- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli
Published: Vol 10, Iss 13, Jul 5, 2020 DOI: 10.21769/BioProtoc.3663 Views: 4953
Reviewed by: Xiaoyi ZhengYing ShiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2901 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3547 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
Glomerulonephritis (GN) is a common pathological condition in chronic kidney diseases that often leads to end stage renal failure. Mac-1 (CD11b/CD18)-mediated neutrophil, macrophage, and dendritic cell glomerular infiltration leading to cellular dysfunction and destruction is an important disease mechanism. The cellular distribution and dynamics of the expression of Mac-1 ligands ICAM-1 and ICAM-2 in GN have not been well studied because of the difficulties in tissue staining and colocalizing glomerular cells with surface antigens. To improve the visualization of cell surface marker and antigen expression in kidney compartments, we have devised an even but mild fixation procedure employing p-formaldehyde-lysine-periodate (PLP) perfusion. A large panel of antibodies (Ab) against cell surface markers was used to identify kidney cell types and adhesion molecules. When confocal microscopy was used in visualizing glomerular adhesion molecule staining, the endothelial cells were found to specifically express CD31, and these cells express ICAM-2 constitutively. Though ICAM-1 was not expressed by glomerular endothelial cells in homeostasis, it was highly upregulated in mice with chronic GN and severe proteinuria. VCAM-1, a ligand for VLA-4 important in leukocyte migration, was not expressed in the glomerulus. The results highlight the importance of ICAM-1 in the infiltration of macrophages and dendritic cells in cGN. This report will provide a widely applicable procedure for yielding high quality confocal images and for the identification and quantitation of receptors and other cellular antigens expressed in different kidney compartments and cell types.
Keywords: GlomerulonephritisBackground
The glomerulus plays a central role in the ultrafiltration function of the kidney by the formation of a semipermeable filtration barrier between the circulation and the urinary space (Dickinson, 2016). It is composed of a network of capillaries formed by fenestrated endothelial cells lined by podocytes with interdigitating foot-processes forming slit diaphragms for selective filtration and mesangial cells which provide mechanical support and contractile functions. Most chronic kidney diseases and a large percentage of end stage renal disease exhibit glomerulus involvement and glomerulonephritis (GN) is the underlying cause in 40% of patients with chronic kidney disease (Jha et al., 2013; Foster, 2016; Lindenmeyer and Kretzler, 2017). Leukocyte infiltration and immune complex deposition are largely responsible for the initiation and progression of GN with polymorphonuclear neutrophils (PMN) and macrophages constituting the predominant infiltrating cell types (Devi et al., 2013; Dickinson, 2016; Sung et al., 2017; Almarza Novoa et al., 2018). Leukocyte transmigration into the glomerulus is dependent on chemokine and adhesion molecule functions (Filippi, 2016; Sung et al., 2017). For PMN and monocyte adhesion and diapedesis into the subendothelial space and the mesangium, CD11b function is of particular significance (Ernandez and Mayadas, 2016; Filippi, 2016; Sung et al., 2017). This β2 integrin binds ICAM-1 and ICAM-2 which are expressed by glomerular intrinsic cells, especially the endothelial cells (Devi et al., 2013; Filippi, 2016; Sung et al., 2017). The expression of integrin β1 and β2 ligands including ICAM-1, ICAM-2, and VCAM-1 on endothelial cells are modulated by changes in the inflammatory environments but their expression within the glomerulus is not well-studied. To understand cellular interactions in GN pathogenesis, microscopic examination of the dynamics of cell type-specific expression of adhesion molecules is critical (Devi et al., 2013; Sung et al., 2017). Studies by confocal microscopy have provided a clearer understanding of leukocyte emigration in the glomerulus. Unequivocal definition of expression specificity in this microscopic technique requires (1) availability of glomerular cell type-specific Ab; (2) optimized methods for tissue preparation and fixation; (3) availability of Ab for detecting the antigenic epitopes; and (4) adequate signal to noise ratio. In the past, difficulties arising from one or more of these issues have resulted in suboptimal detection of integrin expression by glomerular cells. Some of the issues in kidney glomerulus staining are summarized below. The first is the high autofluorescence and nonspecific binding of renal tubules. The autofluorescence of cortical tubules is particularly notable. The problem of nonspecific staining is especially severe when secondary Ab are used. Availability of reagents poses another hurdle since fluorescence-tagged Ab for staining kidney glomerular intrinsic cell types such as podocyte and mesangial cells are not commercially available. Furthermore, with the staining of frozen sections, as is often the case, the images are diffuse and lack resolution compared to fixed tissues (Figure 1, cf. HLA-DR staining (arrows) in panel A–frozen and panel B–fixed sections). This report describes a mild kidney fixation method for preserving tissue architecture and Ab-binding antigenic epitopes. Ab conjugated with fluorescence tags for background reduction were used to identify glomerular cell expression of integrins in homeostatic and inflamed kidneys. The procedure yielded sharp images of glomerular endothelial cells expressing ICAM-1 and ICAM-2 and extraglomerular tubular cells expressing VCAM-1. The method is applicable to studies on cellular interactions and receptor and cytokine expression by other kidney cell types such as inflammatory cells, fibroblasts, and epithelial cells.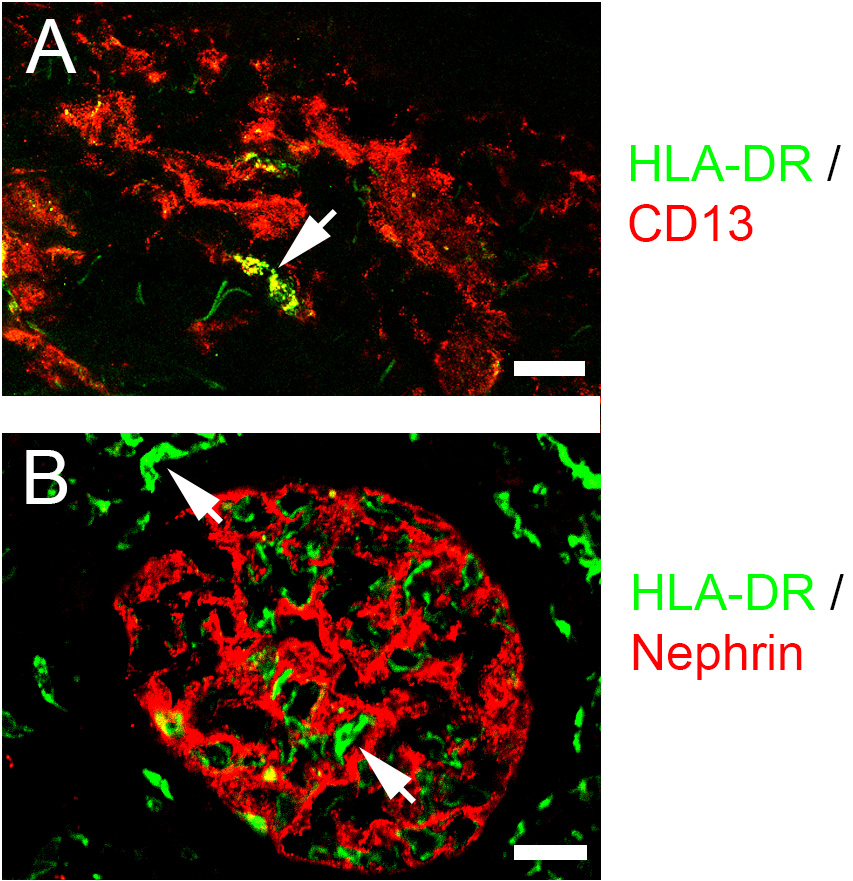
Figure 1. Comparison of immuno-fluorescence staining in frozen and fixed tissues. A. Human skin tissue was frozen in OCT, sectioned in 5 μm slices, fixed for 10 min in acetone at -20 °C, and stained with the indicated Ab as described in Procedure B. B. Human kidney tissue was fixed for 3 h in 2% phosphate-lysine-periodate (PLP), equilibrated in sucrose and OCT as described in Procedure A, sectioned, and stained with the indicated Ab as described in Procedure B. Confocal images were captured as described in Procedure C. Arrows indicate HLA-DR-stained cells. Scale bars = 25 μm.
