrifuging at 1,124 x g for 2 min at 4 °C.
- Elute FUS by adding 5 ml FUS Elution Buffer (Recipe 2) and incubating for 20 min at room temperature. Centrifuge the beads in Elution Buffer at 1,124 x g for 2 min at 4 °C in Bio-spin chromatography columns (Bio-Rad) over glass tubes to collect the elution.
- Measure purified FUS via Bradford Assay. Flash freeze purified FUS in liquid nitrogen and store at -80 °C. Protein can be used for up to 3 years. Freeze-thaw cycle should be minimized.
- Kapβ2 Purification
- Generate a Kapβ2 expression construct in a pGEX-TEV vector with a TEV-cleavable site, resulting in a GST-TEV-Kapβ2 construct. Express and purify this Kapβ2 construct from E. coli BL21-CodonPlus(DE3)-RIL cells (Agilent) as follow.
- Grow Kapβ2-expressing cells in 6 L of Lennox Broth (LB) at 37°C with shaking at 250 rpm until the OD600 is between 0.6-0.8. Induce cells with 1 ml of 1 M IPTG (final conc. 1 mM IPTG), then grow overnight at 25 °C with shaking at 250 rpm. Spin cells down at 4,000 rpm for 20 min at 4 °C.
- To lyse cells, resuspend pellet in Resuspension Buffer (Recipe 3), add 20 µg of lysozyme per 1 ml resuspended cells, and incubate on ice for 30 min. Sonicate cells for 1 min 30 s at amplitude 15, 30 s on/off, and then spin lysed cells down at 16,000 rpm for 60 min at 4 °C.
- Incubate lysate supernatant with 5 ml Glutathione Sepharose 4 Fast Flow beads (GE Healthcare) for 90 min at 4 °C. Centrifuge beads at 4,500 x g for 3 min at 4 °C and discard the supernatant.
- Wash beads four times with 25 ml Resuspension Buffer each, by centrifuging at 1,124 x g for 3 min at 4 °C. Add 10 ml ATP Buffer (Recipe 4) and let stand at room temperature for 10 min before centrifuging at 1,124 x g for 3 min at 4 °C. Wash beads twice with 20 ml ATP Buffer each, by centrifuging at 1,124 x g for 3 min at 4 °C. Wash beads three time with 10 ml Buffer A each time (Recipe 5), by centrifuging at 1,124 x g for 3 min at 4 °C.
- Elute Kapβ2 by adding 20 ml Kapβ2 Elution Buffer (Recipe 6) and incubating for 30 min at 4 °C. Centrifuge the beads in Elution Buffer at 1,124 x g for 2 min at 4 °C in Bio-spin chromatography columns (Bio-Rad) over glass tubes to collect the elution.
- Measure the concentration of the purified Kapβ2, then add 1 µg TEV per 10 µg Kapβ2. Aliquot the mixture into Low-Bind Tubes and incubate overnight at 30 °C to cleave the GST tag.
- Cleaved Kapβ2 is further purified by HiTrap-Q anion exchange chromatography column (GE healthcare).
- Inhibition of FUS aggregation by Kapβ2
- Spin down FUS at 16,100 x g for 10 min at 4 °C and transfer supernatant to a fresh tube. Measure the concentration of FUS and Kapβ2 via Bradford Assay with calibration curve generated using BSA.
- Set up the aggregation assay in a 96-well plate, with a blank well, a standard FUS well, and experimental wells.
- In each experimental well, add 4 µM FUS (Table 1), 4 µM Kapβ2 (Table 1), 5 µl TEV Buffer (Recipe 7), 1.5 µl 100 mM DTT, and 0.8 µg of TEV. Fill to 100 µl with FUS Buffer (Recipe 2). Pipet up and down gently to mix.
- In the blank well, add 5 µl TEV Buffer, 1.5 µl 100 mM DTT, and 0.8 µg TEV. Match the volume of Kapβ2 added to the experimental wells with Kapβ2 Buffer. Fill to 100 µl with FUS Buffer. Pipet up and down gently to mix.
- In the standard FUS well, add 4 µM FUS, 5 µl TEV Buffer, 1.5 µl 100 mM DTT, and 0.8 µg TEV. Match the volume of Kapβ2 added to the experimental wells with Kapβ2 Buffer. Fill to 100 µl with FUS Buffer. Pipet up and down gently to mix.
