- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Cultivation and Visualization of Malaria Liver Stages in Primary Simian Hepatocytes
Published: Vol 10, Iss 16, Aug 20, 2020 DOI: 10.21769/BioProtoc.3722 Views: 5066
Reviewed by: Alexandros AlexandratosLaurent DembeleRAMESH KUDIRA

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantifying Intracellular Distributions of HaloTag-Labeled Proteins With SDS-PAGE and Epifluorescence Microscopy
Julia Shangguan and Ronald S. Rock
Jul 20, 2025 2501 Views

Fluorescence Lifetime-Based Separation of FAST-Labeled Cellular Compartment
Aidar R. Gilvanov [...] Yulia A. Bogdanova
Oct 5, 2025 1323 Views

Generation of Intestinal Epithelial Monolayers From Single-Cell Dissociated Organoids
Neta Felsenthal and Danijela Matic Vignjevic
Oct 5, 2025 2300 Views
Abstract
Human liver is the primary and obligatory site for malaria infection where sporozoites invade host hepatocytes. Malaria hepatic stages are asymptomatic and represent an attractive target for development of anti-malarial interventions and vaccines. However, owing to lack of robust and reproducible in vitro culture system, it is difficult to target and study this imperative malaria liver stage. Here, we describe a procedure that allow cultivation and visualization of malaria hepatic stages including dormant hypnozoites using primary simian hepatocytes. This method enables sensitive and quantitative assessment of different hepatic stages in vitro.
Background
Malaria is transmitted to humans after bite of female Anopheles mosquito injecting sporozoites into the bloodstream, which migrate to the liver and invade host hepatocytes. Inside hepatocytes, sporozoites undergo first round of asexual multiplication and transformed into multinucleated hepatic schizonts. Fully matured hepatic schizonts burst and release merozoites that enter bloodstream and infect red blood cells (RBCs). Inside the RBCs, the parasites undergo second round of asexual multiplication and completion of blood stage cycle eventually elicit the clinical symptoms associated with malaria. Exceptionally, of all the Plasmodium species, sporozoites of P. vivax, P. cynomolgi and P. ovale can produce dormant hepatic forms known as hypnozoites (Prudêncio et al., 2011).
P. vivax is the second major malaria parasite with a broader geographical distribution of all the malaria species including tropics, sub-tropics and temperate climate. The biggest challenge to elimination of P. vivax malaria is the periodic malaria relapse caused by activation of dormant hypnozoites that initiate onset of liver stage proliferation (Wells et al., 2010). Due to lack of robust in vitro culture system, we have little understanding of the mechanism regulating dormancy and hypnozoite activation. As a result, there are no rationale target-based drug discovery approaches to develop therapeutics to treat and prevent relapsing P. vivax malaria. The P. cynomolgi simian malaria parasite, genetically related to P. vivax, is an excellent model that has been previously employed to study hypnozoite biology (Voorberg-van der Wel et al., 2017 and 2020) as well as used for discovery of liver stage active compounds (Zeeman et al., 2014) and novel anti-relapse compounds (Campo et al., 2015). Currently, primaquine and tafenoquine are the only two commercially approved drugs that can eliminate hepatic hypnozoites and consequently prevent relapses of P. vivax malaria. However, both drugs have major limitations, as they are contraindicated in pregnancy, lactating mothers, and patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency (Baird et al., 2019). In addition, primaquine requires CYP2D6 bio-activation in the liver, and thus cannot be administered to poor CYP2D6 metabolizers (Baird et al., 2018). Hence, novel radical cure anti-malarial drugs with improved safety profile are critically needed to enable malaria elimination.