Materials and Reagents
- 24-well plates (Sarstedt, catalog number: 83.1836 )
- Fisher Superfrost Plus Microscope Slide (Fisher Scientific, catalog number: 12-550-15 )
- Sharpie ultra fine point marker (Amazon.com, catalog number: 37001 )
- Gold Seal #3317 22 × 50 mm #1 cover glass (VWR, catalog number: 48404-053 )
- Tissue Cassettes (Fisher Scientific, catalog number: 15-200-403E )
- Base Molds, 15 × 15 mm, disposable (Fisher Scientific, catalog number: 22-363-553 )
- Pasteur pipet (Fisher Scientific, catalog number: 13-678-20D )
- Diamond glass cutter (Diamond knife) (EMS, catalog number: EMS 70038 )
- Coplin staining jar (Thermo, catalog number: E94 )
- BioAssay dish, square, 245 mm (Corning, catalog number: 431111 )
- Whatman grade 1 circular filter paper, 18.5 cm diameter (GE Healthcare, catalog number: 1001185 )
- 0.2 µm filter (Thermo, catalog number: 565-0020 )
- Whatman 3 MM Chromatography paper, 25 cm square (Fisher, catalog number: 05-716-6B )
- Microscope slide box (EMS, catalog number: 71470-W )
- NZM2328 mice (Originally from Jackson Laboratories; currently maintained in-house)
- O.C.T. Compound (Tissue-Tek OCT) (Sakura, catalog number: 4583 )
- Everbrite Hardset Mounting Medium without DAPI (Biotium, catalog number: 23003 )
- Polyclonal Ab, Goat Ab:
- Monoclonal anti-mouse Ab:
- Rat mAb: Alexa Fluor (A)-488-anti-mouse ICAM-2 [CD102, clone 3C4 (MIC2/4)] (Biolegend, catalog number: 105609 )
- Rat mAb: A-647-anti-mouse VCAM-1 (CD106, clone 429) (Biolegend, catalog number: 105712 )
- Hamster mAb: BV421 anti-mouse ICAM-1 (CD54 clone 3E2) (BD, catalog number: 564704 )
- Rat mAb: anti-mouse CD16/CD32 (clone 2.4G2) (Bio-X-Cell, catalog number: BE-0307 )
- Anti-human Ab
- mAb labeling kits for labeling unconjugated Ab:
- Alexa FluorTM 488 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20181 )
- Alexa FluorTM 555 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20187 )
- Alexa FluorTM 647 Antibody Labeling Kit (Thermo Fisher, Invitrogen, catalog number: A20186 )
- Pacific BlueTM Antibody Labeling Kit (Thermo Fisher, PacBlue, catalog number: P30013 )
- Phosphate-buffered formalin (10%; Fisher Scientific, catalog number: 22-110-869 )
- DAPI (Sigma, catalog number: 10236276001 )
- HCl (MP Biomedical, catalog number: 194697 )
- Triton X-100 (Sigma, catalog number: X100-500ML )
- Horse serum (Gibco, catalog number: 16050 )
- Chicken serum (Gibco, catalog number 16110-082)
- HEPES (free acid) (ICN, catalog number: 101926 )
- Dulbecco’s modified Eagle’s medium (DMEM; Gibco, catalog number: 11965-092 )
- Sucrose (Sigma, catalog number: S0389 )
- Ketamine (Ketaset; Zoetis, catalog number: EA2489-564 , 100 mg/ml)
- Xylazine (AnaSed; Akorn, catalog number: 59399-110-20 , NADA #139-236, 20 mg/ml)
- Heparin (Elkins-Sinn, catalog number: 6505-00-153-9740 , 1,000 units/ml)
- Monobasic sodium phosphate (NaH2PO4·H2O) (Baker, catalog number: 3818-5)
- Dibasic sodium phosphate (Na2HPO4·7H2O) (USB, catalog number: 20232 )
- NaCl (Fisher, catalog number: S271-1 )
- KH2PO4 (Baker, catalog number: 3246-5)
- KCl (Baker, catalog number: 3040-05)
- EDTA·H2O (Baker, catalog number: 4040-04)
- Lysine HCl (MP Biomedical, catalog number: 194697 )