- Set up the TECAN plate reader. Identify the plate type as Costar 96 Flat Black with no lid and no humidity cassette. Program for a kinetic loop of 90 cycles with fixed intervals of 00:01:00 with no shaking. Program absorbance readings at 395 nm at each cycle. Insert the plate into the plate reader and run the aggregation assay at room temperature.
- After 1.5 h, the data will be automatically stored in an excel spreadsheet (Figure 3).
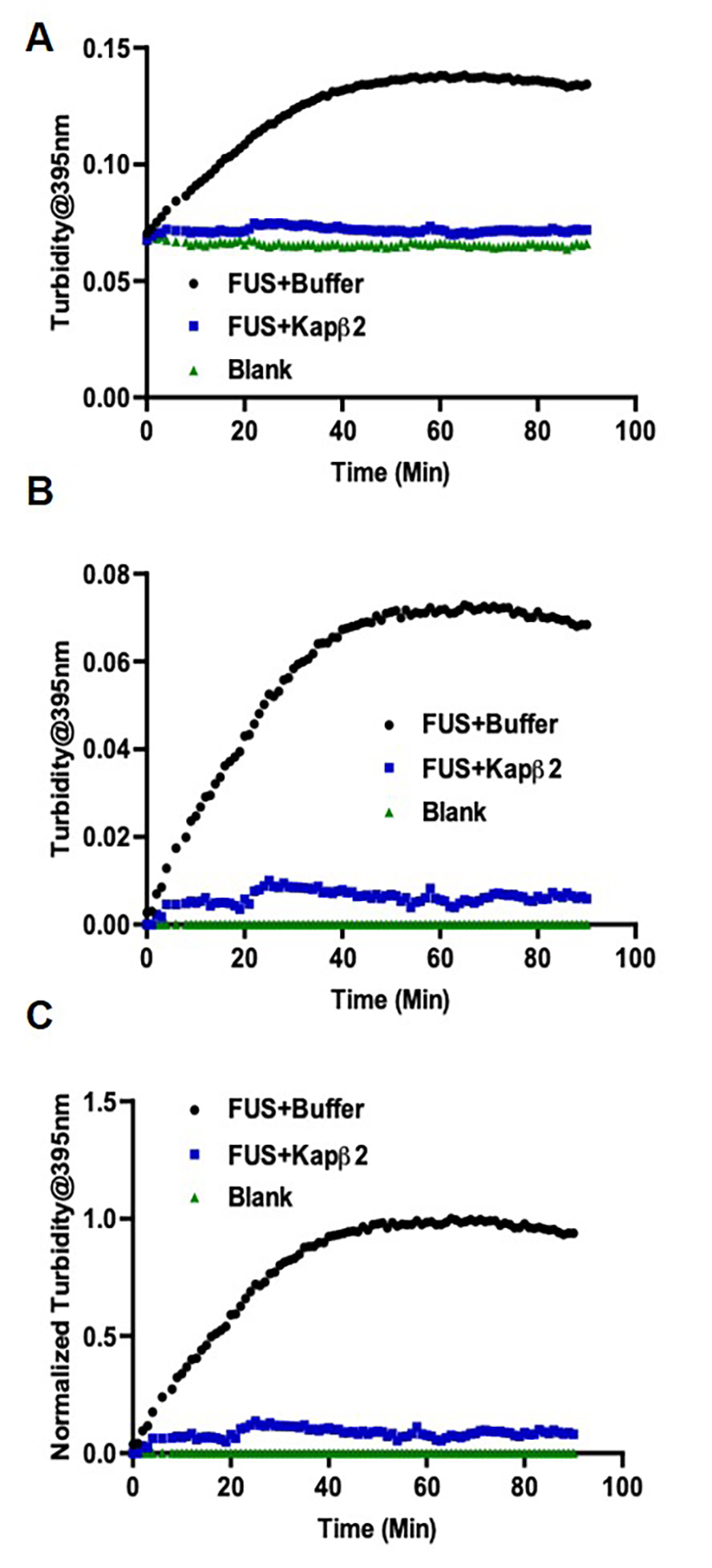
Figure 3. Turbidity measurement of FUS aggregation shows Kapβ2 inhibits FUS aggregation. A. Raw data as exported from TECAN. B. Data after background subtraction. C. Data after normalization.