Due to the inherent difficulties associated with studying relapsing parasites there is a dearth of stage specific protein markers. Transcriptomics (Voorberg-van der Wel et al., 2017) studies with relapsing malaria parasites yielded some differential markers of schizont development and hypnozoite maintenance. Ferredoxin, an apicoplast electron transport chain protein and GAP45 (Glideosome Associated Protein) an inner membrane complex (IMC) protein expressed differentially in liver stages. Antibodies generated against these proteins revealed a contrasting staining pattern in P. cynomolgi , while Ferredoxin is expressed in schizonts, GAP45 is expressed in hypnozoites and early developing liver stages (Voorberg-van der Wel et al., 2017). However, these findings have not been confirmed in P. vivax. Intracellular liver stage parasites reside in a membranous compartment known as parasitophorous vacuole (PV) that separates parasite from host hepatocyte cytoplasm (Plattner et al., 2008). UIS4 (upregulated in infectious sporozoite 4) and EXP1 (exported protein-1) are PVM (parasitophorous vacuole membrane) proteins expressed in both hypnozoites and schizonts in P. cynomolgi (Bertschi et al., 2018). UIS4, HSP70 (heat shock protein) and H3K9ac, a transcriptional activity marker (Gupta et al., 2016) are other known protein markers expressed across all liver developmental stages in both P. vivax (Mikolajczak et al., 2015) and P. cynomolgi (Gupta et al., 2019). None of the above markers distinguishes dormant hypnozoites from developing hepatic stages.
Recently, we demonstrated that LISP2 (Liver specific protein 2) expression coincides with liver stage development and serves as a molecular marker to differentiate hypnozoites from earliest developing hepatic stages or schizonts in relapsing malaria parasites, in vitro (P. cynomolgi ) and in vivo (P. vivax) . We further demonstrated that LISP2 could be used as an early marker suitable for the development of the drug discovery assays predictive of anti-relapse activity (Gupta et al., 2019). Here, we describe the detailed protocol to cultivate and visualize P. cynomolgi malaria liver stages in simian primary hepatocytes including hypnozoites.
Materials and Reagents
- Collagen I coated plates 96-well plate (Greiner Bio-One, catalog number: 655956 )
- Needles and syringes for dissection (Fisher Scientific, catalog number: 08-965B )
- Autoclaved glass slides (VWR, catalog number: 16004-422 )
- 2 ml serological pipette (Fisher Scientific, catalog number: 170354N )
- 50 ml Falcon tube (Corning, catalog number: 14-432-22 )
- Disposable hemocytometer (iNCYTO, catalog number: DHC-N01-5 )
- Adhesive plate seals (Beckman Coulter, catalog number: 538619 )
- Nylon filter, 40 µm mesh size, sterile (BD, Falcon, catalog number: 352340 )
- Filtration unit, 500 ml (Corning, catalog number: 431097 )
- Biocoat matrigel matrix 5 ml (Corning, catalog number: 356234 )
- 10 ml syringes (Fisher Scientific, catalog number: 201635 )
- Trypan blue (Sigma, catalog number: T8154-20ML )
- Primary simian hepatocytes (Cynomolgus monkey hepatocytes (BioIVT) (https://bioivt.com/)
- William’s E medium (Life Technologies, catalog number: 12551-032 )
- Plating medium (Life Technologies, catalog number: CM3000 )
- Recovery medium (Life Technologies, catalog number: CM7000 )
- Fetal calf serum clone II (Hyclone, catalog number: SH30066.03 )
- Hydrocortisone (Sigma, catalog number: H0396-100 mg )
- Penicillin/Streptomycin/L-glutamine (Life Technologies, catalog number: 10378016 )
- Human insulin (Sigma, catalog number: I3536-100 mg )
- Sterile reagent reservoirs (Corning, catalog number: 29442-476 )
- Amphotericin B (Amp B) , 250 µg/ml stock in sterile water (Sigma, catalog number: A2942 )
- FisherbrandTM Easy ReaderTM Conical Polypropylene centrifuge tube 15 ml (Fisher Scientific, catalog number: 05-539-12 )
- FisherbrandTM Easy ReaderTM Conical Polypropylene centrifuge tube 50 ml (Fisher Scientific, catalog number: 05-539-13 )
- Methanol (Sigma, catalog number: 34860-1L-R )
- 1x PBS (Life Technologies, catalog number: 10010-023 )
- DAPI (Sigma, catalog number: D9542-5MG )
- Anti-P. cynomolgi HSP70 (PcyM_0515400) polyclonal antibody (custom synthesized by Genscript)
- Anti-P. cynomolgi LISP2 (PcyM_0307500) monoclonal antibody (custom synthesized by Genscript)
- Alexa 488 conjugated goat anti-rabbit immunoglobulin (Invitrogen, catalog number: 11034 )
- Alexa 594 conjugated goat anti-mouse immunoglobulin (Invitrogen, catalog number: 11032 )
- Rabbit anti-H3K9ac antibody (Abcam, catalog number: ab177177 )
- Anopheles dirus clipped wing mosquitoes infected with P. cynomolgi (Armed forces research institute of medical sciences , AFRIMS Bangkok)
- Leibovitz L-15 media (Gibco, catalog number: 11415049 )
- Leibovitz L-15 media, 4 x 50 ml, supplemented with 2.5 µg/ml Amp B.