- p-formaldehyde (Fisher, catalog number: 04042-500 )
- Sodium m-periodate (Sigma, catalog number: S-1147 )
- Dulbecco’s phosphate-buffered without Ca2+ and Mg2+ (PBS): Dilute 10× PBS (see Recipes) with double distilled (dd) H2O
- Sodium phosphate buffer, 1 M, pH 7.4 (1 M phosphate buffer) (see Recipes)
- Sodium phosphate buffer, 0.375 M, pH 7.4 (see Recipes)
- 10× PBS (see Recipes)
- NaOH, 10 N (see Recipes)
- HEPES, 1 M, pH 7.4 (see Recipes)
- EDTA, 0.5 M, pH 8.0 (see Recipes)
- 4% p-formaldehyde in 0.1 M phosphate buffer, pH 7.4 (see Recipes)
- Lysine phosphate (see Recipes)
- p-Formaldehyde-Lysine-sodium periodate (2% PLP) (see Recipes)
- Sucrose (30%) in 50 mM phosphate buffer, pH 7.4 (see Recipes)
- NaN3 (10%) (see Recipes)
- Detergent tissue extraction solution (see Recipes)
- Tissue blocking solution (see Recipes)
- Anesthesia mix (see Recipes)
- DAPI stock and working solutions (see Recipes)
Equipment
- 1 L beaker
- 1 L Erlenmeyer flas
- Stirring bar
- Fume hood
- Water bath
- Dissection tray (Carolina Biological Supply, catalog number: 629005 )
- Cryostat (Leica Biosystems, model: Leica CM1850 UV )
- Zeiss Laser Scanning Confocal Microscopy System with (Carl Zeiss, model: LSM 700 ):
- 405 nm, 488 nm, 555 nm, and 639 nm laser excitation lines
- EC Plan-Neofluar 10×/0.3. M27 objective
- Plan Apochromat 20×/0.8 M27 objective
- EC Plan-Neofluar 40×/1.30 Oil objective
- Plan-Apochromat 63×/1.4 Oil DIC M27 objective
- Revco Ultima II -70 °C Freezer (Thermo Scientific, model: ULT-2186-9-A )
- Constant pressure perfusion assembly with reservoirs in “Mariotte’s bottle” configuration:
A constant pressure reservoir assembly is constructed as shown in Figure 2A. It is consisted of 60 ml syringes with inlets made from #6 rubber stoppers and trimmed 5 ml pipets (Figure 2B). The syringe reservoirs are connected by 1/8” i.d. silicone tubings (Cole-Parmer, catalog number: EW-95886-03 ) through 1/16” i.d. Luer Fitting Adapters (Cole-Parmer, catalog number: GH41507-62 ) and regulated by a 3-way stopcock (Cole-Parmer, catalog number: 30600-08 ). The draining outlet is consisted of a 40” silicone tubing (3/32” i.d., Cole-Parmer, catalog number: EW-96201-84 ) with a 16 G needle (needle point trimmed) at one end to connect to the luer slip outlet of the 3-way valve and a luer lock adapter (Cole-Parmer, catalog number: GH41507-62 ) on the other end to connect with a 21 gauge butterfly vacutainer (BD, catalog number: 367296 ) for perfusion. The needle sheath of the vacutainer needle is trimmed to expose the needle head and fitted on the needle to stop excessive penetration (Figure 2C). The hydrostatic pressure is set at 32” H2O.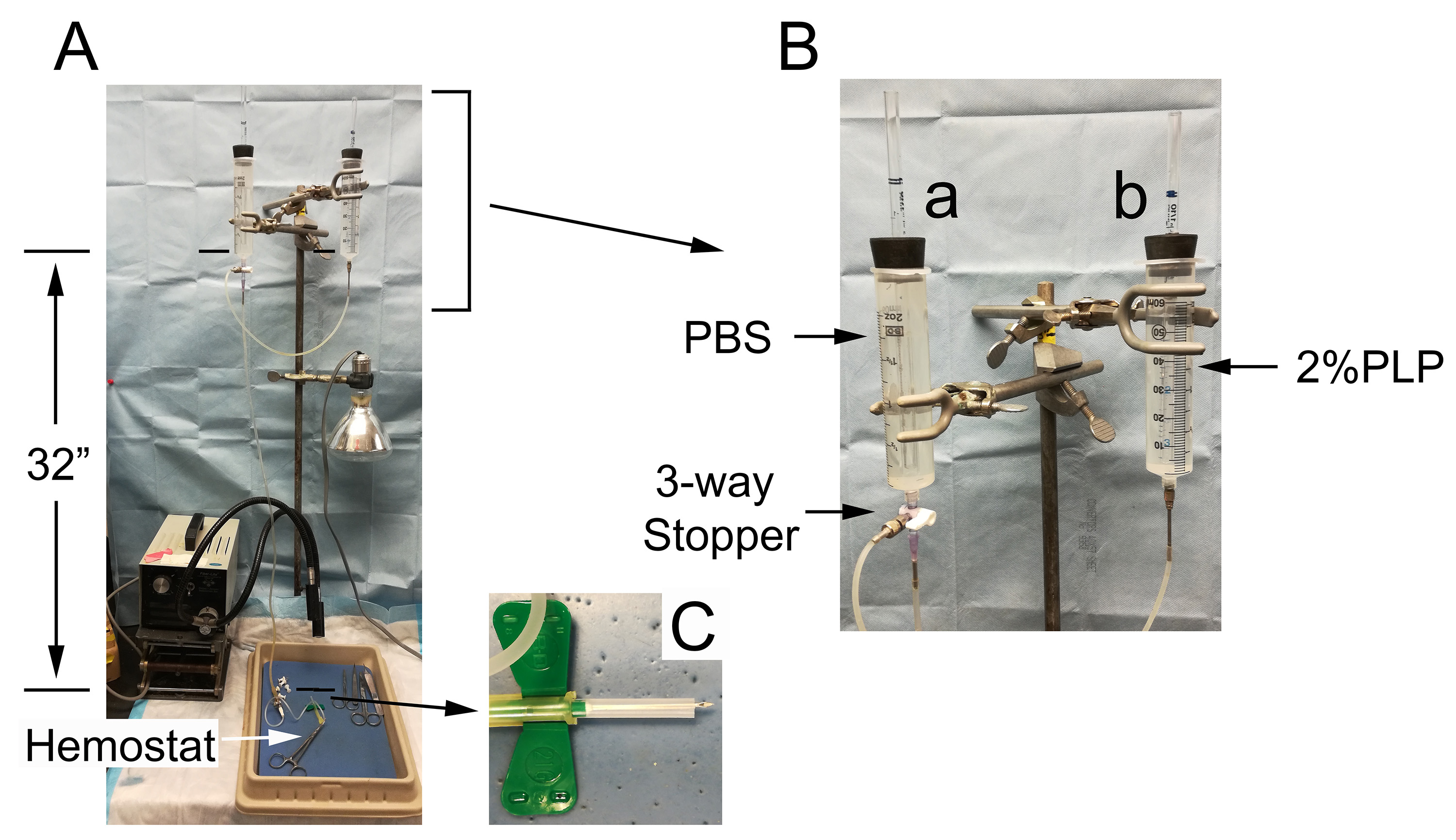
Figure 2. Mouse perfusion assembly. The perfusion assembly (A) is consisted of 2 connected 60 ml syringes in a Mariotte’s bottle configuration. Syringe a in (B) is fitted with a 3-way valve to regulate flow. The draining tubing outlet is connected with a 21 G vacutainer. The needle sheath is cut to size to expose the needle point (C) and left in place to prevent over-penetration of the heart. Perfusion through the vacutainer is stopped by clamping with a hemostat.