- Disaggregation of FUS fibrils by Kapβ2
- Spin down FUS at 16,100 x g for 10 min at 4 °C and transfer supernatant to a fresh tube. Measure the concentration of FUS and the concentration of Kapβ2.
- Set up the aggregation assay in a 96-well plate, with a blank well, a standard FUS well, and experimental wells.
- In each experimental well, add 4 µM FUS (Table 1), 5 µl TEV Buffer (Recipe 7), 1.5 µl 100 mM DTT, and 0.8 µg of TEV. Fill with FUS Buffer (Recipe 2) so that adding 4 µM Kapβ2 would give a total volume of 100 µl (Table 1). Pipet up and down gently to mix.
- In the blank well, add 5 µl TEV Buffer, 1.5 µl 100 mM DTT, and 0.8 µg TEV. Fill with FUS Buffer so that adding 4 µM Kapβ2 would give a total volume of 100 µl. Pipet up and down gently to mix.
- In the standard FUS well, add 4 µM FUS, 5 µl TEV Buffer, 1.5 µl 100 mM DTT, and 0.8 µg TEV. Fill with FUS Buffer so that adding 4 µM Kapβ2 would give a total volume of 100 µl. Pipet up and down gently to mix.
- Set up the TECAN plate reader. Identify the plate type as Costar 96 Flat Black with no lid and no humidity cassette. Program for a kinetic loop of 90 cycles with fixed intervals of 00:01:00. Program absorbance readings at 395 nm at each cycle. Insert the plate into the plate reader and run the aggregation assay at room temperature.
- At the end of the 90 cycles, add 4 µM of Kapβ2 to each experimental well. Add Kapβ2 Buffer to the blank well and the standard FUS well, match the volume of Kapβ2. Pipet up and down gently to mix. Program for another kinetic loop of 90 cycles with fixed intervals of 00:01:00. Program absorbance readings at 395 nm at each cycle. Insert the plate into the plate reader and run the disaggregation assay (Figure 4).