- Leibovitz L-15 media, 4 x 50 ml, supplemented with 2x P/S (Penicillin/Streptomycin)
- 70% (v/v) ethanol in water
- Liquid nitrogen
- William’s E maintenance media (Life Technologies, catalog number: 12551-032 ), 50 ml, supplemented with 0.5 µg/ml Amp B
- Phenol Red (Life Technologies, catalog number: A1217601 )
- Plating medium (filter-sterilize, shelf life of 1 month) (see Recipes)
- William’s E maintenance medium (filter-sterilize, shelf life of 1 month, store at 4 °C) (see Recipes)
Equipment
- Fine tip forceps (Fisher Scientific, catalog number: 12-000-122 )
- Multichannel pipettes (Thermo Fisher, catalog number: 4661050N )
- Water bath at 37 °C
- Refrigerated centrifuge with swing bucket rotor and buckets with caps (Thermo Scientific, model: Megafuge 40R )
- Nucleocounter (ChemoMetec, model: NC-100 )
- Potter-Elvehjem Tissue Homogenizer, 30 ml
- Incubator set at 37 °C, 5% CO2
- Fluorescence microscope
We used Leica DMI6000B inverted fluorescence microscope equipped with a DFC365FX camera using a HC PL APO 63x/1.40-0.60 oil objective. - Refrigerated bench top centrifuge with fixed angle rotor for Eppendorf tubes (Eppendorf, model: 5415R ), pre-cooled to 4 °C
- Ice box with dry ice
- Biosafety cabinets (BSC)
Software
- For image acquisition: Leica Application Suite X (LAS X) (Leica microsystems) www.leica-microsystems.com
- For microscope image processing: Fiji (Schindelin et al., 2012; Schneider et al., 2012)
https://imagej.net/Fiji
Procedure
- Seeding of simian primary hepatocytes
- Pre-incubate the collagen coated 96-well plates in the 37 °C incubator until ready for use. See Figure 1 for summary and workflow of the protocol.
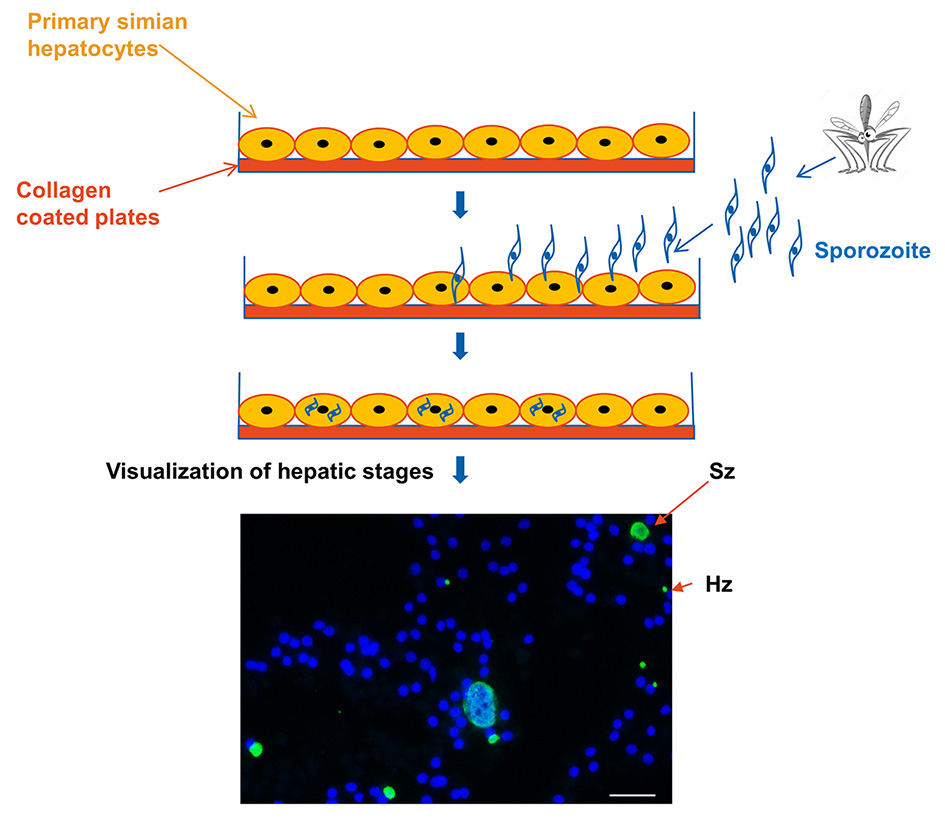
Figure 1. Summary of the workflow for in vitro cultivation and visualization of malaria liver stages. Briefly, primary simian hepatocytes plated on pre-collagen coated plates. Post 24 h, freshly isolated sporozoites from A. dirus mosquitoes incubated with primary hepatocytes. After 3 h of incubation/infection, hepatocytes are washed to remove remaining sporozoites. Sporozoites that invaded hepatocytes and developed into hepatic stages over the period of 3-7 days post infection are visualized with immunofluorescence assay. Merged IFA image exhibiting P. cynomolgi liver stage parasites at day 6 post infection visualized with DAPI for DNA content (blue) and a polyclonal antibody specific for P. cynomolgi HSP70 protein (green). Scale bar, 35 µm. Hz refers to single- nucleus containing hypnozoite and Sz refers to multi-nucleated Schizont. - Pre-incubate the recovery medium (25 ml per vial of hepatocytes) and plating medium (13 ml per vial of hepatocytes) in the 37 °C water bath for at least 15 min.
- Take out cryovials of hepatocytes from the liquid nitrogen tank, place into an ice box containing dry ice for transport to the laboratory.