Software
- ZEN 2.3 SP1 FP3 (Black) 64 bit program for data acquisition (Zeiss, available here)
- ZEN Lite for data analysis (Zeiss, available here)
- Adobe Photoshop Version 20.0.4 20190227.r.76 (available here)
Procedure
- Mouse tissue collection and fixation
Note: Because most Ab used in this report are monoclonal Ab, the single epitope recognized by the Ab are often destroyed by harsh fixation. Thus a mild fixation method with limited reaction period was used. This fixation method also improves tissue staining by polyclonal Ab.- Fill reservoirs a and b (Figure 2B) in the perfusion assembly with PBS and 2% PLP respectively. Expel air bubbles by first opening the 3-way stopper to allow 2% PLP to flow into the vacutainer. Switch valve position to allow PBS to flush the perfusion line and to stop 2% PLP flow. Ensure that all bubbles are purged. Clamp the vacutainer line with a hemostat.
- Inject mice i.p. with 0.225 ml anesthesia mix (Recipe 14). Wait for 3-5 min until the mouse loses the foot pinch reflex. Pin the mouse on a vinyl dissection pad in a dissection tray (Figure 2A; Figure 3A)
- The surgical procedure is illustrated in Figure 3. Open skin by first making a nick in the posterior end of the abdomen at the midline (Figure 3A). Insert the closed scissors blades underneath the skin towards the anterior end of the thorax (Figure 3B). Open the blades (Figure 3C) and slide in the posterior direction to separate the skin from the abdominal and thoracic walls.
- Slit open the skin along the midline (Figure 3D) and pin the skin on the side to keep the rib cage exposed (Figure 3E).
- Nick the abdominal wall with a pair of scissors (Figure 3F). Insert the pointed blade into the opening and cut open the abdomen wall short of the diaphragm. Cut sideways to open up the abdomen.
- Lift the rib cage at the diaphragm to identify the location of the heart. Poke gently through the diaphragm with the pointed blade of the scissors toward the heart to release air into the rib cage. This will create space in the thoracic cavity and facilitate incisions without damaging the heart and lungs (Figure 3G).
- Cut the rib cage along the midline until the larynx (Figure 3H). Excise the rib cage from the side to expose the heart (Figures 3I and 3J).
- Open blades of a small pair of scissors and press down on the heart to position the overhanging segment of the right atrium between the blades (Figure 3K). Excise the atrium segment to create the perfusion outlet.
- Grab the heart with a pair of #3 forceps (Figure 3L) and insert the needle of the vacutainer into the apex of the left ventricle (Figure 3M). Release the vacutainer clamp to commence PBS flush. Ensure that no air bubble drains into the heart.
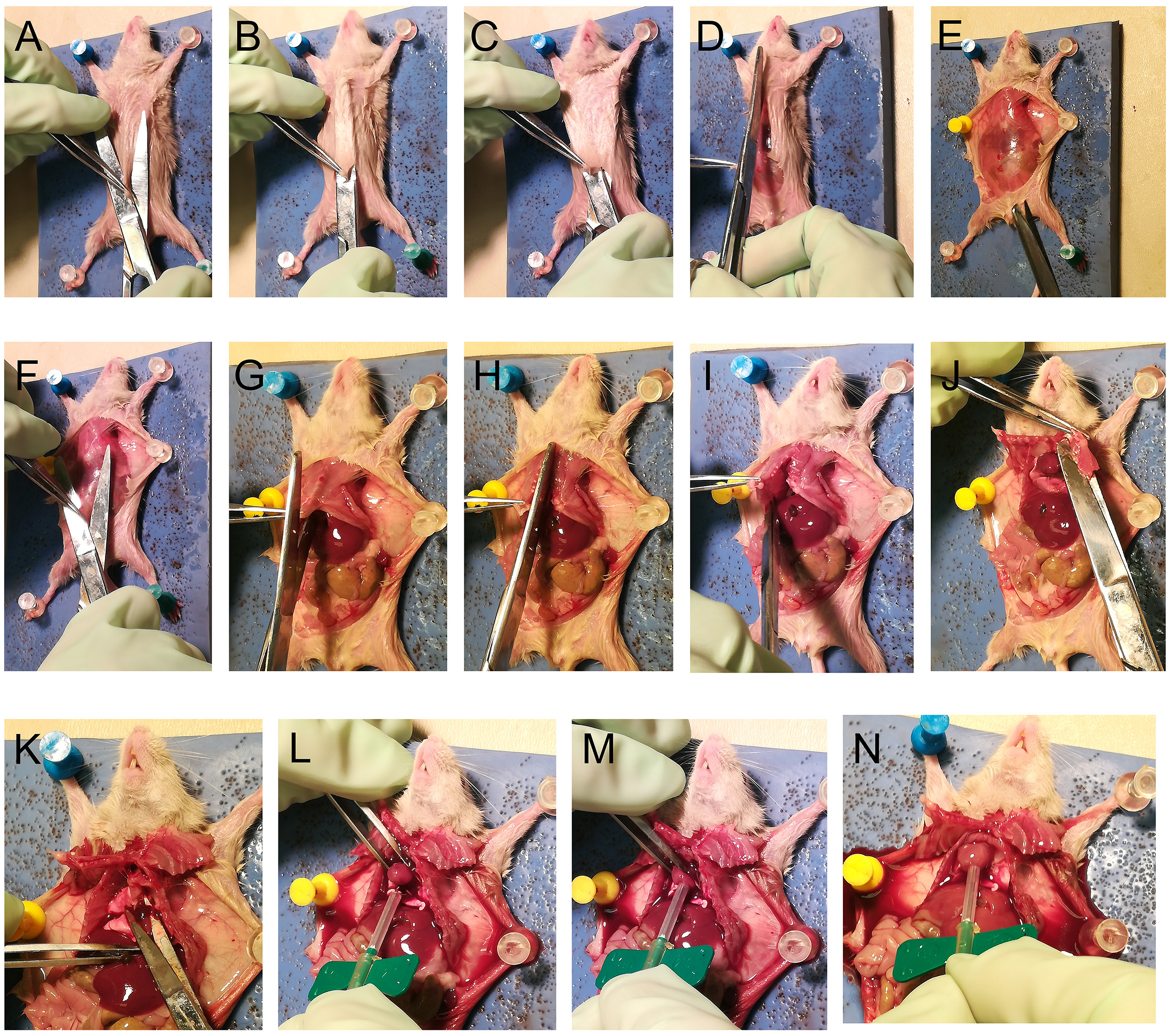
Figure 3. Kidney perfusion through the left ventricle. The details of the method are described in Steps A2-A9. - After the 20 ml PBS flush, turn valve to flush with 20 ml 2% PLP.
