- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Production and Isolation of Magnetic Protein Crystals in HEK293T Cells
Published: Vol 10, Iss 14, Jul 20, 2020 DOI: 10.21769/BioProtoc.3684 Views: 4172
Reviewed by: Samantha E. R. DundonShalini Low-NamGundeep Kaur

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

ZnCl2 Precipitation-Assisted Sample Preparation for Proteomic Analysis
Qiqing He [...] Fuchu He
Jul 20, 2025 2726 Views

Protocol for the Preparation of a Recombinant Treacle Fragment for Liquid–Liquid Phase Separation (LLPS) Assays
Nadezhda V. Petrova [...] Artem K. Velichko
Sep 20, 2025 1814 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1229 Views
Abstract
Advances in protein engineering have enabled the production of self-assembled protein crystals within living cells. Our recent publication demonstrates the production of ftn-PAK4, which is a ferritin-containing crystal that can mineralize iron and become magnetic when isolated. We have developed an optimized protocol for the production and isolation of PAK4-based crystals. The crystals are first grown in low-passage HEK293T cells, released using a lysis buffer containing NP-40 and DNase, and collected under careful centrifugation conditions. Our protocol maximizes the purity and yield of crystals and is quick and straightforward.
Background
Recent works have reported the production and isolation of “in cellulo” crystals through the heterologous expression of proteins in living cells. These crystals have varied applications, such as cargo delivery (Ijiri et al., 2009) or x-ray structure determination (Baskaran and Ang, 2015). The properties of the crystals vary, but they are generally quite large relative to the cell, ranging from 1-2 μm up to hundreds of μm in size (Schönherr et al., 2015). In our recent work, we modified inka-PAK4 crystals to create ftn-PAK4, which is a ferritin-containing crystal that can mineralize enough iron to be attracted to a nearby permanent magnet (Li et al., 2019).
The production and isolation of intact protein crystals poses several unique experimental challenges. Because the crystals are so large, purification methods such as gel electrophoresis cannot be used. Likewise, because the crystals are protein-based, harsher lysis conditions such as SDS will disassemble the crystals. However, clean suspensions can be critical for downstream applications. For example, excessive debris in an inka-PAK4 suspension can trap auto-oxidized iron and stick to crystals, generating spurious magnetic attraction results.
Here, we present a protocol for the production, isolation, and iron loading of ftn-PAK4 and inka-PAK4 crystals. It is possible to achieve results using only deionized water as the lysis buffer, but we present several optimizations that significantly improve crystal yield and minimize unwanted debris. These considerations should inform future work on other protein crystals, both for their production and isolation as well as their functional applications.
Materials and Reagents
- Pipettes and Pipette Tips
- 1.5 ml Eppendorf centrifuge tubes
- Glass slides
- 6 Well Tissue Culture Plate, Tissue Culture Treated, Flat Bottom (Fablab, catalog number: FL7105 )
- HEK 293T cells (< 10 passages) (ATCC, catalog number: CRL-3216 )
- DMEM High Glucose without Sodium Pyruvate (Gibco, catalog number: 11965092 )
- Penicillin/Streptomycin (Gibco, catalog number: 15140122 )
- Heat Inactivated Fetal Bovine Serum (Gibco, catalog number: 10438034 )
- Lipofectamine 2000 (Invitrogen, catalog number: 11668030 )
- Opti-MEM Reduced Serum Medium (Gibco, catalog number: 31985070 )
- GFP-PAK4, ftn-PAK4, and inka-PAK4 plasmids
- HEPES Buffer, 1 M, pH 7.2-7.5 (Gibco, catalog number: 15630130 )
- NP-40 (Sigma, catalog number: I8896 , also known as Igepal CA-630)
- Deoxyribonuclease I (DNase) (Worthington, catalog number: LS002007 )
- Earle’s Balanced Salt Solution (EBSS) (Life Technologies, catalog number: 14155-063 )
- Ferrous Ammonium Sulfate Hexahydrate (FAS) (Sigma-Aldritch, catalog number: FX0245 )
- D-Mannitol (Sigma-Aldrich, catalog number: M4125 )
- Concentrated Hydrochloric Acid (Fisher Scientific, catalog number: A144-500 )
- Potassium Ferrocyanide Trihydrate (MP Biomedicals, catalog number: 0 215256080 )
- Lysis buffer (see Recipes)
- DNase stock solution (see Recipes)
- Iron loading stock solution (see Recipes)
- Prussian Blue stain (see Recipes)
Equipment
- Centrifuge (Eppendorf, model: 5804 )
- Rocker (VWR Scientific Rocking Platform, Model 100)
- Microscope with Brightfield and Fluorescence Imaging (Leica, model: DMI 6000B )
Procedure
- Transfection
- Prepare cells
Plate HEK293T cells at approximately 60% confluency in a 6-well culture plate. Ensure that the cells have not been passaged more than 10 times before transfection.