- Remove the cryovials from the dry ice and quickly open the cap halfway to completely release liquid nitrogen trapped within. Close back the cap.
- Thaw cells in the 37 °C water bath and shake continuously until a small piece of ice remains in the vial.
- With a 2 ml serological pipette, carefully transfer the cells to a 50 ml Falcon tube in Biosafety cabinet (BSC).
- Drop by drop, with a rate of 1 ml every 10 s, gently add 25 ml warm recovery medium to the cells.
- Invert the tube once to gently mix the cells with the media.
- Spin down at 100 x g (677 rpm), 28 °C for 4 min (Acceleration = 9, Brake = 9).
- Carefully aspirate the supernatant with a serological pipette.
- With a serological pipette, gently re-suspend the cell pellet with sufficient warm plating medium.
- Count the cells with Trypan blue and a Neubauer improved hemocytometer chip.
- Adjust hepatocyte suspension to 1 x 106 cells/ml with additional pre-warmed plating medium, if needed.
- Seed 83 µl per well, equivalent to 83,000 hepatocytes per well in a 96-well plate.
- Incubate the plates at 37 °C, 5% CO2 overnight. Restrict access to the incubator.
- Next day, wash (one time) and replace old spent media with 100 µl pre-warmed maintenance media.
- Observe under a light microscope to determine the well coverage.
- Incubate plates at 37 °C, 5% CO2 until needed for infection.
- Pre-incubate the collagen coated 96-well plates in the 37 °C incubator until ready for use. See Figure 1 for summary and workflow of the protocol.
- Isolation of P. cynomolgi sporozoites from Anopheles dirus mosquitoes
- A. dirus mosquitoes fed on blood from the P. cynomolgi (strain B) infected monkey were obtained from AFRIMS, Thailand. Once the mosquitoes have been deliver to the lab, proceed immediately to dissection and isolation of salivary glands (Coleman et al., 2007; Dembélé et al., 2014).
- Keep the isolated salivary glands on ice.
- Transfer the entire salivary gland preparation into the Teflon homogenizer, placed on ice.
- Homogenize the salivary gland suspension with an up and down twisting motion for at least 10 strokes.
- Filter the homogenized sporozoites through a 40 µm nylon filter on top of a pre-chilled 50 ml Falcon tube.
- Wash remainder of sporozoites in the Teflon homogenizer with 1 ml of cold maintenance medium with amphotericin B (Amp B).
- Pour through the 40 µm filter into the Falcon tube as before.
- Spin at 16,128 x g for 3 min, 4 °C.
- Aspirate the supernatant and re-suspend the pellet with maintenance medium.
- Aliquot 10 µl of the 1:10 and 1:100 diluted homogenized suspension into a disposable hemocytometer and determine sporozoite count and viability with a light microscope at 40x magnification. Keep the remainder on ice.