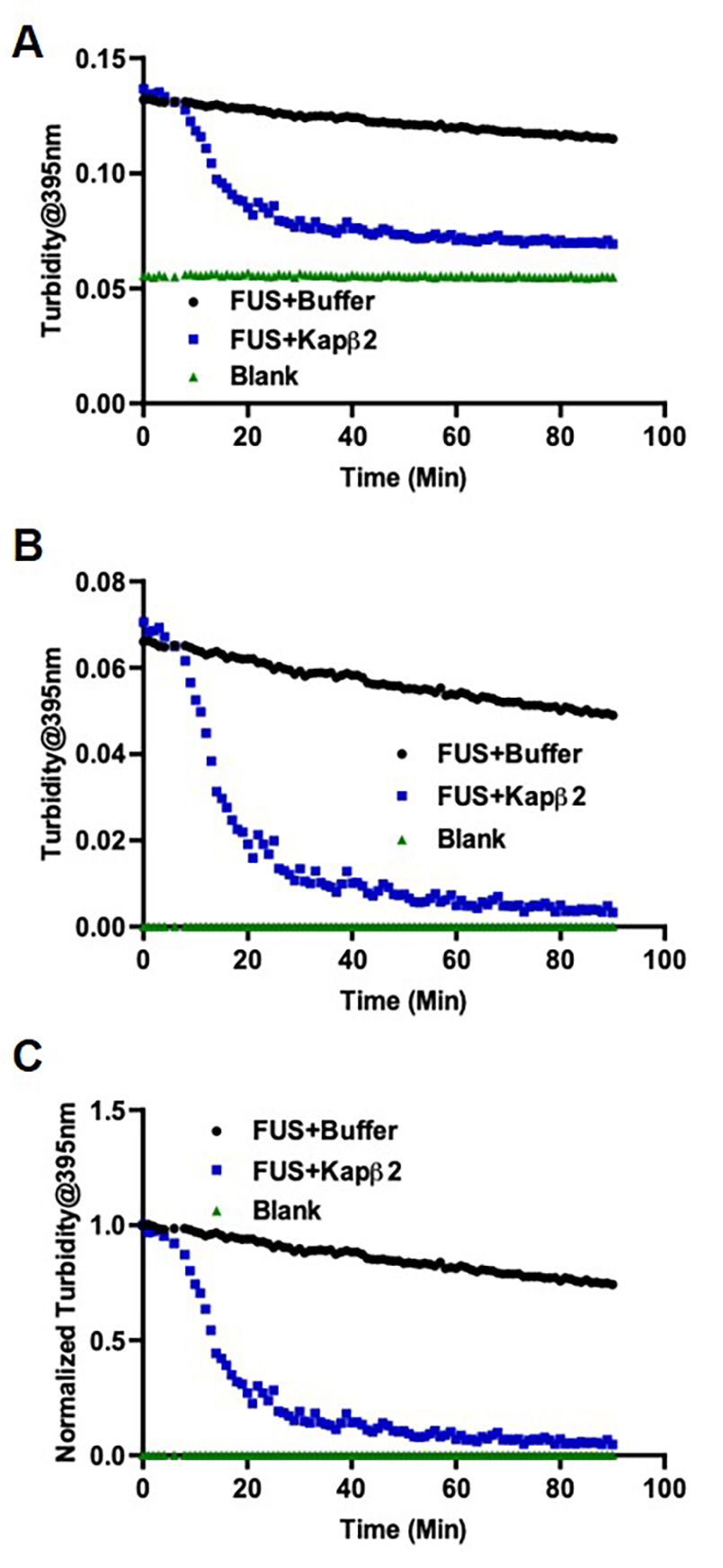
Figure 4. Turbidity measurement of FUS disaggregation shows Kapβ2 reverses FUS aggregation. A. Raw data as exported from TECAN. B. Data after background subtraction. C. Data after normalization.
- Kapβ2 reverse FUS liquid-liquid phase separation and aberrant phase transition
- Spin down FUS at 16,100 x g for 10 min at 4 °C and transfer supernatant to a fresh tube. Measure the concentration.
- Dilute FUS to a concentration of 10 µM (Table 1) with FUS Buffer (Recipe 2). Pipette 30 µl of 10 µM FUS into a fresh tube. Leave the tube on the bench at room temperature for approximately 2 h.
- Set up the LEICA microscope to observe the liquid droplets using a 100x oil immersion objective.
- Pipette 10 µl of the 10 µM FUS sample onto the cover slide above the drop of oil.
- Observe liquid-liquid droplet formation. Droplets move fluidly and merge upon contact.
- Add 1 µl of 100 μM Kapβ2 (Table 1) into the 10μl FUS sample on the coverslip to disassemble FUS liquid droplets and reverse FUS liquid-liquid phase separation (Figure 5A). Kapβ2 can disassemble preformed FUS liquid droplets within 30 s.
- Liquid droplets age into hydrogel after 6 hours of incubation at room temperature. Add 10 µl of 10 µM FUS sample onto the coverslip to observe hydrogel. Add 1 µl of 100 μM Kapβ2 into the 10μl FUS sample on the coverslip to disassemble FUS hydrogel and reverse FUS aberrant phase separation (Figure 5B).
