- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Intestinal Enteroid Culture for Human Astroviruses
(*contributed equally to this work) Published: Vol 10, Iss 14, Jul 20, 2020 DOI: 10.21769/BioProtoc.3687 Views: 4766
Reviewed by: David PaulGundeep KaurAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2457 Views

Flow Cytometric Quantification of HIV-1-Infected Cells Expressing Either Abortive or Elongated HIV-1 Transcripts Using Flow-FISH
Shirley Man [...] Neeltje A. Kootstra
Jul 20, 2025 2156 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3033 Views
Abstract
Human astroviruses (HAstV) are non-enveloped, positive-sense single stranded RNA viruses that typically cause gastroenteritis in children, the elderly and among immunocompromised individuals. Some HAstV species have also been implicated in neurological diseases. It is important to study these viruses to understand the pathogenesis and develop therapeutics. Here we describe HAstV infection in epithelium-only human intestinal enteroids (HIE) isolated from biopsy-derived intestinal crypts. Although different HAstV clades have been propagated in transformed immortalized cell lines such as A549, Caco-2, HEK293T and Huh7.5, we chose HIE because they better mimic the human intestine and thus are more physiologically relevant. Additionally, HIE support the replication of all HAstV clades including clinical samples, thus making HIE a valuable potential universal model to study HAstV biology.
Keywords: AstrovirusBackground
Human astroviruses (HAstV) are enteric viruses that are highly prevalent, causing widespread infections ranging from diarrhea to encephalitis. Asymptomatic, systemic and extra-intestinal infections are common (Madeley and Cosgrove 1975; Bosch et al., 2014; Kolawole et al., 2019). Despite their medical importance, HAstV are some of the least characterized enteric viruses. Currently, three groups of human AstV are recognized: classic HAstV, and non-classic HAstV-MLB (Melbourne), and HAstV-VA/HMO (Virginia/Human-Mink-Ovine-like) with each group containing multiple strains. Several immortalized cell lines have been developed for successful propagation of multiple HAstV strains. Human colon carcinoma Caco-2 cells are commonly used to propagate all eight serotypes of classic HAstV. As for the non-classic HAstV, Caco-2 cells, human embryonic kidney HEK293T, adeno-carcinomic human lung epithelial A549, and primary astrocytes were only recently described to support VA1 propagation (Janowski et al., 2017 and 2019). In addition, A549 and human liver Huh7 were developed to propagate MLB1 and MLB2 (Vu et al., 2019). However, no single culture system supports the replication of all tested species. The lack of a robust, physiologically relevant viral propagation system for genetically diverse viruses from all clades has created a fundamental gap in our understanding about the biology of HAstV. For example, little or no information is available on the in vivo cell tropism, pathophysiology, and host responses to HAstV infection.
Human intestinal enteroids (HIE) are nontransformed in vitro models that recapitulate many characteristics of the gastrointestinal tract. HIE are derived from stem cells isolated from human intestinal biopsy tissues or surgical resections. Thus, this system exhibits multiple advantages over traditional transformed intestinal cell lines (e.g., Caco-2 cells). These include the non-transformed status, the presence of multiple cell types, the specific regional characteristics and host genetics of the donor and the ability to differentiate from crypt-like into villus-like cell populations (Kolawole et al., 2019; Kolawole and Wobus, 2020). There are some drawbacks involved with using HIE. Examples include donor-to-donor variability, inability of HIE to completely mimic the cellularity of the intestine in vivo and the major investment needed for culture maintenance. However, HIE have been used to culture human rotavirus, norovirus and astrovirus by breaking apart the 3D spheres in which they are normally organized in culture (Figure 1, left) and seeding as 2D monolayer (Figure 1, right) (Kolawole and Wobus, 2020). Here, we describe our methods of HIE maintenance and AstV infection. Laboratory adapted samples of HAstV1, MLB1 and VA1 represented HAstV clades that were propagated in HIE. HAstV5-positive stool sample was also used to propagate clinical HAstV in HIE. All infections were performed in 2D monolayers. These protocols, with the required specific adjustments, should facilitate the study of HAstV and other enteric viruses.