- Clamp vacutainer tubing.
- Remove kidney capsule by grabbing the capsule near the hilum with a #3 forceps. Carefully puncture the capsule with a fine pair of scissors. Insert blade and push outward and upward to remove capsule. Excise kidneys. Cut 1 mm traverse sections with a razor blade.
Note: Select central kidney slices near the traverse midline such that cortex, medulla and papilla regions are included in each slice. Two such kidney slices are fixed in 2% PLP for immunofluorescence staining and 2 slices are fixed in 10% phosphate-buffered formalin (Fisher) for histological staining. Two additional slices flanking the central slices on each side are combined with the 2 central pieces for 2% PLP fixation for added glomeruli scoring. Usually 1 kidney is cut into slices for immunofluorescence and histological staining and the other kidney is cut in half, snap frozen in liquid nitrogen and stored for biochemical analyses. - Place the tissue slices into tissue cassettes and fix in 2% PLP on ice for 3 h.
- Remove p-formaldehyde and equilibrate the tissue in sucrose for preservation by washing tissues twice in PBS followed by soaking in 5% sucrose at 4 °C overnight.
Note: It is critical to remove p-formaldehyde by soaking in low percentages of sucrose for at least several hours. Residual p-formaldehyde causes high background, especially at the shorter wavelengths. Graded percentages of sucrose solutions are then used to bring the tissue sucrose content to 30% to minimize ice crystal formation during freezing for better tissue architecture preservation and for extracting water to reduce swelling. Avoid prolonged soaking in 30% sucrose because the high osmolarity causes shrinkage of the tubules. The tissue is finally dipped briefly in 4 successive wells of OCT compound before embedding to remove surface sucrose for easier sectioning on the cryostat. - Change solutions and equilibrate the tissue in 15% sucrose for 2 h followed by 30% sucrose for 3 h.
- Dip tissue slices briefly in 4 consecutive wells of OCT in 24-well plates to coat tissue slices thoroughly with OCT. Immerse tissue in OCT in base molds with the cut surface facing down. Press tissue down towards the sectioning face of the tissue block. Freeze on dry ice.
- Store in -70 °C freezers.
- Tissue staining
- Cut 5 µm sections and place on Superfrost plus slides, 2 sections per slide.
- Label slides on the frosted surface with a Sharpie marker.
- Encircle tissues by etching with a diamond knife on the underside of the slide to facilitate tissue localization during microscopy.
- Place slides in Coplin staining jars and wash 2 ×, 5 min each with PBS.
- Add detergent extraction medium and incubate at room temperature (RT) for 30 min.
- Wash 2 times, 5 min each, with PBS.
- Block non-specific binding with blocking solution for 2 h at RT.
- Prepare Ab mixes using 2 µg/ml of each Ab and up to 4 Ab labeled with respective fluorochromes of BV421, A-488, A-555, and A-647. Dilute Ab in blocking medium.
Notes:- DAPI for nucleus localization can be used instead of BV421-conjugated Ab (see Recipes for DAPI use).
- Avoid using secondary Ab for kidney staining. For direct staining with purified unconjugated Ab, label Ab with Thermo Fisher (Invitrogen) mAb labeling kits as described by the suppliers.
- Wash slides 2 times, 5 min each, with PBS. Suction off liquid around the tissue slices with a Pasteur pipet and place slides in a 24.5 cm × 24.5 cm Bioassay dish lined at the bottom with a square wetted #3 Whatman filter paper. Do not allow the kidney tissue to go dry.
- Pipet 50 µl Ab mix onto the tissue section and spread the solution with the pipet tip to cover all areas of the tissue.
- Incubate for 2 h to overnight at 4 °C in the dark.
- At the end of the Ab staining, stand slide lengthwise upright and tap on a paper towel to drain off staining solutions.
- Place in PBS. Wash 4 times, 5 min each.
- Suction off liquid and put a small drop of mounting medium on the tissue slices. Place a #1 coverglass on top. Invert slide and press down on a paper towel to spread out the mounting medium.
- Place in a slide box.
- Image capture
- Set up the confocal microscope using the “Smart Setting”. Images were captured with 3 track excitation: track 1, PacBl, A-647, T-PMT; track 2, A-488; track 3, A-555. Other settings were: Image size, 1,024 × 1,024; scan speed, 7; scan mode, frame; averaging, 2, frame, mean, 8 bit; gain, 750. Other parameters are at default or set automatically by “Smart Setting”. Most images were captured with the 20× objective.
Note: Settings on the confocal microscope can be varied to optimize the collection time, resolution, and photobleaching attenuation. - In the locate mode, use a 10× objective to locate the field of interest and approximate focus with brightfield or a single red or green fluorescence channel.
- Switch to the 20× lens. Locate the field of interest and focus.
- In the “Acquisition” mode, set Scan Area to maximum. Turn on one laser and switch the others off. Increase laser power for image visualization. Click “Live” to set focus with fine focusing knob and image brightness with laser power. Turn on the “Range indicator” in the laser power adjustment to minimize off scale fluorescence.
- Set brightness for all channels by adjusting the laser power one color at a time (see Figures 4A-4C for optimal exposure).
- Turn on all 3 lasers and scan with “Live” to ensure optimal color intensities.
- Capture the image using “Snap”.
- Save image in LSM or CZI format.
- Use the “Crop” button under the “Dimensions” tab to magnify subregions or structures in the captured image.
