- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessing in vitro and in vivo Trogocytosis By Murine CD4+ T cells
Published: Vol 10, Iss 9, May 5, 2020 DOI: 10.21769/BioProtoc.3607 Views: 6012
Reviewed by: Andrea PuharAndrea IntroiniAndrea GramaticaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
Melissa Dolan [...] John M.L. Ebos
Aug 20, 2025 3859 Views

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1413 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1441 Views
Abstract
Recognition of antigens by lymphocytes (B, T, and NK) on the surface of an antigen-presenting cell (APC) leads to lymphocyte activation and the formation of an immunological synapse between the lymphocyte and the APC. At the immunological synapse APC membrane and associated membrane proteins can be transferred to the lymphocyte in a process called trogocytosis. The detection of trogocytosed molecules provides insights to the activation state, antigen specificity, and effector functions and differentiation of the lymphocytes. Here we outline our protocol for identifying trogocytosis-positive CD4+ T cells in vitro and in vivo. In vitro, antigen presenting cells are surface biotinylated and pre-loaded with magnetic polystyrene beads before incubating for a short time with in vitro activated CD4+ T cell blasts (90 min) or naïve T cells (3-24 h). After T cell recovery and APC depletion by magnetic separation trogocytosis positive (trog+) cells are identified by streptavidin staining of trogocytosed, biotinylated APC membrane proteins. Their activation phenotype, effector function, and effector differentiation are subsequently analyzed by flow cytometry immediately or after subsequent incubation. Similarly, trogocytosis-positive cells can be identified and similarly analyzed by flow cytometry. Previous studies have described methods for analyzing T cell trogocytosis to identify antigen-specific cells or the antigenic epitopes recognized by the cells. With the current protocol, the effects of trogocytosis on the individual T cell or the ability of trog+ T cells to modulate the activation and function of other immune cells can be assessed over an extended period of time.
Keywords: TrogocytosisBackground
Trogocytosis is the intercellular transfer of plasma membrane and membrane-associated molecules. This phenomenon has been widely studied in the analysis of immune cell interactions and has been observed to include transfer to CD4+ (Wetzel et al., 2005; Shi et al., 2006; Adamopoulou et al., 2007; Hudrisier et al., 2007; Umeshappa and Xiang, 2010; Baba et al., 2011; Osborne and Wetzel, 2012; Reed and Wetzel, 2019), CD8+ (Hudrisier et al., 2007 and 2011; Riond et al., 2007; Gary et al., 2012; Uzana et al., 2012) and γδ T cells (Espinosa et al., 2002; Gertner et al., 2007). This phenomenon is not limited to T lymphocytes as B cells (Aucher et al., 2008; Gardell and Parker, 2017), NK cells (Carlin et al., 2001; Poupot et al., 2008; Nakayama et al., 2011; Miner et al., 2015), basophils (Miyake et al., 2017), macrophages (Daubeuf et al., 2010; Sarvari et al., 2015), neutrophils (Li et al., 2016; Valgardsdottir et al., 2017; Mercer et al., 2018), and dendritic cells (Zhang et al., 2008; Bonaccorsi et al., 2014) all perform trogocytosis (Ambudkar et al., 2005; Lis et al., 2010; LeMaoult et al., 2015).
For adaptive lymphocytes, trogocytosis leads to the presence antigen-presenting cell derived molecules on the cells. This raises the possibility that these molecules may impact the biological functions of those cells. Trogocytosis-positive (trog+) CD4+ T cells can themselves act as antigen presenting cells (Xiang et al., 2005; Shi et al., 2006; LeMaoult et al., 2007; Helft et al., 2008; Ford McIntyre et al., 2008; Umeshappa and Xiang, 2010) and we have also shown that these molecules (such as peptide-loaded MHC molecules) can interact with receptors on the cell surface (such as the T cell antigen receptor) and trigger intracellular signaling (Wetzel et al., 2005; Osborne and Wetzel, 2012; Reed and Wetzel, 2019). For T cells, acquisition of APC membrane has been used as an indicator of activation (Hudrisier et al., 2005; Puaux et al., 2006) and has been used to identify peptide-specific T cells (Puaux et al., 2006; Dhainaut and Moser, 2014) and identify T cell epitopes (Li et al., 2019). Previous methods have been published to analyze trogocytosis (Puaux et al., 2006), but with the in vitro protocol outlined here (Wetzel et al., 2005; Osborne and Wetzel, 2012; Reed and Wetzel, 2019) investigators can analyze the impact that trogocytosis has on cells for up to 7 days after recovery of APC. The in vivo protocol outlined here also makes it possible to examine how trogocytosis modulates immune responses in vivo (Kennedy et al., 2005; Adamopoulou et al., 2007; Zhou et al., 2011; Romagnoli et al., 2013; Dhainaut and Moser, 2014).