Materials and Reagents
- 1.7 ml popsi-click centrifuge tubes (Denville, catalog number: C2170 )
- U-bottom 96-well plates (Falcon, catalog number: 353077 )
- 48-well plates (Costar, catalog number: 3548 )
- 24-well plates Nunclon delta surface (Thermo Scientific, catalog number: 142475 )
- 6-well plates (CytoOne, catalog number: CC7682 )
- P1000 pipette tips (Denville, catalog number: 1159M42 )
- P200 pipette tips (Denville, catalog number: 1159M40 )
- P20 pipette tips (Denville, catalog number: 1159M43 )
- 1 ml disposable syringe (BD, catalog number: 309659 )
- 25G x 5/8″ PrecisionGlide needle (BD, catalog number: 305122 )
- Cell strainer, 40 μm nylon mesh (Fisherbrand, catalog number: 22363547 )
- 15 ml conical tubes (Falcon, catalog number: 352096 )
- Sterile 500 ml vacuum filtration systems (0.22 μm, PES membrane; Corning, catalog number: 431097 )
- Sterile 250 ml vacuum filtration systems (0.22 μm, PES membrane; Corning, catalog number: 431096 )
- Sterile 50 ml vacuum filtration systems (0.22 μm; Millipore, catalog number: SCGP00525 )
- Matrigel matrix (Corning, catalog number: 354234 )
- Fetal bovine serum (FBS; HyClone, catalog number: SH30396.03 )
- LWRN cells (ATCC, catalog number: CRL-3276 )
- Advanced DMEM/F-12 (Invitrogen, catalog number: 12634-028 )
- N-2 media supplement (Invitrogen, catalog number: 17502-048 )
- Phosphate buffered saline (PBS-/-; Gibco, catalog number: 10010-023 )
- Dulbecco’s phosphate buffered saline with calcium and magnesium (PBS+/+; Gibco, catalog number: 14040-133 )
- HEPES (1 M; Invitrogen, catalog number: 15630-080 )
- 5000 units/ml penicillin/streptomycin (Invitrogen, catalog number: 15070-063 )
- Geneticin, G418 (Gibco, catalog number: 10131-035 )
- Hygromycin B (Invitrogen, catalog number: 10687010 )
- Puromycin (Sigma-Aldrich, catalog number: P8833 )
- GlutaMax (Invitrogen, catalog number: 17504-044 )
- B-27 supplement minus vitamin A (Invitrogen, catalog number: 17504-044 )
- N-Acetyl-L-cysteine (Sigma-Aldrich, catalog number: A9165-5G )
- Mouse EGF (Invitrogen, catalog number: PMG8043 )
- SB202190 (Sigma-Aldrich, catalog number: S7067 )
- A83-01 (Tocris, catalog number: 2939 )
- Nicotinamide (Sigma-Aldrich, catalog number: N0636 )
- Leu15-Gastrin I (Sigma-Aldrich, catalog number: G9145 )
- Y-27632 (Sigma-Aldrich, catalog number: Y0503 )
- Human collagen IV (Sigma, catalog number: C5533 )
- Porcine trypsin (Sigma-Aldrich, catalog number: T-0303 )
- 0.05% Trypsin-EDTA (Gibco, catalog number: 25300-054 )
- Recovery cell culture freezing medium (Gibco, catalog number: 12648-010 )
- Glacial acetic acid (Thermo Scientific, catalog number: A38-211 )
- UltraPure distilled water (Invitrogen, catalog number: 10977-015 )
- Dimethyl sulfoxide (DMSO; Sigma, catalog number: D2650 )
- Accumax (STEMCELL technologies, catalog number: 0 7921 )
- LIVE/DEAD fixable aqua dead cell stain kit (Thermo Fisher Scientific, catalog number: L34957 )
- Fixation/Permeabilization concentrate (eBioscience, catalog number: 00-5123-43 )
- Biotin-conjugated primary dsRNA (J2; Scicons, catalog number: 10010200 )
- Rabbit anti-ZO1 primary antibody (Thermo Fisher Scientific, catalog number: 61-7300 )
- Mouse anti-VA1 polyclonal serum (primary antibody; made in our lab)
- Secondary anti-rabbit AlexaFluor 594 (Thermo Fisher Scientific, catalog number: A32740 )
- Secondary anti-mouse AlexaFluor 647(Thermo Fisher Scientific, catalog number: A32728 )
- Ulex europeus agglutinin 1 (UEA-1) lectin conjugated with FITC (Thermo Fisher Scientific, catalog number: L32476 )
- 4’,6-Diamidino-2-phenylindole (DAPI; Sigma-Aldrich, catalog number: D9542 )
- ProLong Gold antifade reagent (Thermo Fisher Scientific, catalog number: P36930 )
- Transwell permeable support (Costar, catalog number: 3413 )
- Stock solutions (see Recipes)
500 mM N-acetyl cysteine
50 μg/ml Mouse EGF
500 mM A-83-01
10 mM SB202190
1 M Nicotinamide
10 μM Leu15-Gastrin
10 mM Y-27632 - Media (see Recipes)
Advanced DMEM-20
LWRN conditioned medium
Noggin conditioned medium
LWRN- medium (1%)
LWRN+ (with growth factors)
Differentiation medium
Equipment
- 37 °C incubator (Thermo Scientific Heracell 150i CO2 Incubator)
- 37 °C water bath (Thermo Electron Corporation)
- -80 °C freezer
- Benchtop Centrifuge (Sorval legend RT)
- Hemocytometer (Reichert)
- P1000 pipette (Eppendorf Research)
- P200 pipette (Eppendorf Research)
- P20 pipette (Eppendorf Research)
- Vacuum aspirator
- Biosafety hood (SterilGARD® III Advance)
- Light microscope (Fisher Scientific)
- Cell counter
Procedure
- Reviving enteroids from liquid nitrogen storage
- Thaw Matrigel aliquot overnight at 4 °C.