- Collect 1 cycle of image capture with Snap for brightness adjustment. Collect final cropped image with “Snap” and save.
- Set up the confocal microscope using the “Smart Setting”. Images were captured with 3 track excitation: track 1, PacBl, A-647, T-PMT; track 2, A-488; track 3, A-555. Other settings were: Image size, 1,024 × 1,024; scan speed, 7; scan mode, frame; averaging, 2, frame, mean, 8 bit; gain, 750. Other parameters are at default or set automatically by “Smart Setting”. Most images were captured with the 20× objective.
- Confocal image analysis
- Open image files by ZEN Lite blue, a free download from the Zeiss website.
- Adjust brightness and background of each channel with the slider in the Histogram panel. Avoid offscale adjustment and keep gamma at 1.0 (see Figure 4 for optimal settings).
- Set pseudocolor for each channel. For confocal images, limit the single colors to red, green, and blue for easy colocalization determination. Colocalization colors are: green and red, yellow; green and blue, cyan; red and blue, magenta; green, red, and blue, white.
- Place scale bars in images.
- Export single color and confocal images as TIFF files.
- Use Photoshop to trim images, compile composite figures, and label panels.
- Save composite images with LZW and ZIP for image and layer compression respectively.
- Merge layers, adjust image size, and save as TIFF files for publication.
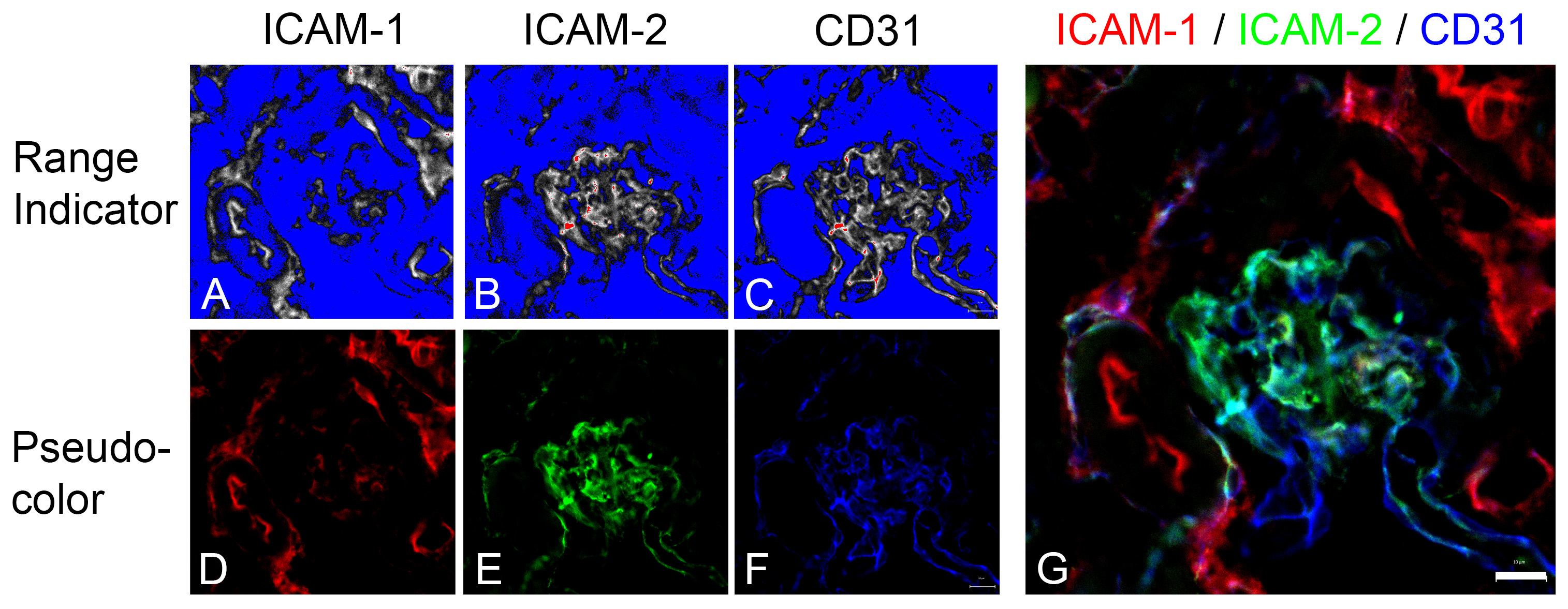
Figure 4. Setting channel brightness in confocal images. The brightness and background intensities were adjusted with the sliders in the Histogram panel in Zeiss Zen Lite software. Brightness is increased until red regions which designates offscale intensity barely appears (panels A-C). Scale bar in panel G equals 10 μm.
Data analysis
Results similar to Figure 5 has been published as Figure 5 in Sung et al. (2017).
- In non-inflamed kidneys in the NZM2328 lupus-prone mouse, glomerular endothelial cells identified by anti-CD31 staining (Figures 5A-a, b, d, blue) expressed ICAM-2 (Figures 5A-a, e, green) but little ICAM-1 (Figures 5A-a, b, c, red). VCAM-1 staining was restricted to the extraglomerular interstitial region (Figures 5A-b, f). Podocytes and mesangial cells identified by nephrin and integrin α8 respectively expressed little ICAM-1 and ICAM-2 (not shown). The results suggest that in homeostasis PMN and monocyte adhesion to glomerular endothelial cells through CD11b is largely dependent on Mac-1/ICAM-2 but not Mac-1/ICAM-1 interaction. The results also show that β1 integrins such as VLA-4 (α4β1) which binds VCAM-1 play negligible roles in myeloid cell adhesion in the glomerulus.
- In mice with anti-glomerular basement membrane-induced nephritis, little change from control mice in the localization of ICAM-1, ICAM-2, and VCAM-1 was found (Figure 5B).
- There were remarkable changes in the ICAM-1 expression in NZM2328 mice with chronic GN from non-diseased mice (cf., Figures 5C with 5A, panels a, b, c). In sick mice, ICAM-1 was highly expressed and occurred predominantly in the glomeruli. ICAM-1 colocalized with endothelial cells expressing CD31 (Figure 5C, panels a, b, c) instead of being periglomerular as in control mice (Figure 5A, panels a, b, c). This ICAM-1 upregulation may play significant roles in the infiltration of macrophages in the glomeruli of mice with chronic GN (Figure 5 in Sung et al., 2017). ICAM-2 expression in the glomerulus of mice with chronic GN (Figure 5C, panels a, e) was similar to that of nondiseased mice (Figure 5A, panels a, e). Since macrophage glomerular infiltration is correlated with ICAM-1 and not ICAM-2 expression in the glomerulus, it is likely that ICAM-2 plays minor or insignificant roles in macrophage glomerular emigration in cGN. By the same token, VCAM-1 expression remained extraglomerular (Figures 5C-b, f), indicating that VLA-4 plays little or no role in macrophage glomerular emigration in mice with severe GN.