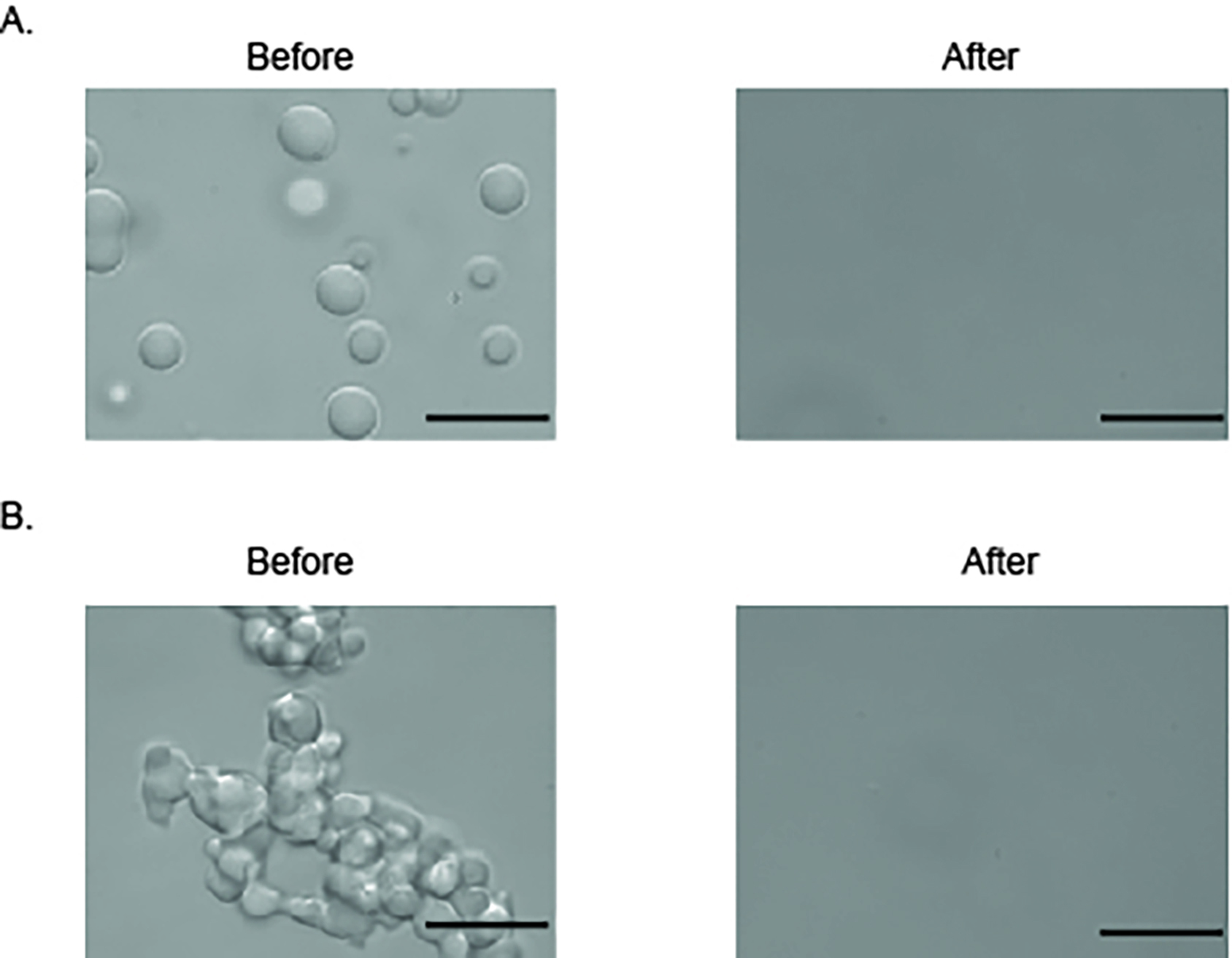
Figure 5. Kapβ2 reverses the LLPS and aberrant phase transition of FUS. A. DIC image of FUS liquid droplets before and after addition of Kapβ2. B. DIC image of “aged” FUS droplets (> 6 h) before and after addition of Kapβ2. Scale bars: 10 μm.
Data analysis
Analysis of FUS Aggregation
- Upon completion of any turbidity assay, data will automatically be imported from Tecan SparkControl into Microsoft Excel.
- Plot the absorbances of each well as a function of time. Subtract absorbances of the blank well from that of the standard well and the experiment wells to get signal.
- Normalize the signals to the maximum signal of the standard reaction to determine the relative extent of aggregation/disaggregation (Figures 3 and 4).