Note: Cells can be plated any time before transfection, including immediately beforehand, as long as the density is 60% at the time of transfection. - Transfection
Transfect HEK293T cells using Lipofectamine 2000 according to the manufacturer's instructions.- Use 2 μg of total plasmid, and 6 μl of Lipofectamine 2000 per well, for a 1:3 plasmid to reagent ratio.
- To produce ftn-PAK4, use 1 μg of ftn-PAK4 plasmid and 1 μg of inka-PAK4 plasmid. To produce inka-PAK4, use 2 μg of inka-PAK4 plasmid. The plasmid concentration is not important, only the total mass of DNA.
- To aid in crystal visualization through fluorescence microscopy, include 0.2 μg GFP-PAK4 plasmid in the plasmid mix. It is not necessary to increase the amount of Lipofectamine used.
- Wait for crystal growth
The first crystals should appear 24 h after transfection. While inka-PAK4 crystals should be immediately visible, ftn-PAK4 crystals may not be obvious after 24 h. To maximize crystal yield and size, maintain cells in culture for 72 h after transfection before isolation (Figure 1).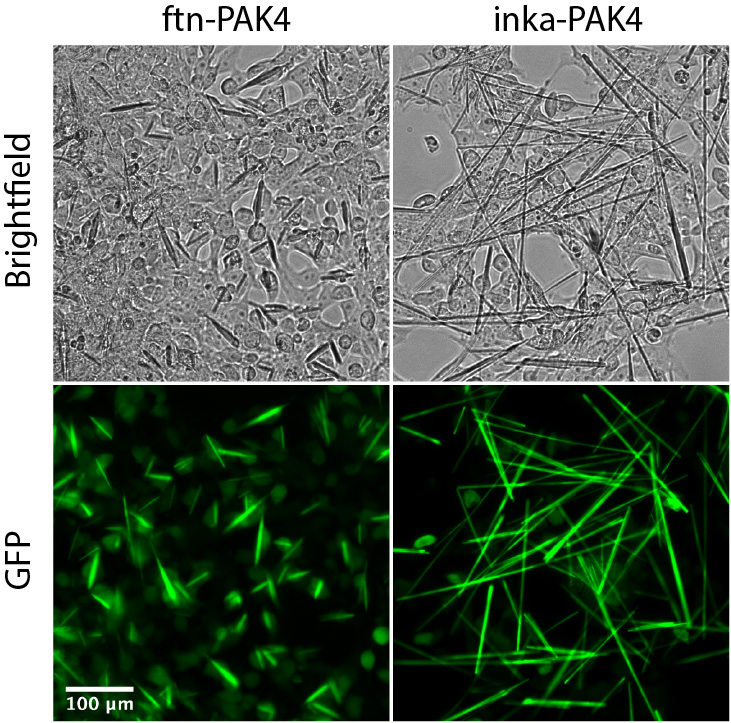
Figure 1. HEK293T cells containing ftn-PAK4 and inka-PAK4 crystals, with GFP-PAK4, 72 h after transfection. These crystals are ready for isolation.
- Prepare cells
- PAK4 isolation
- Lyse Cells
Remove the media from the cells, and gently rinse once with 1 ml room-temperature sterile deionized water. Add 500 μl lysis buffer and 10 μl DNase solution directly to the cell culture well, and place on a rocker at 50 tilts/min at room temperature for 40 min.