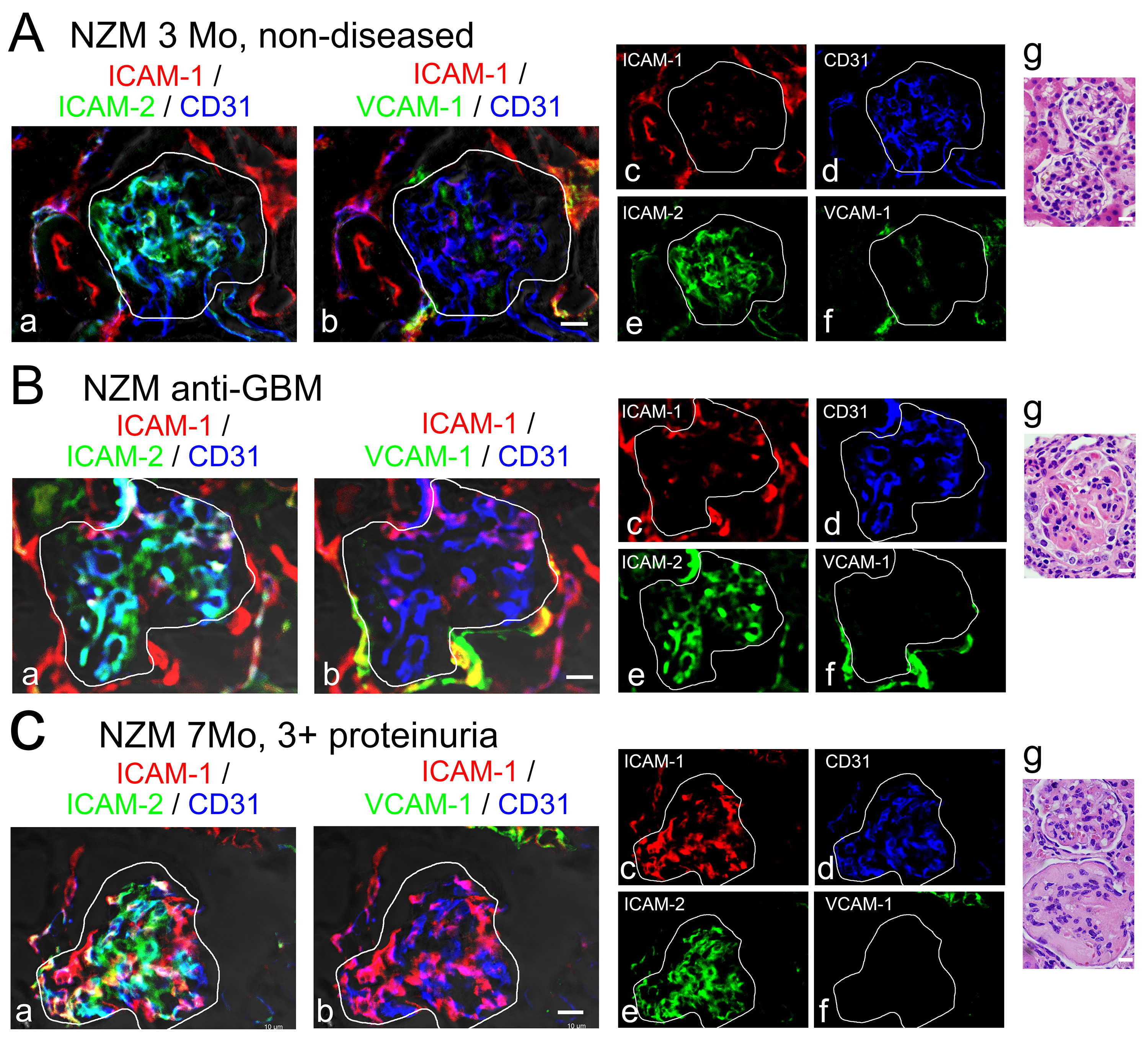
Figure 5. Expression of ICAM-1, ICAM-2, and VCAM-1 by kidney glomerular cells. Kidneys in NZM2328 mice were fixed in 2% PLP and stained with BV421-anti-ICAM-1, A-488-anti-ICAM-2, A-555-anti-CD31, and A-647-anti-VCAM-1. Micrographs were captured on an LSM700 Confocal Microscope. Merged and single color channels of the same field are shown. In panels g, hematoxylin and eosin-stained glomeruli are shown. A. 3 month old control mice with no treatment. B. Day 8 after treatment of 3 month old mice with anti-GBM nephrotoxic serum. C. 7 month old NZM mice with spontaneous chronic GN and severe proteinuria (3+ by dipstick). Similar results were found in multiple mice in multiple experiments. The glomeruli were encircled. Scale bars = 10 µm in panels b and 20 μm in panels g.