- Add 10 ml of LWRN- into a 15 ml conical tube and leave on ice.
- Transfer frozen vial containing enteroids from liquid nitrogen under running tap water at room temperature (a frozen vial usually contains about 150-200 enteroids).
- Transfer content into the 15 ml conical tube containing LWRN-.
- Spin down cells in a refrigerated centrifuge at 80 x g for 5 min at 4 °C.
- Aspirate medium and suspend pellet in Matrigel (calculate the required volume of Matrigel based on the total number of wells that will be seeded with enteroids).
- Plate enteroids in 10 µl Matrigel drops in a 24-well plate, four drops per well (seed 3 wells of 4 drops from one cryovial of 500 µl).
- Allow the Matrigel to solidify in a 37 °C incubator for 10 min.
- Add 1 ml of LWRN+ medium to each well containing enteroids suspended in Matrigel.
- Incubate at 37 °C, 5% CO2.
- Replace medium every two days until enteroids are ready to be passaged after 6 to 7 days (Figure 1, left).
- Passaging and maintaining enteroids (procedure for 1-10 wells)
- Preparation: Keep pipette tips in refrigerator for about 30 min before start. Warm up the plates.
- Carefully aspirate medium from wells without disturbing Matrigel.
- Add 500 µl of cold LWRN- to each well and mechanically break up Matrigel by pipetting up and down 3-5 times with a P1000 pipette. Optimize the level of pipetting for each enteroid line.
- Using a 1 ml syringe with a 25G x 5/8″ needle, push broken Matrigel in and out 3-4 times per well, transfer the contents of the well into a 15 ml conical tube (cells will not be damaged by the needle; if there are multiple wells of the same enteroid line, combine up to 10 wells in the same tube).
- Add another 500 µl of cold LWRN- to each well and transfer the content into the 15 ml tube with the syringe. Add at least 5 ml total volume to 15 ml tube.
- Spin down cells in a refrigerated centrifuge at 80 x g at 4 °C for 5 min.
- Carefully remove and discard supernatant and keep enteroid pellet on ice.
- Suspend enteroid pellet in Matrigel on ice. Matrigel must be kept cold to avoid solidification.
- Using cold P20 pipette tips, plate enteroids in 10 µl Matrigel drops in a 24-well plate, four drops per well.
- Transfer plate into a 37 °C incubator and allow the Matrigel to set for 10 min.
- Add 500 µl of room temperature LWRN+ to each well and incubate in a 37 °C, 5% CO2 incubator