Recipes
- FUS Wash Buffer
- Make 1x PBS prior to purifying FUS
- Add 1 L MilliQ H2O to a 1 L bottle
- Add 5 Phosphate-Buffered Saline tablets to the MilliQ H2O
- Stir until the tablets have fully dissolved. Filter and store at 4 °C
- Add fresh reagents to 300 ml 1x PBS the day of purification
- Add 6 protease inhibitor tablets to 1x PBS
- Stir until the tablets have fully dissolved
- Filter and store at 4 °C
- Make 1x PBS prior to purifying FUS
- FUS Elution Buffer
- Add 1 ml 1 M Tris (pH 8) buffer to a 50 ml beaker
- Add 1.513 g of D-(+)-trehalose [final conc. 4.4 mM] to increase solubility of FUS
- Add 112.9 mg reduced glutathione [final conc. 364 µM] to remove GST-tagged protein from the Glutathione Sepharose 4 Fast Flow beads
- Add Millipore water to a final total volume of 20 ml. Stir until reagents have fully dissolved and pH to pH 8. Filter and store at 4 °C
- Kapβ2 Resuspension Buffer
- Make a 1 L stock solution prior to purifying Kapβ2
- Add 50 ml of 1 M Tris (pH 7.5) [final conc. 50 mM]
- Add 5.844 g of NaCl [final conc. 100 mM]
- Add 2 ml of 0.5 M EDTA [final conc. 1 mM]
- Add 200 ml glycerol [final conc. 20%]
- Fill to 1 L with MilliQ water
- Stir until reagents have fully dissolved and pH to pH 7.5. Filter and store at 4 °C
- Add fresh reagents to 300 ml of stock the day of purification
- Add 600 µl 1 M DTT [final conc. 2 mM]
- Add 300 µl of 5 mM Pepstatin [final conc. 5 µM]
- Add 6 protease inhibitor tablets
- Stir solution until protease inhibitor tablets have fully dissolved and pH to pH 7.5. Filter and store at 4 °C
- Make a 1 L stock solution prior to purifying Kapβ2
- Kapβ2 ATP Buffer
- Make a 1 L stock solution prior to purifying Kapβ2
- Add 50 ml of 1 M Tris (pH 7.5) [final conc. 50 mM]
- Add 5.844 g of NaCl [final conc. 100 mM]
- Add 2 ml of 0.5 M EGTA [final conc. 1 mM]
- Add 0.10165 of MgCl2 [final conc. 0.5 mM]
- Add 200 ml glycerol [final conc. 20%]
- Fill to 1 L with MilliQ water
- Stir until reagents have fully dissolved and pH to pH 7.5. Filter and store at 4 °C
- Add fresh reagents to 100 ml of stock solution the day of purification
- Add 200 µl 1 M DTT [final conc. 2 mM]
- Add 2 ml 250 mM ATP stock [final conc. 2 mM]
- Add 2 protease inhibitor tablets
- Stir solution until protease inhibitor tablets have fully dissolved and pH to pH 7.5. Filter and store at room temperature
- Make a 1 L stock solution prior to purifying Kapβ2
- Kapβ2 Buffer A
- Make a 1 L stock solution prior to purifying Kapβ2
- Add 50 ml of 1 M Imidazole (pH 6.5) [final conc. 50 mM]
- Add 4.383 g of NaCl [final conc. 74 mM]
- Add 2 ml of 0.5 M EDTA [final conc. 1 mM]
- Add 200 ml glycerol [final conc. 20%]
- Fill to 1 L with MilliQ water
- Stir until reagents have fully dissolved and pH to pH 6.5. Filter and store at 4 °C
- Add fresh reagents to 100 ml of stock solution the day of purification
- Add 200 µl 1 M DTT [final conc. 2 mM]
- pH to pH 6.5, filter, and store at 4 °C
- Make a 1 L stock solution prior to purifying Kapβ2
- Kapβ2 Elution Buffer
- Add 20 ml of fresh 100 ml Buffer A
- Add 122.9 mg reduced glutathione
- Stir until reduced glutathione has completely dissolved and pH to pH 6.5. Filter and store at 4 °C
- TEV Buffer
- Add 1 M Tris-HCl (pH 8.0)
- Add 10 mM EDTA
- Stir and pH to pH 8.9. Filter and store at room temperature
Table 1. Final Protein Concentrations for Experimental Assays

Acknowledgments
This protocol was described briefly in Guo et al., 2018. This study was supported by grants from the NIH R21NS090205 (to J. Shorter), the G. Harold and Leila Y. Mathers Charitable Foundation (to J. Shorter), Target ALS (to J. Shorter), ALSA (to J. Shorter), and the Packard Center for ALS Research (to J. Shorter). We also acknowledge the Alzheimer's Association (AARF-16-441196) and Target ALS Springboard Fellowship to L.G.