Materials and Reagents
- 1 ml syringe with 5/8 inch long, 25 gauge needle
- 5 ml and 10 ml serological pipettes
- Pipet for serological pipets (such as a Drummond pipette aid)
- Sterile 15 and 50 ml conical centrifuge tubes
- Sterile 12 x 75 polystyrene snap cap culture tubes (Falcon, catalog number: 352054 )
- Sterile 12 x 75 polystyrene culture tube with cell strainer cap (Falcon, catalog number: 352235 )
- Sterile tissue culture flask (T-25 and T-75)
- Tissue culture treated 100 mm diameter polystyrene Petri plates
- Sterile, non-tissue culture treated 100 mm polystyrene Petri plates
- Autoclaved microscope slides with ground glass ends (wrap a pair in aluminum foil with a small section between the slides to facilitate separation after autoclaving)
- SpheroTM 2.22 µm Polystyrene magnetic beads (Spherotech, Green Oaks, IL, catalog number: PM-20-10 ), store at RT
- SpheroTM 3.75 µm streptavidin-coated magnetic particles (Spherotech, Green Oaks, IL, catalog number: SVM-30-10 )
- Wild type mice to serve as donors of primary murine splenocytes and bone marrow, and recipients of adoptively transferred T cells and/or antigen immunization.
- AND or 5C.C7 T cell antigen receptor transgene mice (or other TCR transgenic, if desired)
- Bone Marrow Derived Dendritic Cells (BMDC). Preparation described in Step A1
- MCC:FKPB transfected fibroblast APC (Wetzel et al., 2005)
- EZ-Link Sulfo-NHS-Biotin (Thermo Scientific, Waltham, MA, catalog number: A39256 )
- Purified antibodies to analyze T cell phenotype and trogocytosed molecules of interest such as:
- Anti-CD4 AlexaFluor 700 (clone RM4-5, BioLegend, catalog number: 100536 )
- Anti-CD69 PE-Cy7 (clone H1.2F3, BioLegend, catalog number: 104512 )
- Anti-CD80 Pacific Blue (16-10A1, BioLegend, catalog number: 104724 )
- Anti-CD86 APC (clone GL1, BioLegend, catalog number: 105012 )
- Anti-IA/IE Brilliant Violet 421 (clone M5/114.15.2, BioLegend, catalog number: 107632 )
- To stimulate T cells, purified, azide-free anti-CD3 (clone 145.2C11, BioLegend, catalog number: 100302 ) and anti-CD28 (clone 37.51, BioLegend, catalog number: 102112 )
- To detect biotin-labeled APC membrane proteins trogocytosed by T cells, fluorochrome-conjugated streptavidin (PE-Dazzle 594, BioLegend, catalog number: 405248 ). The anti-I-Ek PE (clone 17-3-3, Thermo Fisher, catalog number: OB189809 ). The exact antibodies will be determined by the experimental parameters employed.
- Zombie Aqua Fixable Viability Stain (BioLegend, catalog number: 423101 ). Store aliquots at -20 °C, shelf life ~1 year
Note: A fixable live/dead stain is critical when performing intracellular staining. - Lympholyte-M (Cedarlane Labs, catalog number: CL5035 ). Store at 4 °C in the dark, shelf life ~5 years
- Trypan blue solution (0.4% in Phosphate Buffered Saline) (Sigma, catalog number: T6416 )
- Powdered RPMI 1640 Media (without L-glutamine or NaHCO3) (Sigma, catalog number: R6504 )
- Fetal Bovine Serum (FBS) (Atlanta Biologicals, catalog number: S1550 )
- 1 mM L-glutamine (Sigma, catalog number: G6784 )
- 100 mg/ml sodium pyruvate (Sigma, catalog number: S8636 )
- 50 mM β-Mercaptoethanol (Sigma, catalog number: P7522 )
- Essential amino acids (Sigma, catalog number: M5650 )
- Nonessential amino acids (Sigma, catalog number: M7145 )
- 100 U/ml penicillin G (Sigma, catalog number: P3032 )
- 100 U/ml streptomycin (Sigma, catalog number: S9137 )
- 50 mg/ml gentamicin (Sigma, catalog number: G1264 )
- Dulbecco’s Modified Eagle’s Media (DMEM) (Sigma, catalog number: M0518 )
- Recombinant murine Granulocyte/Monocyte Colony Stimulating factor (Peprotech, catalog number: 315-03 )
- Sigma Adjuvant System (Sigma, catalog number: S6322 )
- 500 µM moth cytochrome c 88-103 (MCC88-103) antigenic peptide* solution (in PBS, pH 7.4) Genscript (Piscataway, NJ)
Note: *Choose appropriate peptide for experimental system (insuring it binds to specific MHC Class II molecule and, if used, specific transgenic T cell antigen receptor). - Albumin from chicken egg (Ovalbumin) (Sigma, catalog number: A5503 )