Recipes
- Sodium phosphate buffer, 1 M, pH 7.4 (1 M phosphate buffer)
- Make 1 M monobasic sodium phosphate (NaH2PO4·H2O, 137.99 g/L) and 1 M dibasic sodium phosphate (Na2HPO4·7H2O, 268.07 g/L)
- Mix 190 ml 1 M NaH2PO4 and 810 ml 1 M Na2HPO4
- Store at room temperature
- Sodium phosphate buffer, 0.375 M, pH 7.4
- Dilute 375 ml of each of 1 M NaH2PO4 and 1 M Na2HPO4 (from Recipe 1) to 1 L with ddH2O to make 0.375 M NaH2PO4 and 0.375 M Na2HPO4 respectively
- Mix 190 ml 0.375 M NaH2PO4 and 810 ml 0.375 M Na2HPO4 to make 1 L of 0.375 M sodium phosphate buffer, pH 7.4
- Store at room temperature
- 10× PBS
- Add 80 g NaCl, 25.6 g Na2HPO4·7H2O, 2.4 g KH2PO4, 2 g KCl to 800 ml of ddH2O to dissolve
- Make to 1 L with ddH2O
- Autoclave in liquid cycle at 121 °C for 40 min
- Store at room temperature. Dilute aseptically to keep stock solutions sterile
- NaOH, 10 N
- Add 40 g of NaOH to 90 ml ddH2O with stirring
- Cool and make to 100 ml
- HEPES, 1 M, pH 7.4
- Dissolve 23.8 g of HEPES (free acid) in 60 ml ddH2O
- Titrate to pH 7.4 with 10 N NaOH
- Make to 100 ml and sterilize with a 0.2 µm filter (Thermo)
- Store at 4 °C
- EDTA, 0.5 M, pH 8.0
- Place 186.12 g of disodium EDTA·H2O in 800 ml dd water and titrate to pH 8.0 with 10 N NaOH
- Add ddH2O to 1 L and store at RT
- Lysine phosphate, 0.375 M lysine and 0.1875 M phosphate buffer, pH 7.4 (5× lysine phosphate)
- Dissolve 68.48 g lysine HCl in ddH2O and make up to a final of 500 ml
- Titrate in a 1 L beaker with 0.375 M Na2HPO4 (dibasic sodium phosphate) to pH 7.4
- Make up to 1 L with 0.375 M sodium phosphate buffer, pH 7.4
- Add 2 mM EDTA, pH 8.0 for preservation
- Store at 4 °C
- 4% p-formaldehyde in 0.1 M phosphate buffer, pH 7.4
- Weigh 20 g p-formaldehyde and place in a 1 L Erlenmeyer flask with a stirring bar
- Add 100 ml ddH2O and heat to 70 °C in a fume hood
- Add 1 drop of 10 N NaOH
- When solution clears up, filter through a #1 Whatman filter paper (Whatman)
- Add 250 ml 0.2 M phosphate buffer, pH 7.4
- Make volume up to 500 ml with ddH2O
- Store at 4 °C and make fresh every 2 weeks
- p-Formaldehyde-Lysine-sodium periodate (2% PLP)
- Mix 50 ml 4% p-formaldehyde, 20 ml 5× lysine phosphate, 30 ml ddH2O, and 0.2 g sodium m-periodate
- Swirl container to dissolve sodium m-periodate
- Store on ice
- Sucrose (30%) in 50 mM phosphate buffer, pH 7.4
- Weigh 300 g of sucrose (Sigma) and place into a 1 L beaker with stirrer
- Add 20 ml 1 M phosphate buffer, pH 7.4 and make volume up to 950 ml with ddH2O. Stir and warm mildly if necessary
- Add 4 ml 0.5 M EDTA for preservation
- Make final volume to 1 L
- Store at 4 °C
- Dilute 30% sucrose solution with 50 mM phosphate buffer, pH 7.4 to make 5% and 15% sucrose solutions
- NaN3 (10%)
Dissolve 10 g of NaN3 (Fisher) in ddH2O and make up to 100 ml - Detergent tissue extraction solution
0.3% Triton X-100
10% horse serum
25 mM HEPES, pH 7.4
0.1% sodium azide in DMEM
Store at 4 °C - Tissue blocking solution
25 µg/ml anti-mouse CD16/CD32 mAb
10% horse serum
10% chicken serum
25 mM HEPES, pH 7.4
0.1% NaN3 in DMEM
Store at 4 °C - Anesthesia mix
1.4 ml ketamine (Ketaset, Zoetis, 100 mg/ml)
0.7 ml xylazine (AnaSed, NDC, Akorn, 20 mg/ml)
9 ml heparin (Elkins-Sinn, 1,000 units/ml) - DAPI stock and working solutions
- Prepare a stock of 25 mg/ml DAPI (25,000×) in ddH2O and make 10 µl aliquots
- Store at -20 °C
- For use, dilute an aliquot with ddH2O to 250 µl and use at 1 µg/ml (1,000× dilution)
Acknowledgments
The author thanks Jessica Handy for technical assistance. This study is supported in part by NIH grant U19-AI083024. An abbreviated version of the current protocol was published in Sung et al., 2017.
Competing interests
The author has no conflict of interests.
Ethics
Animal use and manipulation followed protocols approved by the University of Virginia Institutional Animal Use and Care Committee (Protocol #2483; 1995-2020). Human tissues were obtained from the U. of Virginia Biorepository Facility through an Exempt Protocol for surgical discard tissues.
References
- Almarza Novoa, E., Kasbekar, S., Thrasher, A. J., Kohn, D. B., Sevilla, J., Nguyen, T., Schwartz, J. D. and Bueren, J. A. (2018). Leukocyte adhesion deficiency-I: A comprehensive review of all published cases. J Allergy Clin Immunol Pract 6(4): 1418-1420 e1410.
- Devi, S., Li, A., Westhorpe, C. L., Lo, C. Y., Abeynaike, L. D., Snelgrove, S. L., Hall, P., Ooi, J. D., Sobey, C. G., Kitching, A. R. and Hickey, M. J. (2013). Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med 19(1): 107-112.
- Dickinson, B. L. (2016). Unraveling the immunopathogenesis of glomerular disease. Clin Immunol 169: 89-97.
- Ernandez, T. and Mayadas, T. N. (2016). The changing landscape of renal inflammation. Trends Mol Med 22(2): 151-163.
- Filippi, M. D. (2016). Mechanism of diapedesis: importance of the transcellular route. Adv Immunol 129: 25-53.
- Foster, M. H. (2016). Optimizing the translational value of animal models of glomerulonephritis: insights from recent murine prototypes. Am J Physiol Renal Physiol 311(3): F487-495.
- Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., Saran, R., Wang, A. Y. and Yang, C. W. (2013). Chronic kidney disease: global dimension and perspectives. Lancet 382(9888): 260-272.
- Lindenmeyer, M. T. and Kretzler, M. (2017). Renal biopsy-driven molecular target identification in glomerular disease. Pflugers Arch 469(7-8): 1021-1028.
- Sung, S. J., Ge, Y., Dai, C., Wang, H., Fu, S. M., Sharma, R., Hahn, Y. S., Yu, J., Le, T. H., Okusa, M. D., Bolton, W. K. and Lawler, J. R. (2017). Dependence of glomerulonephritis induction on novel intraglomerular alternatively activated bone marrow-derived macrophages and Mac-1 and PD-L1 in lupus-prone NZM2328 Mice. J Immunol 198(7): 2589-2601.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sung, S. J. (2020). Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli. Bio-protocol 10(13): e3663. DOI: 10.21769/BioProtoc.3663.
Category
Immunology > Immune cell function > Macrophage
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link