Competing interests
The Authors declare that there is no conflict of interest.
References
- Altmeyer, M., Neelsen, K. J., Teloni, F., Pozdnyakova, I., Pellegrino, S., Grofte, M., Rask, M. B., Streicher, W., Jungmichel, S., Nielsen, M. L. and Lukas, J. (2015). Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun 6: 8088.
- Burke, K. A., Janke, A. M., Rhine, C. L. and Fawzi, N. L. (2015). Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol Cell 60(2): 231-241.
- Da Cruz, S. and Cleveland, D. W. (2011). Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr Opin Neurobiol 21(6): 904-919.
- Guo, L., Kim, H. J., Wang, H., Monaghan, J., Freyermuth, F., Sung, J. C., O'Donovan, K., Fare, C. M., Diaz, Z., Singh, N., Zhang, Z. C., Coughlin, M., Sweeny, E. A., DeSantis, M. E., Jackrel, M. E., Rodell, C. B., Burdick, J. A., King, O. D., Gitler, A. D., Lagier-Tourenne, C., Pandey, U. B., Chook, Y. M., Taylor, J. P. and Shorter, J. (2018). Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell 173(3): 677-692 e620.
- Hofweber, M., Hutten, S., Bourgeois, B., Spreitzer, E., Niedner-Boblenz, A., Schifferer, M., Ruepp, M. D., Simons, M., Niessing, D., Madl, T. and Dormann, D. (2018). Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 173(3): 706-719 e713.
- King, O. D., Gitler, A. D. and Shorter, J. (2012). The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res 1462: 61-80.
- Lin, Y., Protter, D. S., Rosen, M. K. and Parker, R. (2015). Formation and maturation of Phase-separated liquid Droplets by RNA-Binding Proteins. Mol Cell 60(2): 208-219.
- Mackenzie, I. R., Rademakers, R. and Neumann, M. (2010). TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9(10): 995-1007.
- Molliex, A., Temirov, J., Lee, J., Coughlin, M., Kanagaraj, A. P., Kim, H. J., Mittag, T. and Taylor, J. P. (2015). Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163(1): 123-133.
- Murakami, T., Qamar, S., Lin, J. Q., Schierle, G. S., Rees, E., Miyashita, A., Costa, A. R., Dodd, R. B., Chan, F. T., Michel, C. H., Kronenberg-Versteeg, D., Li, Y., Yang, S. P., Wakutani, Y., Meadows, W., Ferry, R. R., Dong, L., Tartaglia, G. G., Favrin, G., Lin, W. L., Dickson, D. W., Zhen, M., Ron, D., Schmitt-Ulms, G., Fraser, P. E., Shneider, N. A., Holt, C., Vendruscolo, M., Kaminski, C. F. and St George-Hyslop, P. (2015). ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88(4): 678-690.
- Patel, A., Lee, H. O., Jawerth, L., Maharana, S., Jahnel, M., Hein, M. Y., Stoynov, S., Mahamid, J., Saha, S., Franzmann, T. M., Pozniakovski, A., Poser, I., Maghelli, N., Royer, L. A., Weigert, M., Myers, E. W., Grill, S., Drechsel, D., Hyman, A. A. and Alberti, S. (2015). A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162(5): 1066-1077.
- Yoshizawa, T., Ali, R., Jiou, J., Fung, H. Y. J., Burke, K. A., Kim, S. J., Lin, Y., Peeples, W. B., Saltzberg, D., Soniat, M., Baumhardt, J. M., Oldenbourg, R., Sali, A., Fawzi, N. L., Rosen, M. K. and Chook, Y. M. (2018). Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. Cell 173(3): 693-705 e622.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Robinson, E., Shorter, J. and Guo, L. (2020). Karyopherin-β2 Inhibits and Reverses Aggregation and Liquid-liquid Phase Separation of the ALS/FTD-Associated Protein FUS. Bio-protocol 10(16): e3725. DOI: 10.21769/BioProtoc.3725.
Category
Neuroscience > Nervous system disorders > Neurodegeneration
Biochemistry > Protein > Self-assembly
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