- Adjust the sporozoite (spz) suspension to 1 x 106 sporozoite /ml with maintenance medium.
- Infection of simian primary hepatocytes with isolated P. cynomolgi sporozoites
- Aspirate spent medium from the wells and add 60 µl per well of the 1 x 106 spz/ml suspension in the plate.
- Add 60 µl per well of William’s E maintenance medium for uninfected control wells.
- Centrifuge the plate at 448 x g for 10 min at 4 °C (Acceleration = 9, Brake = 1).
Note: Spin down at low brake to settle the sporozoites onto the cells. - Cautiously remove the plates from the centrifuge and incubate at 37 °C, 5% CO2 for three hours before the first medium replacement. Limit access to the incubator.
- After three hours of incubation, cautiously remove medium from the wells with a multichannel pipette and add 150 µl of fresh medium (maintenance medium + Amp B) into every well.
Alternative step: Addition of Matrigel overlay to increase the longevity of primary hepatocytes (Dembélé et al., 2014).- Thaw the Matrigel on ice.
Note: Keep matrigel on ice to prevent polymerization. - After three hours of incubation with sporozoites, wash hepatocytes three times with maintenance medium (100 µl per wash).
- Gently add Matrigel (60 µl per well) over the cells so that entire well is covered with Matrigel
- Keep the Matrigel plates for 15 min at 37 °C.
- Add 150 µl of fresh medium (maintenance medium + Amp B) over Matrigel wells.
- Thaw the Matrigel on ice.
- Incubate plates at 37 °C, 5% CO2 and change medium after every 48 h with 150 µl maintenance medium + Amp B.
- Immunofluorescence assay for visualization of infected liver stages
- Remove the spent medium from the plates and wash 2 times with 1x PBS (150 µl/well each time).
- Remove PBS and fix cells with 100 µl ice cold methanol at room temperature for 3-5 min.
- Remove the methanol and wash 3 times with 1x PBS. Add 100 µl of PBS in each well and store at 4 °C until needed or proceed with IFA staining (Figure 2).
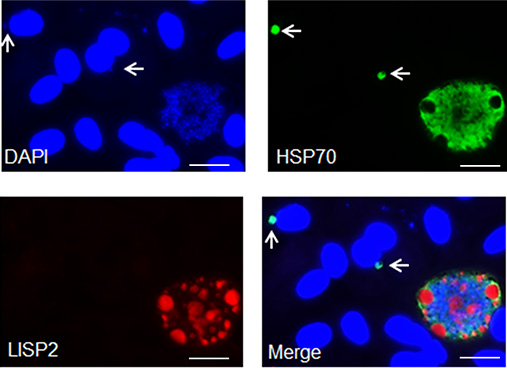
Figure 2. Representative images of P. cynomolgi liver stage parasites in primary hepatocytes after seven days post sporozoite infection visualized with DAPI (blue), anti-P. cynomolgi HSP70 protein antibody (green) and anti-P. cynomolgi LISP2 antibody (red). Scale bars, 35 µm. Arrow shows immunostaining of hypnozoites. - Prepare the primary antibody in 1x PBS: Anti-P. cynomolgi HSP70 rabbit antibody diluted 1:2,000 in 1x PBS and anti-P. cynomolgi LISP2 mouse antibody diluted 1: 500 in 1x PBS.
- Incubate fixed cells with primary antibody (50 µl per well) for one hours at 37 °C.
Note: Avoid incubation for longer duration at 37 °C as it results in higher fluorescence background. Alternatively, incubate at 4 degree for longer incubation if required. - Aspirate and wash three times with 1x PBS (100 µl per wash).
- Prepare secondary antibody dilutions in the same tube as follows: Alexa 488-conjugated goat anti-rabbit immunoglobulin (1:5,000) and Alexa 594-conjugated goat anti-mouse immunoglobulin (1:5,000) and supplemented with 1 μg per ml of DAPI.
- Add 50 µl per well of secondary antibody dilutions and incubate the fixed culture plates for one-two hours at 37 °C.
- Aspirate and wash three times with 1x PBS.
- Visualize malaria liver stages (hypnozoite and schizont) using a fluorescent microscope with 40x, 60x or 100x magnification.
- This staining assay could be used to visualize malaria liver stages from day 1 post infection to days 15-21 depending on the viability of hepatocytes under in vitro conditions (Figure 3).