- EDTA (Sigma, catalog number: ED2SS )
- Bovine Serum Albumin, fraction V (Sigma, catalog number: A9647 )
- Methanol (Fischer Scientific, catalog number: A452-4 )
- Paraformaldehyde (Sigma, catalog number: P6148 )
- Saponin (EMD Millipore, catalog number: 558255 )
- NH4Cl (Mallinckrodt Baker, catalog number: 3384-12 )
- KCl (Mallinckrodt Baker, catalog number: 6858-04 )
- Na2HPO4 (Mallinckrodt Baker, catalog number: 4062-01 )
- KH2PO4 (Sigma, catalog number: P9791 )
- Glucose (Sigma, catalog number: G7021 )
- Phenol Red (Sigma, catalog number: P3532 )
- NaOH (Fischer Scientific, catalog number S318 )
- HCl (Mallinckrodt Baker, catalog number: 9535-05 )
- MgCl2·6H2O (Sigma, catalog number: M2393 )
- MgSO4·7H2O (Sigma, catalog number: M5921 )
- CaCl2 (anhydrous) (Sigma, catalog number: C1016 )
- NaHCO3 (Sigma, catalog number: S5761 )
- Glycine (Sigma, catalog number: G8790 )
- 1x Trypsin-EDTA solution (Sigma, catalog number: 59430C )
- Triton X-100 (Sigma, catalog number: T9284 )
- Gey’s solution A (see Recipes)
- Gey’s solution B (see Recipes)
- Gey’s solution C (see Recipes)
- 1x Gey’s hypotonic solution (see Recipes)
- Spinner minimum essential medium eagle with Joklik modification (S-MEM) (Sigma, catalog number: M0518 ) (see Recipes)
- Complete media supplement (see Recipes)
- Complete RPMI (cRPMI) (see Recipes)
- Complete DMEM (cDMEM) (see Recipes)
- 10x Phosphate Buffered Saline (PBS) (see Recipes)
- 1x Sterile Phosphate Buffered Saline, pH 7.4 (see Recipes)
- 1x Sterile phosphate buffered saline, pH 8.0 (see Recipes)
- Flow cytometry staining buffer (see Recipes)
- 10% Sodium azide solution (NaN3) (see Recipes)
- 4% Paraformaldehyde (see Recipes)
Equipment
- 2 L beaker
- Stem CellTM EasySepTM Magnet (Stem Cell, catalog number: 18000 )
- P10, P20, P200, and P1000 Micropipettes and sterile pipette
- Temperature controlled, humidified cell culture incubator (5% CO2 environment)
- Laminar flow class II biosafety cabinet
- Refrigerated tabletop centrifuge
- Hemacytometer and hand tally
- Inverted phase contrast microscope with 4x, 10x, and 20x objectives
- Multicolor flow cytometer (e.g., Becton Dickinson, model: FACSAria )
- Computer for offline data analysis (Apple iMac or MacMini)
- Fume hood
- Heat plate with integrated stir motor
- Stir bars
- Nitex screen, 60 µm mesh (Elko Filtering Co., catalog number 03-60/42 )
Note: Cut into 1-inch squares. Used to filter out cell clumps before flow cytometry. Can be autoclaved if necessary.
Software
- FlowJo 8.8.7 and FlowJo 10 software (Tree Star, Ashland, OR)
Procedure
- Preparation of APC (including surface biotin labeling) either the day before, or the day of, the trogocytosis assay
Note: Many different antigen presenting cell types can be used in trogocytosis assays. We predominantly use peptide-pulsed primary bone marrow derived dendritic cells (BMDC) or transfected murine fibroblasts expressing CD80, ICAM-1 and an MHC Class II molecule covalently attached to antigenic peptide via a flexible linker (Wetzel et al., 2002). BMDC are grown in cRPMI and fibroblast APC are grown in cDMEM.- To generate the BMDC, culture bone marrow cells isolated from mouse femurs and for 6 days in sterile 100 mm diameter non-tissue culture Petri dishes at 105 cells/ml in cRPMI medium containing 30 ng/ml recombinant murine granulocyte macrophage-colony stimulating factor (GM-CSF) (PeproTech, Rocky Hill, NJ) at 37 °C and 5% CO2. Add fresh culture media and GM-CSF on day 3 and harvest non-adherent cells on day 6. Activate these non-adherent cells by plating on tissue culture-coated 6-well plates with addition of Sigma adjuvant system at 125 ng/ml 18 h prior to use (Inaba et al., 1992 and 2009). Resulting adherent cells were surface biotinylated (as described in A4-A6) and exogenously loaded with antigenic peptide by addition of peptide solution (to a final concentration 2.5 µM) to BMDC culture for at least 2 h before use. Resulting adherent cell population is > 90% CD11c+ (Reed and Wetzel, 2019).