The presence of DNase in the lysis buffer is critical to high yields. Without DNase, a sticky pellet will form from the lysed cells’ DNA, which will trap most of the crystals (Figure 2).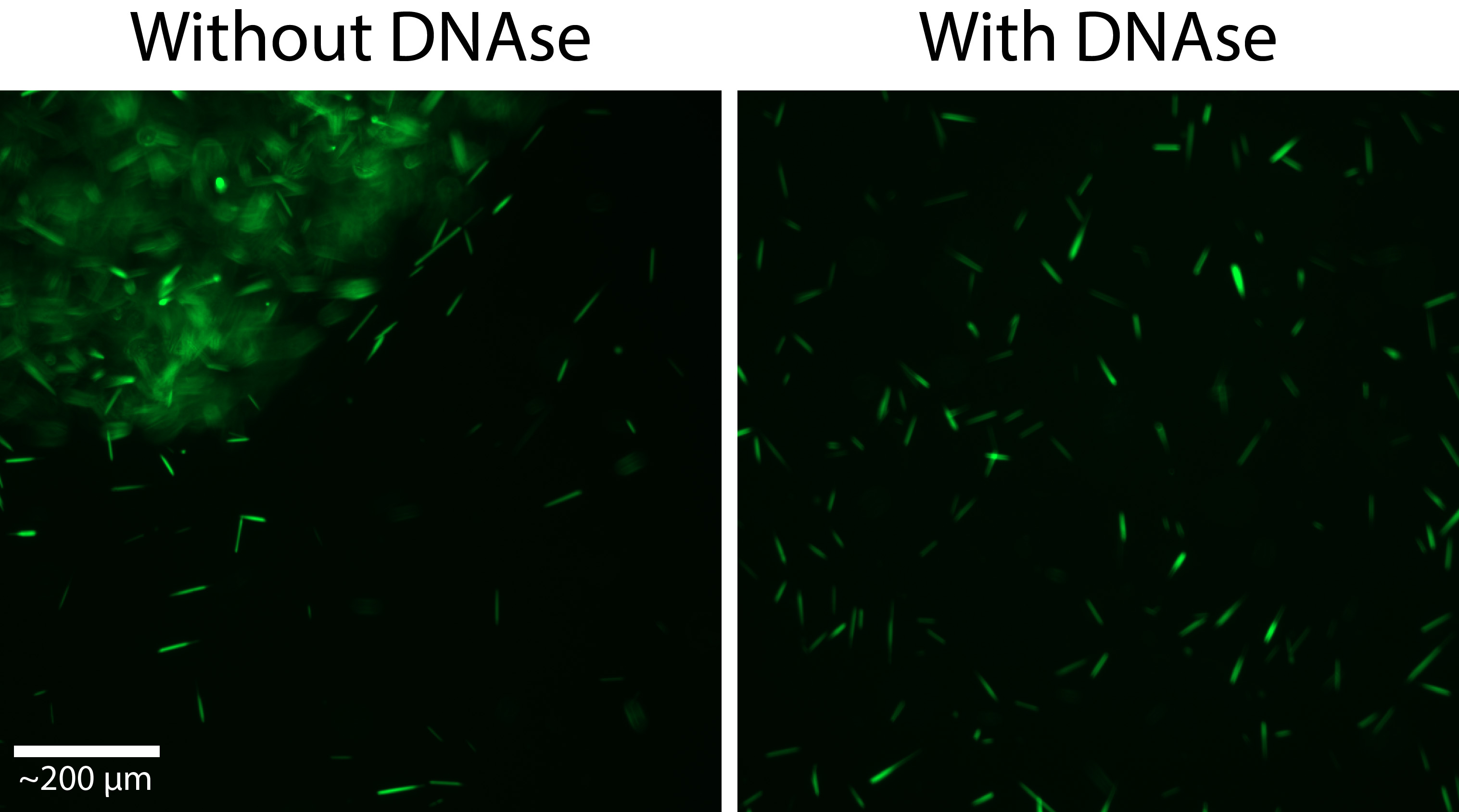
Figure 2. A ftn-PAK4 isolation without, and with DNase. In the DNase-free condition, many of the crystals have been trapped in a sticky pellet, substantially reducing the concentration of free crystals. - Centrifuge
After rocking, transfer the lysed cell solution to 1.5 ml Eppendorf centrifuge tubes. Centrifuge at 400 rcf for 1 min in a swinging-bucket centrifuge. Discard the supernatant and resuspend the pellet in 500 μl of 0.1 M HEPES buffer.
Note: The supernatant will still contain crystals, which can be collected using a second centrifugation. - Verify Isolation
Check crystal yield by pipetting a few μl of the suspension onto a coverslip and looking at it under the microscope. Isolated crystals should be visible through the eyepiece using a 10x objective under both brightfield and GFP illuminations.
- Lyse Cells
- Iron loading and assay
- Add Iron
Prepare a solution of FAS (50 mM, 100x) and mannitol (500 mM, 100x) in sterile deionized water. Add 1 μl of each stock solution to a 100 μl aliquot of the crystal suspension. Incubate at room temperature for exactly 15 min.
Notes:- The final concentrations are 500 μM FAS and 5 mM mannitol. The mannitol reduces the autooxidation of the iron.
- The FAS stock solution must be made immediately before use, as the Fe2+ will rapidly oxidize to the insoluble Fe3+.
- The final iron-loaded suspension may be a light yellow/orange color. The darker this color is, the more Fe3+ precipitate there is.
- Wash Iron
Centrifuge samples at 400 rcf in a swinging bucket centrifuge for 1 min. Discard the supernatant and resuspend the pellet in 100 μl of HEPES buffer.
Notes:- It is important to match the volume of the wash buffer with the original suspension volume. If a larger volume, like 500 μl, is used, the ftn-PAK4 suspension may not pellet during centrifugation, though the WT-PAK4 will.