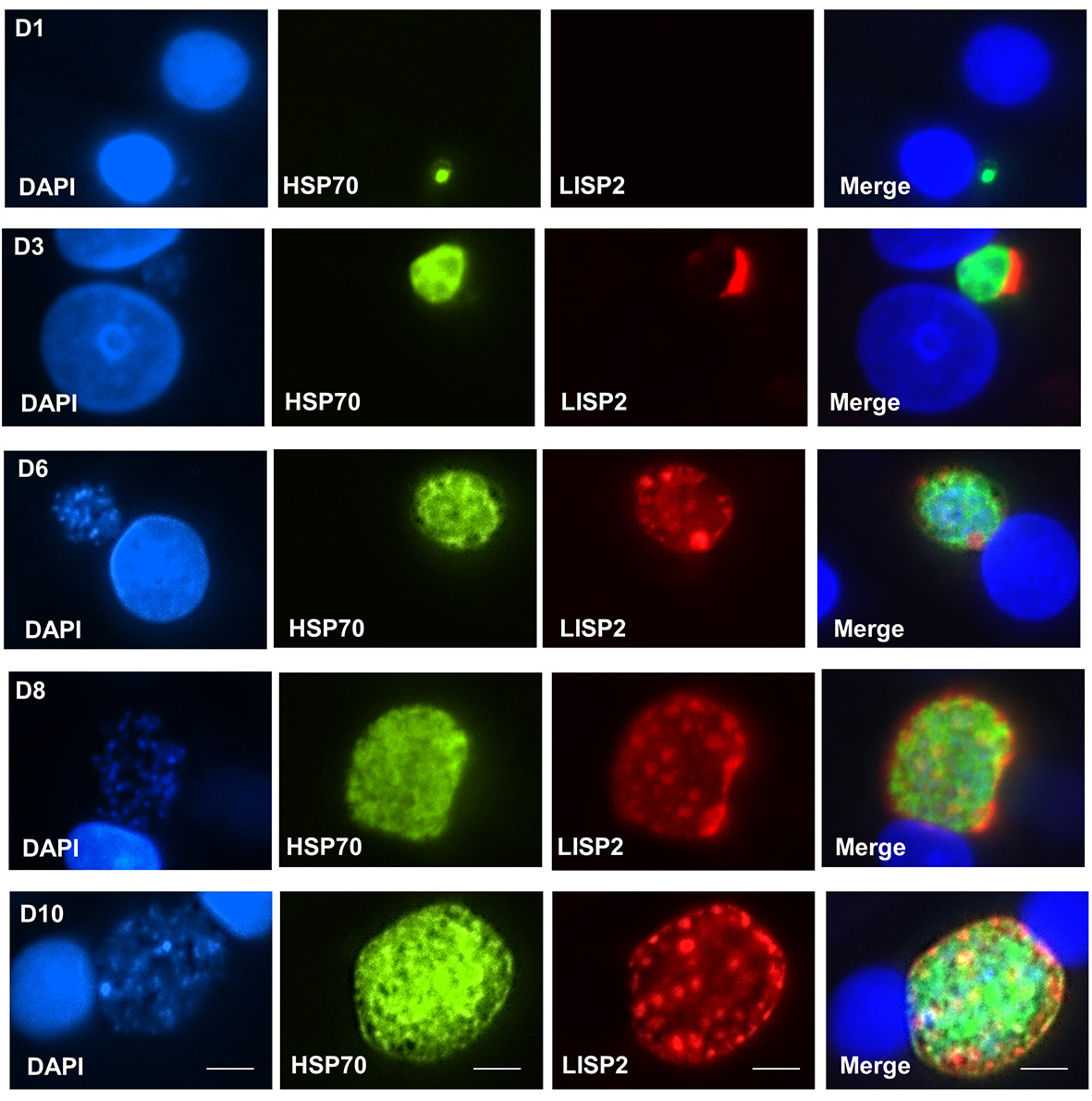
Figure 3. Time course kinetics of liver stage development. Cultivated simian hepatocytes infected with P. cynomolgi sporozoites fixed and analyzed in IFA assay with anti-LISP2 (red) and anti-HSP70 (green) antibodies to monitor liver stage development from day 1 to day 10 post sporozoite infection. LISP2 expression first detected at day 3 post infection in a crescent shape located on one side of earliest developing parasites. For subsequent time points, LISP2 accumulates in peripheral vacuolar membrane structures in multinucleate schizonts. The heat shock protein (HSP70) is expressed and detected during all the hepatic stages throughout all the time points. The Plasmodium HSP70 antibody used in this manuscript is specific for malaria parasites and does not cross-react with human and primate hepatocytes. Scale bars, 35 µm.