- Replace medium every two days until enteroids are ready to be passaged (usually after 6 or 7 days) (Figure 1, left).
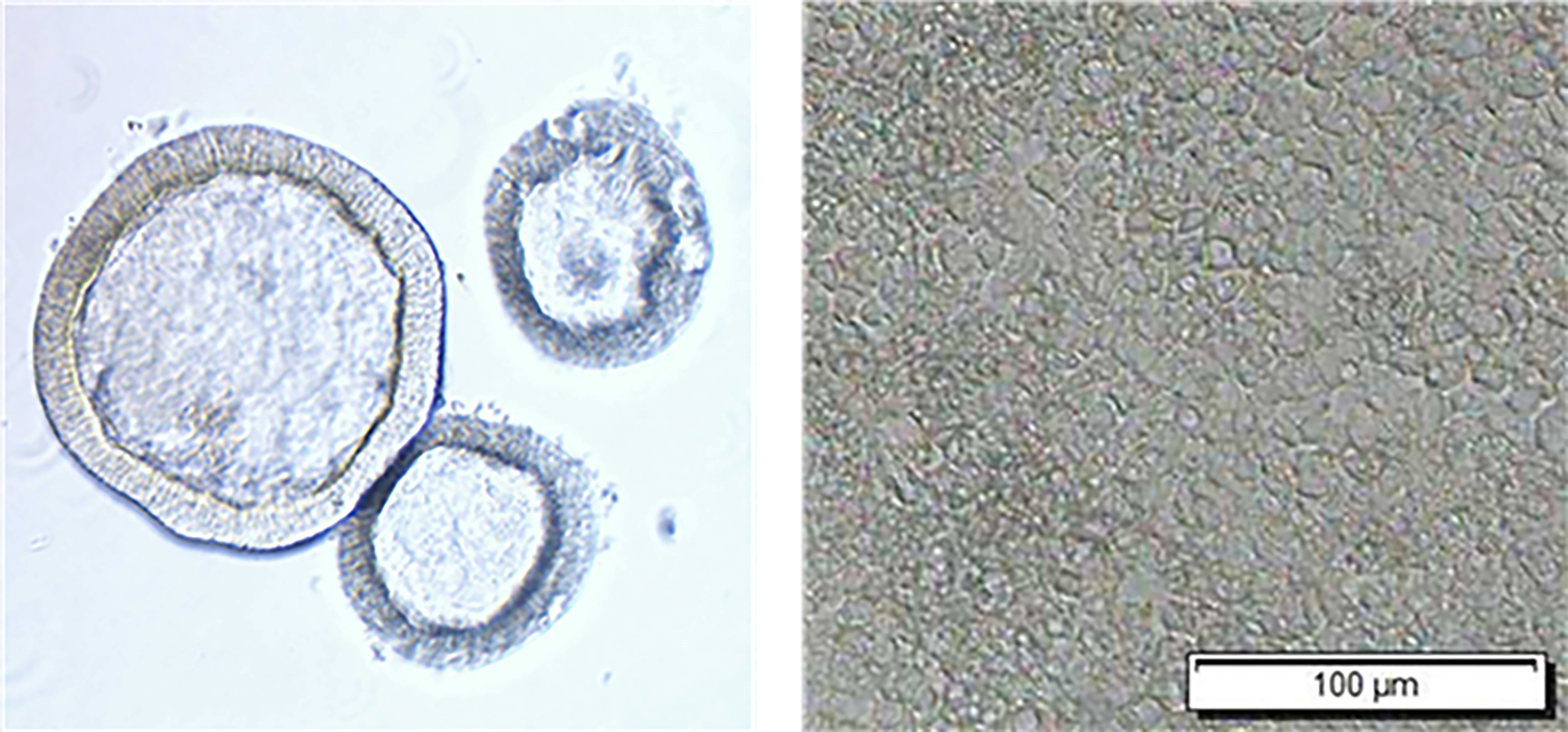
Figure 1. 3D and 2D human intestinal enteroids. Fetal ileum line were kept in LWRN+ culture media for 4 days (left; 3D) or seeded in 48-well plate and kept in differentiation media for 6 days (right; 2D)
- Making and maintaining 2D enteroid monolayers on Transwells and 48-well plates
- Prepare a collagen IV stock solution (1 mg/ml in 100 mM glacial acetic acid).
- Prepare 100 mM acetic acid by adding 60 µl of 100% acetic acid to 10 ml sterile water.
- Suspend 1 mg of collagen IV in 1 ml of 100 mM acetic acid to make collagen IV stock solution and vortex to mix.
- Aliquot in 500 µl and store at -20 °C.
- Prepare 100 mM acetic acid by adding 60 µl of 100% acetic acid to 10 ml sterile water.
- Prepare a 1:30 dilution of collagen IV stock solution in cold sterile water.
- Add 100 µl of the cold diluted collagen IV solution to each Transwell or 200 µl to each well in the 48-well plate and incubate at 37 °C for 2 h for the collagen to solidify.
- Take off the culture medium from well, and break enteroids apart by adding 500 µl/well of cold 0.5 mM EDTA wash solution (made by diluting 10 µl of 500 mM EDTA to 10 ml PBS-/-) and pipetting up and down 5 times.
- Transfer the enteroid solution into a sterile 15 ml tube.
- Add another 500 µl/well of cold 0.5 mM EDTA wash solution to each well and transfer into the 15 ml tube. Add at least 5 ml total volume to 15 ml tube.
- Spin at 300 x g for 5 min at 4 °C to pellet the cells (if pellet is not compact, then re-spin).
- Carefully remove and discard supernatant using a 10 ml pipette. A small amount of residual liquid is okay.
- Re-suspend pellet in 500 µl 0.05% trypsin by pipetting up and down with P1000 (500 µl trypsin for 1-5 wells combined or 1 ml trypsin for 6-10 wells of enteroids).
- Incubate at 37 °C for 4-6 min (optimize the incubation time for each enteroid line).
- Inactivate trypsin by adding 1 ml of LWRN- medium containing 10% FBS.
- Dissociate the enteroids by vigorously pipetting up and down 40-50 times using P1000 pipette (optimize the specific number of times for each enteroid line; avoid forming bubbles by pipetting against the wall of the tube).
- Put a 40 µm cell strainer over a 50 ml conical tube.
- Pre-wet the strainer by passing 1 ml of LWRN- medium supplemented with 10% FBS.