- Grow APC to 70-80% confluence in tissue-culture grade flasks or Petri dishes. Following surface biotinylation, typical recovery of live APC is ~70%.
- Remove APC from flasks/plates with 0.2% EDTA in PBS, or a cell scraper. Trypsin-EDTA can be used, but ensure that molecules of interest are not targets of enzymatic cleavage.
- Remove protein from culture media by washing cells ~1 x 106 cells/ml, wash 3 times in PBS pH 7.4. Centrifuge cells at 500 x g for 5 min in 15 ml conical tubes, decant supernatant, resuspend cell pellet and repeat for each wash. The last wash may be performed in PBS pH 8.0, or 0.1 M carbonate buffer depending on recommended buffer for labeling the specific substrate.
- Resuspend cells according to protocol provided with the NHS-LC-Biotin manufacturer (typically between 0.5 and 1 x 107 cells/ml) in PBS pH 8.0 containing 0.1 mg/ml NHS-LC-Biotin reagent. Incubate at room temperature for 15-20 min.
Note: Alternatively cells may be labeled at 4 °C to minimize internalization of biotinylated proteins, but this is generally not necessary. - Quench the reaction with 10x volume of PBS pH 7.4 containing 100 mM glycine. Centrifuge cells at 500 x g for 5 min in 15 ml conical tubes. Wash again in the same 100 mM glycine buffer for 5 min before centrifuging.
- Resuspend cells in cell culture media containing 10% FBS, and count live cells using trypan blue exclusion.
- At this point, cells can be plated and incubated overnight for use the following day, or may be used on the same day, providing enough time (~2 h) is allowed for APC to strongly adhere to surfaces. If B cells are to be used as APC, this incubation time is not necessary. We have found that a 2.5:1 T cell:APC ratio to be effective in stimulating T cells during the trogocytosis assay (4), though this number may be titrated out, and ratios between 10:1 and 1:10 T:APC may be used, if desired.
- Plating of APC: Resuspend cells at 0.25 x 106 cells/ml and plate 2 ml/well in 6-well plates (assuming doubling time of cells is ~12 h for cell line). If primary bone marrow derived dendritic cells (BMDC) are used, or the trogocytosis assay is to be performed the same day, double the concentration to 0.5 x 106 cells/ml for a final count of 106 cells/well at the time of the trogocytosis-assay. Primary BMDC are cultured in cRPMI and transfected fibroblasts are cultured in cDMEM.
- If loading cells with peptide, peptide can be added at desired concentration at the time of APC plating, or 1-2 h prior to use in the trogocytosis assay.
- To activate APC, plate BMDC on tissue-culture treated plates or Petri-dishes and add Sigma Adjuvant System or pattern recognition receptor (PRR) agonists, such as LPS, at the time of APC plating.
- Incubate cells at 37 °C in 5% CO2 until use in the trogocytosis assay.
- (Optional) If magnetic separation of phagocytic APC following the trogocytosis assay is desired, 2.22 µm magnetic polystyrene beads can be added to cultures immediately prior to plating of APC (Step A1 above) at a final concentration of ~0.01% w/v. If using Spherotech beads as listed in the materials section, a recommended volume is 5 µl/well. The concentration needed will vary between APC type, and titrations should be performed to establish optimal concentrations prior to performing trogocytosis assays. If the number is too high, it may result in APC death due to over internalization. Visualize APC using a 10x objective on an inverted phase contrast microscope prior to the trogocytosis-assay, generally 4-10 beads/APC results in efficient magnetic separation. Bead diameter is between 1/10th and 1/4th the diameter of the cells and are very easily visualized. Alternatively, magnetic beads can be added 2-6 h prior to the trogocytosis assay, however overnight incubation typically yields in better results. Mix thoroughly before plating each well, as the magnetic beads rapidly settle to the bottom of tubes.