- Common failure: If the resulting pellet is a yellow/orange color, the iron incubation step was likely too long, and analysis will be confounded by the presence of Fe3+ precipitates.
- Prepare Prussian Blue (PB) Stain
Prepare a solution of 5% concentrated HCl v/v and 1% potassium ferrocyanide w/v in water.
Note: It is important to prepare the PB stain immediately before use, because Fe2+ will oxidize and create Prussian Blue over time. While this oxidized solution will still work for approximately 48 h, it will contain a significant suspension of Prussian Blue particles. - Add PB Stain
To verify iron loading, pipet a 10 μl droplet of iron-loaded PAK4 crystals onto a glass slide or coverslip and add 1 μl of the PB stain. The droplet can be immediately checked under brightfield optical microscopy (Figure 3).
Note: If iron loading is successful, ftn-PAK4 crystals should have a deep blue color in the center. Inka-PAK4 crystals may be a faint blue, depending on the optics of the microscope.
Figure 3. Ideal colors for ftn-PAK4 and inka-PAK4 crystals after iron loading and PB stain
- Add Iron
Notes
- The passage number of the cells used is a critical variable in crystal yield and purity. High-passage cells will grow fewer, smaller crystals, and crystal suspensions from high-passage cells will contain significantly more debris.
- After iron loading, if the mannitol is not removed from the solution before PB stain, it will react with the PB stain to create a precipitate over several min. This precipitate doesn't interfere with staining as a brief check, but it will interfere with clean imaging.
- When imaging the PB stained crystals, it is important to use brightfield microscopy and not phase contrast, as phase contrast will distort colors.
- Some variables are more important than others.
- The following variables are highly sensitive to variation: cell passage number, centrifugation speed and time, iron loading stock concentrations, and iron loading duration.
- The following variables are less sensitive to variation: Lysis duration and rocking speed, and PB stain concentrations and duration.
- While the presence of mannitol helps to obtain cleaner results, its presence is not necessary to achieve iron loading of ftn-PAK4.
Recipes
- Lysis buffer
0.1 M HEPES Buffer
1% v/v NP-40
Store at room temperature - DNase stock solution
12,500 Units/ml
Dissolved in EBSS
Store at -20 °C - Iron loading stock solution
1 ml MilliQ water
19.6 mg Ferrous Ammonium Sulfate (500 mM stock)
91 mg Mannitol (500 mM stock)
Note: Do not store–make fresh each time. - Prussian Blue stain
1 ml MilliQ water
50 μl Concentrated Hydrochloric Acid
5 mg Potassium Ferrocyanide
Note: Do not store–make fresh each time.
Acknowledgments
Protocol was based on “Engineering a Genetically Encoded Magnetic Protein Crystal”, in Nano Letters 2019 (Li et al., 2019). This work was supported Stanford Interdisciplinary Graduate Fellowship in association with the Wu Tsai Neurosciences Institute (T.L.L.), Packard Fellowship and Engineering (B.C.), and NIH 1R01GM125737 (B.C.).
Competing interests
The authors declare that there are no competing interests or conflicts of interest.
References
- Baskaran, Y. and Ang, K. C. (2015). An in cellulo-derived structure of PAK4 in complex with its inhibitor Inka1. Nat Commun 6: 8681.
- Ijiri, H., Coulibaly, F., Nishimura, G., Nakai, D., Chiu, E., Takenaka, C., Ikeda, K., Nakazawa, H., Hamada, N., Kotani, E., Metcalf, P., Kawamata, S. and Mori, H. (2009). Structure-based targeting of bioactive proteins into cypovirus polyhedra and application to immobilized cytokines for mammalian cell culture. Biomaterials 30(26): 4297-4308.
- Li, T. L., Wang, Z., You, H., Ong, Q., Varanasi, V. J., Dong, M., Lu, B., Pasca, S. P. and Cui, B. (2019). Engineering a Genetically Encoded Magnetic Protein Crystal. Nano Lett 19(10): 6955-6963.
- Schönherr, R., Klinge, M., Rudolph, J. M., Fita, K., Rehders, D., Lubber, F., Schneegans, S., Majoul, I. V., Duszenko, M., Betzel, C., Brandariz-Nunez, A., Martinez-Costas, J., Duden, R. and Redecke, L. (2015). Real-time investigation of dynamic protein crystallization in living cells. Struct Dyn 2(4): 041712.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Li, T. L. and Cui, B. (2020). Production and Isolation of Magnetic Protein Crystals in HEK293T Cells. Bio-protocol 10(14): e3684. DOI: 10.21769/BioProtoc.3684.
Category
Biophysics > Bioengineering > Protein encapsulation
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