- Add the cells onto the strainer and allow the cells to flow through by gravity.
- Rinse the strainer with additional 1 ml LWRN-.
- Pellet the cell suspension by centrifuging at 400 x g at 4 °C for 5 min.
- Remove and discard supernatant with a 10 ml pipette and carefully remove the remaining liquid with a P200 pipette without disturbing the pellet.
- Suspend the cell pellet in 100-1,000 µl LWRN+ medium containing 10 µM Y-27632. Take a small aliquot of the cells and count with hemocytometer.
- Remove the collagen coating solution from the Transwell or plate.
- Plate by adding 1 x 105 cells dropwise into the middle of the collagen-coated Transwell or plate (100 µl per Transwell or 200 µl per 48-well). The cells will spread out and coat the well so do not rotate or swirl the seeded plate.
- Add 600 µl of LWRN+ medium containing 10 μM Y-27632 in the lower compartment of the Transwell.
- The next day, remove the LWRN+ medium containing 10 μM Y-27632 and replace with LWRN+ medium only.
- Replace medium every two days until enteroids are ready to be infected (usually when cells are about 80-90% confluent).
- If there was a need to infect differentiated cells, add differentiation medium to the upper (100 µl) and lower (600 µl) compartments of Transwells or 200 µl to 48-well plate to differentiate 2D enteroids when the wells are 80-90% confluent.
- Replace differentiation medium every two days until enteroids are ready to be infected (usually after 3-7 days) (Figure 1, right).
- Prepare a collagen IV stock solution (1 mg/ml in 100 mM glacial acetic acid).
- Human astrovirus infection of enteroid 2D monolayer
- Thaw HAstV1, MLB1, VA1 and mock cell lysates plus HAstV5-positive stool filtrate at room temperature (expand HAstV1 and VA1 in Caco-2 cells; expand MLB1 in Huh7.5 cells; freeze and lyse infected cells to yield virus stock; filter HAstV5-psitive stool sample collected in AMES buffer through 0.22 µm; titer virus stock by RT-qPCR to determine the genome copies).
- Dilute MLB1 and VA1 as 105 genome copies per 100 µl of LWRN- medium only while HAstV1 and HAstV5 diluent should contain 5 µg/ml porcine trypsin.
- Incubate HAstV1 and HAstV5 in the trypsin solution above for 1 h at 37 °C, 5% CO2.
- Rinse the enteroid monolayer once with cold LWRN- medium.
- Remove media and gently add 100 µl diluted virus.
- Incubate the infected cells for 1 h at 37 °C, 5% CO2.
- Remove the inoculum and gently wash the monolayer once with LWRN- medium.
- Store a plate containing few wells with infected HIE at -80 °C as 0 h to measure the initial inoculum.
- Add 200 µl (100 µl in Transwell insert) LWRN+ or differentiation medium to the remaining wells (± 2 µg/ml porcine trypsin) and incubate at 37 °C, 5% CO2 till harvest.
- Collect supernatant and cells separately during harvest.
- Store at -80 °C during harvest until analysis.
- Immunofluorescent staining of astrovirus-infected 2D enteroid monolayers on Transwells
- Remove media from wells in both apical and basal compartment.
- Wash once with warm 100 µl DPBS+/+.
- Fix cells with 100% methanol for 15 min at -20°C in both apical and basal compartment.
- Aspirate fixative and wash twice with 100 /500 µl (apical/basal) DPBS+/+.
- Block with DPBS+/+ containing 0.1% Triton-X100 and 3% BSA for 1 h.
- Add 100 µl DPBS+/+ containing 0.1% Triton-X100, 3% BSA and the appropriate primary antibodies overnight at 4 °C on the apical side (example of primary antibodies include VA1 for virus, ZO1 for tight junction, UEA-1 for multiple intestinal cells, DAPI for nucleus). Keep the blocking buffer in the basal compartment.
- Aspirate antibody and wash twice each with 100 µl DPBS+/+.
- Add 100 µl DPBS+/+ containing 0.1% Triton-X100, 3% BSA and the appropriate secondary antibodies for 1 h at RT and away from light.
- Wash thrice with DPBS+/+.
- Add 100 µl DPBS+/+ containing appropriate amount of DAPI for 5 min at RT.
- Wash thrice with DPBS+/+.
- Cut out membrane from the Transwell with care and place on microscope slide with apical side face up.
- Place a drop of Prolong gold (without DAPI) on the membrane and place a coverslip to cover the cells.
- Drain excess prolong and seal with nail polisher.
- Allow the cells to dry at RT overnight and place at 4 °C before imaging with the confocal microscope (Figure 2).