- Preparation of T cell blasts
- Pre-coat 6-well plates with 1 ml/well of PBS pH 7.4 containing 1 or 2 µg/ml of anti-CD3 and anti-CD28. Insure the entire surface of each well is covered with buffer by gently rotating the plate. We generally use red blood cell depleted splenic cultures as T cell source, but if using cultures of purified T cells, CD28 can be added at the time of T cell plating. For optimal coating of wells, seal plates with parafilm to minimize evaporation and incubate plates at 4 °C overnight. Coating can also be done by incubating plates at 37 °C for a minimum of 2 h, if preferred.
Note: As an alternative to antibody coated wells, antibody-coated beads may be used, but we do not have experience with this technique. Soluble anti-CD3 is not recommended as it may result in anergy induction rather than activation (Smith et al., 1997). - Prior to addition of T cells to anti-CD3 + anti-CD28 coated wells, in a biosafety cabinet (tissue culture hood) remove the coating buffer and allow wells to completely dry.
- Collect spleen(s) from donor animals and generate single cell suspensions via rupturing the capsule by gently grinding between the ground glass (labeling) section of sterile microscope slides. This is done in a sterile Petri dish and cells are liberated into approximately 10 ml cRPMI. The techniques necessary to collect spleens and prepare single cell spleen cell suspensions are described in Wetzel et al., 2002 and 2005; Doherty et al., 2010; Osborne and Wetzel, 2012; Thueson et al., 2015.
- Mix splenocytes thoroughly and filter debris through a 60 µm sterile Nitex screen or filter-top tube, or allow debris to settle to the bottom of the conical tube before transferring cell suspension to a new 15 ml conical centrifuge tube. If debris is not removed, cells in culture will stick to them, which may significantly reduce activation and/or cell recovery.
- Lyse red blood cells (RBC) using Gey’s buffer. Resuspend cell pellet in 5 ml of Gey’s solution and incubate 5-10 min at room temperature or 5 min at 37 °C. Add an equal volume of cRPMI and centrifuge for 5 min at 500 x g. The resulting cell pellet should be an off-white color and may have a thin layer of red blood cells on the top. If the pellet is uniformly brick red (indicating insufficient RBC lysis), repeat this step.
- If desired, purified CD4+ T cells can be generated by negative selection of CD8+ T cells and other unwanted cell types at this time using your desired method. This is not necessary as with whole splenocyte cultures, typically > 90% of the recovered viable cells after the Ab-stimulation period are T cells.
- Adjust cell concentration to at 1.25 x 106 T cells, or 1.5 x 106 total splenocytes/ml in cRPMI.
- Add 2 ml of cell suspension to each well of the pre-coated plates.
- Incubate cells at 37 °C for 36-48 h. Check cells at 24 h and ensure the T cells are adhering to the bottom of the wells, which is consistent with activation. If T cells are floating and their cytoplasm are optically dense or crenated when viewed at 10x using an inverted microscope, the cells have not been activated and the experiment must terminated and subsequently restarted.
- At 36-48 h, gently rinse the wells with the media in the well to remove cells from 6-well plates and place into new un-coated 6-well plates, or tissue culture flask in cRPMI.
Note: If the media becomes acidic (i.e., phenol-red containing media turns yellow), add between 1/3 volume and an equal volume of fresh cRPMI to cultures. Do not add more than an equal volume of fresh media as critical cytokines such as IL-2 will be diluted out and cells will not continue to proliferate. Check media every 12-24 h until use in the trogocytosis-assay. - Ab-generated blasts can be used 2-4 days after removal from antibody containing plates. Use prior to 2 days may result in higher levels of non-antigen specific trogocytosis due to a heightened state of activation in the T cells.
- Immediately prior to use in the trogocytosis assay, remove dead cells from T cell cultures via density centrifugation using room temperature Lympholyte-M (which is optimal for mouse lymphocytes).
- Resuspend the T cells at 0.5 x 107-1 x 107 cells/ml in room temperature RPMI. To avoid cell clumping, serum-free media is recommended for this step. If cultures contain < 2.5 x 107 cells, resuspend in 5 ml. Add 5 ml of the T cell culture into a 15 ml conical tube. Using a pipette gently underlay 5 ml of Lymphocyte-M below the T cells, being careful to avoid large bubbles, which can disrupt the T cell-Lympholyte interface.