Recipes
- Plating medium (filter-sterilize, shelf life of 1 month)
- William’s E medium without serum and Phenol Red (Life Technologies)
- Thawing/Plating Supplement Pack (Life Technologies)
- Mix both components together, filter-sterilize and store at 4 °C
- William’s E maintenance medium (filter-sterilize, shelf life of 1 month, store at 4 °C)
Component Stock conc. To be added Final concentration William’s E medium N.A 500 ml N.A Fetal calf serum clone II (non-heat inactivated) 100% 50 ml 10% Hydrocortisone in sterile water 50 mM 0.5 ml 50 µM Penicillin/Streptomycin/L-glutamine 100x (10,000 units/ml Pen; 10,000 µg/ml Strep; 200 mM L-glutamine) 5 ml 2x (100 units/ml Pen; 100 µg/ml Strep; 2 mM L-glutamine) Human insulin in sterile water 5 mg/ml 0.5 ml 5 µg/ml
Acknowledgments
The authors would like to thank Bill and Melinda Gates foundation (OPP1141292 and OPP1137694) for their generous support. This protocol was adapted and elaborated from our work previously published (Gupta et al., 2019).
Competing interests
All authors are employed by Novartis Pharma AG.
Ethics
For this study, we have used non-human primates for the source of primary hepatocytes. Novartis Laboratory of Large Animal Services, New Jersey, U.S.A., (Novartis-LAS), SingHealth, Singapore, and The Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand (AFRIMS) had approved the research protocol (Ref No.: 2015/SHS/1024). For details, see the published article (Gupta et al., 2019).
References
- Baird, J. K. (2019). 8-Aminoquinoline Therapy for Latent Malaria. Clin Microbiol Rev 32(4): e00011-00019.
- Baird, J. K., Louisa, M., Noviyanti, R., Ekawati, L., Elyazar, I., Subekti, D., Chand, K., Gayatri, A., Instiaty, Soebianto, S., Crenna-Darusallam, C., Djoko, D., Hasto, B. D., Meriyenes, D., Wesche, D., Nelwan, E. J., Sutanto, I., Sudoyo, H. and Setiabudy, R. (2018). Association of Impaired Cytochrome P450 2D6 Activity Genotype and Phenotype With Therapeutic Efficacy of Primaquine Treatment for Latent Plasmodium vivax Malaria. JAMA Netw Open 1(4): e181449.
- Bertschi, N. L., Voorberg-van der Wel, A., Zeeman, A. M., Schuierer, S., Nigsch, F., Carbone, W., Knehr, J., Gupta, D. K., Hofman, S. O., van der Werff, N., Nieuwenhuis, I., Klooster, E., Faber, B. W., Flannery, E. L., Mikolajczak, S. A., Chuenchob, V., Shrestha, B., Beibel, M., Bouwmeester, T., Kangwanrangsan, N., Sattabongkot, J., Diagana, T. T., Kocken, C. H. and Roma, G. (2018). Transcriptomic analysis reveals reduced transcriptional activity in the malaria parasite Plasmodium cynomolgi during progression into dormancy. Elife 7: e41081.
- Campo, B., Vandal, O., Wesche, D. L. and Burrows, J. N. (2015). Killing the hypnozoite--drug discovery approaches to prevent relapse in Plasmodium vivax. Pathog Glob Health 109(3): 107-122.
- Coleman, J., Juhn, J. and James, A. A. (2007). Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J Vis Exp(5): 228.
- Dembélé, L., Franetich, J. F., Lorthiois, A., Gego, A., Zeeman, A. M., Kocken, C. H., Le Grand, R., Dereuddre-Bosquet, N., van Gemert, G. J., Sauerwein, R., Vaillant, J. C., Hannoun, L., Fuchter, M. J., Diagana, T. T., Malmquist, N. A., Scherf, A., Snounou, G. and Mazier, D. (2014). Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20(3): 307-312.