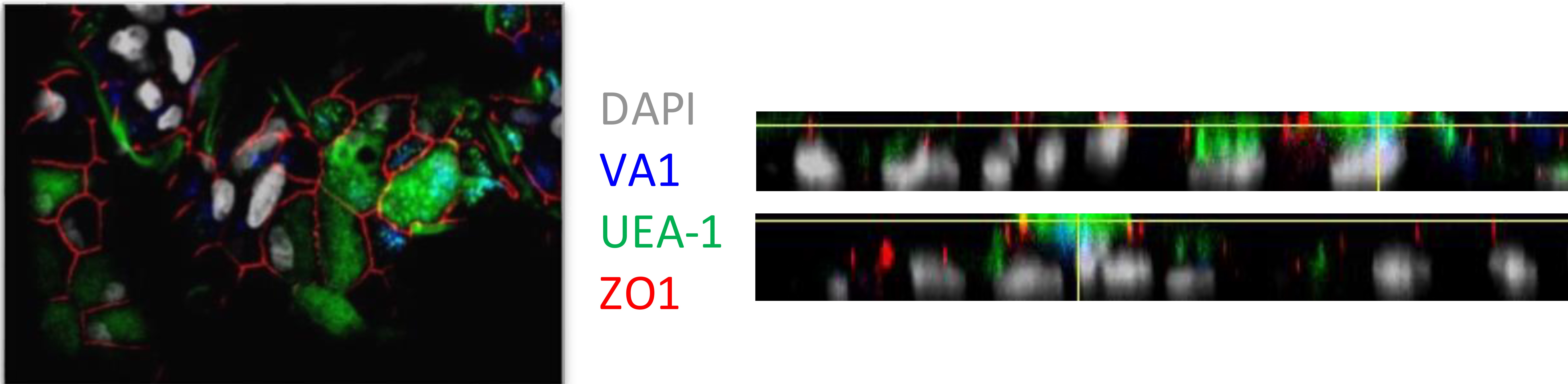
Figure 2. Differentiated fetal ileum 2D enteroids were infected with HAstV-VA1 for 5 days prior to immunofluorescence. Cells were stained with FITC-conjugated UEA-1 (green; multiple intestinal cells), ZO1 (red; tight junction), DAPI (grey; nucleus) and VA1 (blue; virus) antibodies. Image was acquired with a confocal microscope. Z-stack: maximum intensity projection (left) and orthogonal view (right).
- Flow cytometry analysis of astrovirus-infected 2D enteroid monolayers
- Aspirate media from wells.
- Add 100 µl Accumax and incubate at 37 °C for 5 min.
- Collect cells in 1.7 ml centrifuge tube on ice and repeat Step F2 if the cells are not detached.
- Add 500 µl LWRN- supplemented with 2 mM of EDTA and pipette up and down to break big cells aggregates.
- Centrifuge cells at 400 x g for 5 min at 4 °C.
- Re-suspend the pellet in 100 µl LWRN--EDTA and transfer into a round bottom 96-well plate. Pellet the cells by centrifugation at 400 x g for 5 min at 4 °C.
- Add 100 µl of LWRN--EDTA containing live/dead fixable stain for 15 min at RT.
- Spin cells at 400 x g for 5 min at 4°C and wash with LWRN--EDTA twice.
- Add 100 µl LWRN--EDTA containing 0.5% BSA and selected surface markers for 20 min at RT in the dark (example; UEA-1 for multiple intestinal cell types).
- Wash twice with 100 µl LWRN--EDTA each.
- Fix cells with fixation/permeabilization buffer for 20 min at room RT.
- Wash twice each with 100 µl permeabilization buffer and leave the cells for at least 3 h at 4 °C.
- Add primary antibody (anti-dsRNA as virus marker in permeabilization buffer) for 20 min at RT.
- Wash twice each with 100 µl permeabilization buffer.
- Re-suspend in 100 µl permeabilization buffer containing appropriately fluorophore labeled secondary antibodies for 20 min at RT.
- Wash twice each with 100 µl permeabilization buffer.
- Suspend in 500 μl permeabilization buffer. Keep in tubes for flow cytometer analysis (Figure 3).