- Spin tubes containing cells underlayed with Lympholyte for 15 min at 1,000 x g. Approximately 85% of the viable lymphocytes are found at the media–Lympholyte interface (the remainder are suspended in the media layer). Dead cells and non-lymphocytes will pellet at the bottom of the tube. Carefully collect cells at the media:Lympholyte interface using a sterile glass Pasteur pipette or a P1000 micropipette and transfer to a new 15 ml conical tube. Take care to transfer a minimal amount of Lympholyte to the new tube so that the viable lymphocytes will pellet in subsequent wash steps. Centrifuge for 5 min at 500 x g. Wash cells 1 time in cRPMI.
- Resuspend cell pellet in pre-warmed cRPMI and count viable, recovered T cells.
- Adjust the concentration to 1.25 x 106 cells/ml in pre-warmed cRPMI and proceed to in vitro trogocytosis assay
- Pre-coat 6-well plates with 1 ml/well of PBS pH 7.4 containing 1 or 2 µg/ml of anti-CD3 and anti-CD28. Insure the entire surface of each well is covered with buffer by gently rotating the plate. We generally use red blood cell depleted splenic cultures as T cell source, but if using cultures of purified T cells, CD28 can be added at the time of T cell plating. For optimal coating of wells, seal plates with parafilm to minimize evaporation and incubate plates at 4 °C overnight. Coating can also be done by incubating plates at 37 °C for a minimum of 2 h, if preferred.
- Preparation of naïve T cells
- Collect and homogenize spleens, and perform RBC lysis, as above.
- APC and other unwanted cells can be removed from cultures by positive selection using biotinylated antibody cocktail (anti-MHC, anti-B220, etc.) followed by incubation with streptavidin-coated micro beads and magnetic removal labeled cells. Commercial kits may also be used at this step to obtain an enriched culture of desired (i.e., CD4+ T) cells.
- (Optional) Lympholyte-M may also be used to enrich lymphocyte populations at this step.
- Once enriched T cell cultures are obtained, resuspend cells at 1.25 x 106 cells/ml in cRPMI and proceed to the in vitro trogocytosis assay
- In vitro trogocytosis assay
- *Critical step* Remove non-adherent APC and magnetic beads from wells. If non-adherent/dead APC are present at the time of T cell addition, T cells will stick to these clumps to form large aggregates. To remove non-adherent APC, gently wash wells containing APC 2x by running pre-warmed PBS pH 7.4 from top-to-bottom of wells held at a ~45 degree angle. Carefully aspirate buffer at bottom of wells. Vigorous washing may lead to dislodging of APC.
Note: This step should be done carefully, but quickly as wells should not be allowed to fully dry. - Gently add 2 ml of resuspended T cells (1.25 x 106 cells/ml) to APC, and place immediately in a 37 °C incubator containing 5% CO2. Incubate in vitro T cell blasts with APC for 90 min. With T cell blasts we have observed T cell killing of APC by 120 min. For naïve T cells, measurable trogocytosis was first observed at 3 h and after 24 h debris from dying APC can lead to false positive trogocytosis results, so it is recommended that the T be incubated with APC between 3-24 h, depending on desired experimental design.
- Following the co-incubation, gently rinse the surface of the dish to remove non-adherent cells and collect in conical tubes. Wash cells in 10 ml cold PBS pH 7.4 for 5 min before centrifugation at 500 x g for 5 min. Discard the supernatant and pulse-vortex the cell pellet, and proceed with cell staining if cells are to be analyzed immediately. If cells are to be cultured for an extended period of time, magnetic depletion of contaminating APC (as outlined in Procedure E) must be done before establishing the cultures.
- *Critical step* Remove non-adherent APC and magnetic beads from wells. If non-adherent/dead APC are present at the time of T cell addition, T cells will stick to these clumps to form large aggregates. To remove non-adherent APC, gently wash wells containing APC 2x by running pre-warmed PBS pH 7.4 from top-to-bottom of wells held at a ~45 degree angle. Carefully aspirate buffer at bottom of wells. Vigorous washing may lead to dislodging of APC.
- APC removal from recovered T cells
- Re-suspend cells recovered from the in vitro trogocytosis assay at 106 cells/ml in PBS pH 7.4 in a 12 x 75 polystyrene culture tube.
- Using an EasySepTM magnet, remove APC containing phagocytosed magnetic beads. Incubate the tube containing the cells in magnet for 5 min. APC with magnetic beads should form a clear pellet at the magnet. Gently decant the cells suspended in PBS into a sterile 15 ml conical tube. Potential contamination of T cells with APC can quickly assessed using a microscope. If there are visible bead-containing APC present in the T cell suspension after the first magnetic separation, Step E2 must be repeated. Figure 1 shows the efficiency of this method of contaminating APC depletion from the recovered T cells.