- Gupta, D. K., Dembele, L., Voorberg-van der Wel, A., Roma, G., Yip, A., Chuenchob, V., Kangwanrangsan, N., Ishino, T., Vaughan, A. M., Kappe, S. H., Flannery, E. L., Sattabongkot, J., Mikolajczak, S., Bifani, P., Kocken, C. H. and Diagana, T. T. (2019). The Plasmodium liver-specific protein 2 (LISP2) is an early marker of liver stage development. Elife 8: 43362.
- Gupta, D. K., Patra, A. T., Zhu, L., Gupta, A. P. and Bozdech, Z. (2016). DNA damage regulation and its role in drug-related phenotypes in the malaria parasites. Sci Rep 6: 23603.
- Mikolajczak, S. A., Vaughan, A. M., Kangwanrangsan, N., Roobsoong, W., Fishbaugher, M., Yimamnuaychok, N., Rezakhani, N., Lakshmanan, V., Singh, N., Kaushansky, A., Camargo, N., Baldwin, M., Lindner, S. E., Adams, J. H., Sattabongkot, J. and Kappe, S. H. (2015). Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17(4): 526-535.
- Plattner, F. and Soldati-Favre, D. (2008). Hijacking of host cellular functions by the Apicomplexa. Annu Rev Microbiol 62: 471-487.
- Prudêncio, M., Mota, M. M. and Mendes, A. M. (2011). A toolbox to study liver stage malaria. Trends Parasitol 27(12): 565-574.
- Voorberg-van der Wel, A., Roma, G., Gupta, D. K., Schuierer, S., Nigsch, F., Carbone, W., Zeeman, A. M., Lee, B. H., Hofman, S. O., Faber, B. W., Knehr, J., Pasini, E., Kinzel, B., Bifani, P., Bonamy, G. M. C., Bouwmeester, T., Kocken, C. H. M. and Diagana, T. T. (2017). A comparative transcriptomic analysis of replicating and dormant liver stages of the relapsing malaria parasite Plasmodium cynomolgi. Elife 6: 29605.
- Voorberg-van der Wel, A., Zeeman, A. M., I. G. Nieuwenhuis., N. M. Vanderwerff., E. J. Klooster., O. Klop., L. C. Vermaat., Gupta, D. K., L. Dembele., Diagana, T. T. and Kocken, C. H. M. (2020). A dual fluorescent Plasmodium cynomolgi reporter line reveals in vitro malaria hypnozoite activation. Commun Biol 3(7). DOI: https://doi.org/10.1038/s42003-019-0737-3.
- Wells, T. N., Burrows, J. N. and Baird, J. K. (2010). Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol 26(3): 145-151.
- Zeeman, A. M., van Amsterdam, S. M., McNamara, C. W., Voorberg-van der Wel, A., Klooster, E. J., van den Berg, A., Remarque, E. J., Plouffe, D. M., van Gemert, G. J., Luty, A., Sauerwein, R., Gagaring, K., Borboa, R., Chen, Z., Kuhen, K., Glynne, R. J., Chatterjee, A. K., Nagle, A., Roland, J., Winzeler, E. A., Leroy, D., Campo, B., Diagana, T. T., Yeung, B. K., Thomas, A. W. and Kocken, C. H. (2014). KAI407, a potent non-8-aminoquinoline compound that kills Plasmodium cynomolgi early dormant liver stage parasites in vitro. Antimicrob Agents Chemother 58(3): 1586-1595.
Article Information
Copyright
Gupta and Diagana. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gupta, D. K. and Diagana, T. (2020). In vitro Cultivation and Visualization of Malaria Liver Stages in Primary Simian Hepatocytes. Bio-protocol 10(16): e3722. DOI: 10.21769/BioProtoc.3722.
- Gupta, D. K., Dembele, L., Voorberg-van der Wel, A., Roma, G., Yip, A., Chuenchob, V., Kangwanrangsan, N., Ishino, T., Vaughan, A. M., Kappe, S. H., Flannery, E. L., Sattabongkot, J., Mikolajczak, S., Bifani, P., Kocken, C. H. and Diagana, T. T. (2019). The Plasmodium liver-specific protein 2 (LISP2) is an early marker of liver stage development. Elife 8: 43362.
Category
Microbiology > Microbial cell biology > Cell-based analysis
Cell Biology > Cell imaging > Fluorescence
Cell Biology > Cell isolation and culture > Monolayer culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