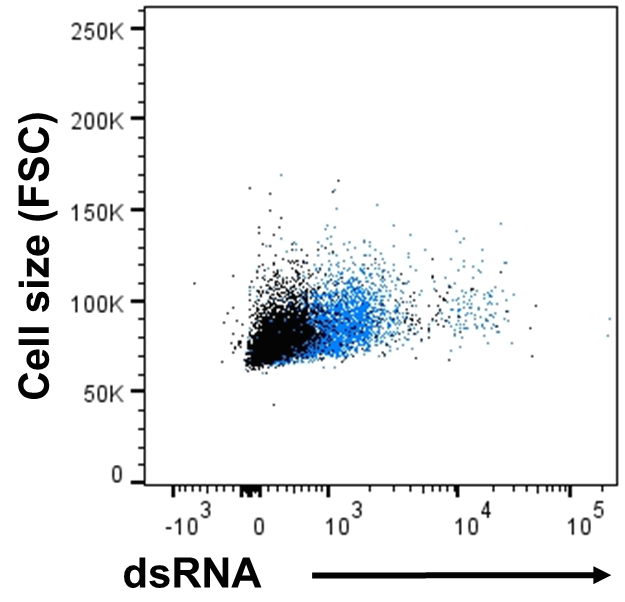
Figure 3. Flow cytometry data of infected HIE. Single cell suspensions of HAstV-VA1-infected fetal ileum enteroids were prepared at 3 dpi. Infected cells were stained with dsRNA (virus replication intermediate) to detect intracellular virus. Representative flow graph of total cell population that was alive (mock, black; VA1 infected, blue).
Recipes
Working Environment
- All preparations and procedures must be done under the sterile conditions of a biosafety hood.
- Appropriate personal protective equipment must be worn.
- Transfer all waste into a biohazard bag in the biosafety hood. Spray the bag with 10% bleach and transfer into a secondary biohazard bag before disposal. Spray the inside of the biosafety hood and all equipment that are used during experiments with 10% bleach. Wipe clean with paper towel. Spray with sterile water to remove residual bleach and wipe off. Spray with 90% ethanol and allow it to dry. Close the sash and turn on the UV for 30 min to completely inactive virus.
Stock solutions Reagent Solvent Stock concentration Working concentration N-acetyl cysteine ddH2O 500 mM 1 mM Mouse EGF PBS 50 μg/ml 50 ng/ml A-83-01 DMSO 500 mM 500 μM SB202190 DMSO 10 mM 10 μM Nicotinamide ddH2O 1 M 1 mM Leu15-Gastrin PBS 10 μM 10 nM Y-27632 ddH2O 10 mM 10 μM
Media
- Advanced DMEM-20
This medium is used to generate conditioned media.- Add 125 ml FBS to 500 ml Advanced DMEM/F-12
- Add 6.25 ml 5,000 units/ml pen/strep (antibiotics)
- Add 6.25 ml GlutaMax
- Mix by gently inverting thrice
- Label container appropriately and store medium at 4 °C. The medium can remain at 4 °C for 1 month
- Add 125 ml FBS to 500 ml Advanced DMEM/F-12
- LWRN conditioned medium
LWRN cells are used to generate conditioned medium that contains L-Wnt3a, R-spondin 1 and noggin, which are used to properly maintain human intestinal enteroids (HIE). The cells are a kind donation from Dr. Jason Spence (University of Michigan, also available at ATCC). LWRN conditioned medium can also be directly purchased from the translational tissue modeling laboratory, University of Michigan Medical School, Ann Arbor, Michigan, USA.- Prepare dual selection medium (0.5 mg/ml both G418 and hygromycin in DMEM-20)
- Thaw LWRN cells stored in liquid nitrogen under running tap water at room temperature
- Transfer content into a 15 ml tube containing 9 ml Advanced DMEM/F12 and centrifuge at 100 x g for 5 min at 4 °C to pellet the cells
- Suspend the cells in 1 ml dual selection medium and transfer into a 15 cm tissue culture treated dish containing 19 ml dual selection medium
- Incubate at 37 °C, 5% CO2 until about 90% confluent (usually ~5 days)
- Split the cells at 1:10 into new tissue culture treated dishes with 20 ml Advanced DMEM-20 and incubate
- At 80% cell confluence, harvest LWRN supernatant by pouring off medium from cells, replace with 20 ml fresh Advanced DMEM-20 and incubate. Consider this harvest as day 0.
- Filter-sterilize the harvested LWRN supernatant with 0.22 µm filtration system. Make 50 ml aliquots in conical tubes and store at -80 °C
- Harvest supernatant and replace medium every 2 days over a maximum of 12 days (about 7 harvests). Repeat Step h after each harvest
- Prepare dual selection medium (0.5 mg/ml both G418 and hygromycin in DMEM-20)
- Noggin conditioned medium
Noggin is an important component of the differentiation medium required for HIE differentiation. HEK293 cells that express Fc-tagged noggin were kindly provided by Dr. Mary Estes (Baylor College, Texas, USA).- Prepare single selection medium (10 µg/ml puromycin in Advanced DMEM-20)
- Thaw HEK293 cells expressing Fc-tagged Noggin stored in liquid nitrogen under running tap water at room temperature
- Transfer content into a 15 ml tube containing 9 ml Advanced DMEM/F12 and centrifuge at 100 x g for 5 min at 4 °C to pellet the cells
- Suspend the cells in 1 ml single selection medium and transfer into a 10 cm tissue culture treated dish containing 9 ml Advanced DMEM-20
- Incubate at 37 °C, 5% CO2 until the plate become confluent
- Split cells at 1:10 into new 10 ml tissue culture treated dishes with 15 ml Advanced DMEM-20 and incubate
- At 80% cell confluence, remove and discard old medium off cells and replace with fresh Advanced DMEM-20.