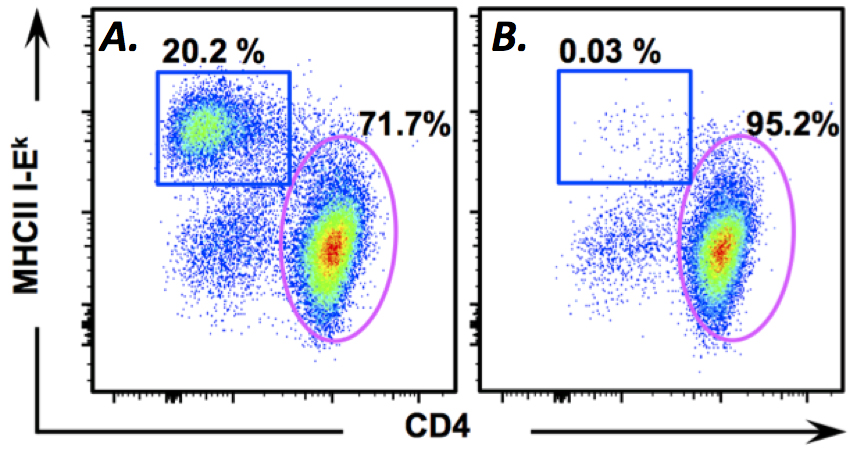
Figure 1. Magnetic depletion of contaminating APC. A. Immediately after recovery from a 90 min. in vitro trogocytosis assay approximately 20% of cells are contaminating APC. B. After magnetic depletion, contaminating APC make up less than 0.05% of cells. - A secondary APC removal step should be performed to remove any residual APC. Resuspend T cell cultures in cRPMI at 105 cells/ml, and place 3 ml/well into a fresh 6-well plate, or 9 ml into a 100 mm tissue-culture treated Petri-dish.
- Incubate for 1 h at 37 °C in the presence of 5% CO2.
- Gently swirl plates to bring non-adherent T cells into suspension, and carefully collect media without disrupting any residual APC that have bound to the surface of the plates/wells.
- Cultures should now contain < 0.5% APC, which can be assessed via flow cytometry.
- Resuspend T cells at low density (104 cells/ml) to avoid cell:cell interactions, and place 2.5 ml/well into fresh 6-well plates. If cultured T cells are observed to undergo significant proliferation, dilute cells in cRPMI to 104 cells/ml and place into fresh wells.
- Collect samples for analysis at desired time points. Typically, between 7-10 days of culture, the vast majority of cells will have died, and beyond 10 days in culture, biotinylated APC proteins may be undetectable.
- Controls
Note: Neutralizing antibodies against trogocytosed-materials can be used to inhibit trogocytosis-mediated signaling. As cells are cultured at low density, neutralizing Abs have not been found to increase Ab-mediated cell killing or complement-mediated lysis. Concentrations of 20 µg/ml have been shown to be sufficient in blocking trogocytosis-mediated signaling, however appropriate concentrations should be determined by titratration. This can be tested by adding neutralizing antibodies to APC 30 min prior to, and during an in vitro trogocytosis-assay, with a 1:5 APC:T cell ratio. Ab-concentrations that are successful in blocking signaling from APC should be expected to be sufficient in blocking trogocytosis-mediated signaling.- Set aside 2 x 106 T cells (naive or T cell blasts) before establishing 90 min in vitro trogocytosis culture. Store on ice until stained along side cells recovered from the trogocytosis assay (Steps H3-H9). These serve as unstimulated controls used to determine baseline exposure of T cell activation markers and trogocytosed molecules.
- Stimulate T cells on anti-CD3 + anti-CD28 pre-coated plates (Step B1) in parallel to the in vitro trogocytosis assay. Recover cells and stain along side the cells recovered from the trogocytosis assay. These cells serve as both a positive control for T cell activation, and a negative control for trogocytosis.
- In vivo trogocytosis
Trogocytosis occurs during T cell activation in vivo (Riond et al., 2007; Ford McIntyre et al., 2008; Rosenits et al., 2010). Trogocytosis+ cells are generated in vivo by routine immunization of wild type (WT) mice.
Note: Trogocytosed molecules have been detected on T cells up to 28 days post-immunization, however the greatest number of detectable trog+ cells will likely be within 5-10 days post-immunization.- Immunize wild type mice subcutaneously at the base of tail with 300 µg of chicken egg white albumin (OVA) (Sigma-Aldrich) in 100 µl Sigma Adjuvant System (SAS), or 100 µl SAS alone as a control using a 1 ml syringe with a 25 G needle (5/8 inch long).