- Harvest supernatant one-time after 5 days, filter sterilize with a 0.22 µm filtration system and store at -80 °C
- Prepare single selection medium (10 µg/ml puromycin in Advanced DMEM-20)
- LWRN- medium (1%)
This is the basal medium that is used to wash enteroids.- To 500 ml Advanced DMEM/F-12, add the following
5 ml GlutaMax
5 ml HEPES
5 ml 5000 units/ml pen/strep - Mix by gently inverting thrice
- Label container appropriately and store medium at 4 °C. The medium stored at 4 °C is good for up to 4 weeks
- To 500 ml Advanced DMEM/F-12, add the following
- LWRN+ (with growth factors)
This is the complete medium with growth factors that is used to maintain enteroids.- To prepare a 100 ml medium, add the following from the stock solutions:
50 ml LWRN conditioned medium
2 ml B27 supplement
1 ml N2 supplement
200 µl N-acetylcysteine
100 µl EGF
100 µl Leu15-Gastrin
100 µl A83-01
100 µl SB202190
1 ml Nicotinamide
45.6 ml LWRN- - Mix by gently inverting
- Filter-sterilize with a 0.22 µm filtration system
- Label container appropriately and store medium at 4 °C. The medium stored at 4 °C is good for up to 2 weeks
- To prepare a 100 ml medium, add the following from the stock solutions:
- Differentiation medium
This is used to differentiate 2D monolayer enteroids from crypt-like into villus-like cell populations in culture.- To prepare a 50 ml medium, add the following from the stock solutions:
2.5 ml noggin conditioned medium
1 ml B27 supplement
500 µl N2 supplement
100 µl N-acetylcysteine
50 µl EGF
50 µl Leu15-Gastrin
50 µl A83-01
45.75 ml LWRN- - Mix by gently inverting
- Filter-sterilize with a 50 ml 0.22 µm filtration system
- Label container appropriately and store medium at 4 °C. The medium stored at 4 °C is good for up to 2 weeks
- To prepare a 50 ml medium, add the following from the stock solutions:
Acknowledgments
This protocol was adapted from the publication of Kolawole et al. (2019). The Michigan Medicine translational tissue modeling laboratory provided intestinal enteroids. Funding for the research was supported by NIH R21-AI141835.
Competing interests
The authors declare no competing interests.
References
- Bosch, A., Pinto, R. M. and Guix, S. (2014). Human astroviruses. Clin Microbiol Rev 27(4): 1048-1074.
- Janowski, A. B., Bauer, I. K., Holtz, L. R. and Wang, D. (2017). Propagation of Astrovirus VA1, a Neurotropic Human Astrovirus, in cell culture. J Virol 91(19).
- Janowski, A. B., Klein, R. S. and Wang, D. (2019). Differential in vitro infection of neural cells by Astroviruses. mBio 10(4).
- Kolawole, A. O., Mirabelli, C., Hill, D. R., Svoboda, S. A., Janowski, A. B., Passalacqua, K. D., Rodriguez, B. N., Dame, M. K., Freiden, P., Berger, R. P., Vu, D. L., Hosmillo, M., O'Riordan, M. X. D., Schultz-Cherry, S., Guix, S., Spence, J. R., Wang, D. and Wobus, C. E. (2019). Astrovirus replication in human intestinal enteroids reveals multi-cellular tropism and an intricate host innate immune landscape. PLoS Pathog 15(10): e1008057.
- Kolawole, A. O. and Wobus, C. E. (2020). Gastrointestinal organoid technology advances studies of enteric virus biology. PLoS Pathog 16(1): e1008212.
- Madeley, C. R. and Cosgrove, B. P. (1975). Letter: Viruses in infantile gastroenteritis. Lancet 2(7925): 124.
- Vu, D. L., Bosch, A., Pinto, R. M., Ribes, E. and Guix, S. (2019). Human astrovirus MLB replication in vitro: persistence in extraintestinal cell lines. J Virol 93(13).
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Owusu, I. A., Mirabelli, C. and Kolawole, A. O. (2020). Intestinal Enteroid Culture for Human Astroviruses. Bio-protocol 10(14): e3687. DOI: 10.21769/BioProtoc.3687.
Category
Microbiology > Microbe-host interactions > Virus
Immunology > Host defense > General
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