- Inject the same mice 14 days later subcutaneously to provide a booster vaccination. The initial OVA-immunized mice are injected s.c. with 300 µg OVA in 100 µl of PBS, while the PBS control mice injected with 100 µl PBS alone.
- Five days after the second immunization, recover the draining inguinal lymph nodes and spleens were from each mouse and prepare single cell suspensions for flow cytometry (Steps B3 and B5).
- Stain the cells and analyze by flow cytometry as in Steps H3-H9.
Notes:- To detect trog+ cells generated in vivo, it is recommended that biotinylated antibodies against trogocytosed target molecules and streptavidin are used to enhance detection. If multiple trogocytosed molecules are to be identified, it is ideal to have the antibodies against these targets be conjugated to brighter fluorophores (i.e., PE-CF594, APC-Cy7/780, or BV421, BV711, BV786).
- Trog+ cells are identified as CD3+ CD4+ CD80/86+ I-A/E+. To confine analysis of cytokine and transcription factor expression to cells with similar activation states, CD4+ CD69High cells were gated on prior to identification of trog+ and trog– populations.
- Staining cells for analysis by flow cytometry
- In TCR transgenic systems approximately 20-40% of naïve cells and 30-40% of activated cells are trog+ following recovery from the in vitro trogocytosis assay. Depending on the time-point observed, the frequency of trog+, Ag-specific activated CD4+ T ranges from 3-20%, while < 1% of control cells (non-Ag specific) are trog+. If extended in vitro culturing of the T cells is performed following the trogocytosis assay, an aliquot of cells should be stained immediately following the trogocytosis-assay to determine the initial frequency of trog+ cells.
- At desired time-points following recovery of cells from either the in vitro or in vivo trogocytosis assay, place cells to be analyzed into 12 x 75 mm polystyrene tubes or individual cells of a round bottom, 96-well plate. Staining should be done in 50 µl of flow staining buffer per 106 cells.
- Wash cells in PBS pH 7.4 (250 µl for 96-well plate, or 0.5 ml if done in tubes) by spinning cells at 300-500 x g for 5 min. After centrifugation discard the supernatant and then gently flick or quickly vortex to loosen pelleted cells.
- Dilute live/dead stain in PBS pH 7.4 and incubate for 10 min at room temperature in the dark. During this incubation, prepare staining reagents as outlined in Step H5 below.
- Dilute desired antibodies in flow staining buffer. Staining panels should include markers on target cells of interest (such as CD4), and antibodies against trogocytosed molecules of interest that are not expressed by target population, such as CD80/CD86, ICOS-L, and MHC class II (mouse). Titrations should be done for each antibody to maximize separation between positive and negative populations. If fluorochrome conjugated streptavidin is to be used to detect the presence of biotinylated APC-derived molecules on the T cells, it should be added to the antibody staining solution.
- Wash cells 2 times as in Step H3.
- Add staining cocktail and incubate cells for 20 min on ice protected from light.
- Following the incubation, wash cells 2 times in flow staining buffer, as in Step H3.
- Resuspend cells in 300 µl of flow staining buffer. Samples should be kept at 4 °C or on ice and analyzed within 2 h. Alternately, cells can be fixed with 2-4% paraformaldehyde diluted in PBS pH 7.4 for 30-45 min washed 2 times in flow staining buffer and resuspended in approximately 300 µl of flow staining buffer overnight at 4 °C in the dark and analyzed the following day.
Note: If examining intracellular targets, surface staining should be completed before cells are treated with appropriate permeabilization reagents for desired targets, i.e., Triton X-100, saponin-containing buffers, or methanol, prior to staining. When non-permanent permeabilization is performed such as 0.1% saponin, staining for intracellular markers, and subsequent washes must include permeabilizing reagents. Following intracellular staining, proceed as in Step H8.
Data analysis
Data analysis is performed using off-line analysis software such as FlowJo. As shown in Figure 2, the identification of trog+ T cells begins with creation of gates to isolate the lymphocyte population (SSC-A vs. FSC-A) followed by rigorous doublet exclusion (FSC-W vs. FSC-H, and SSC-W vs. SSC-A). Exclude dead cells that are positive for live/dead staining, and then isolate target population of cells (i.e., CD3+ and CD4+). APC such as DC, and B cells can be further excluded at this point through additional gates, as trogocytosed molecules are typically at least one to two logs lower in fluorescence intensity compared to APC.
Trogocytosis positive (trog+) cells are identified by the presence of trogocytosed biotinylated-APC membrane protein (greater than stained, anti-CD3/CD28-stimulated, or unstimulated controls), or the presence of CD80/CD86 + MHC Class II, as shown in Figure 3.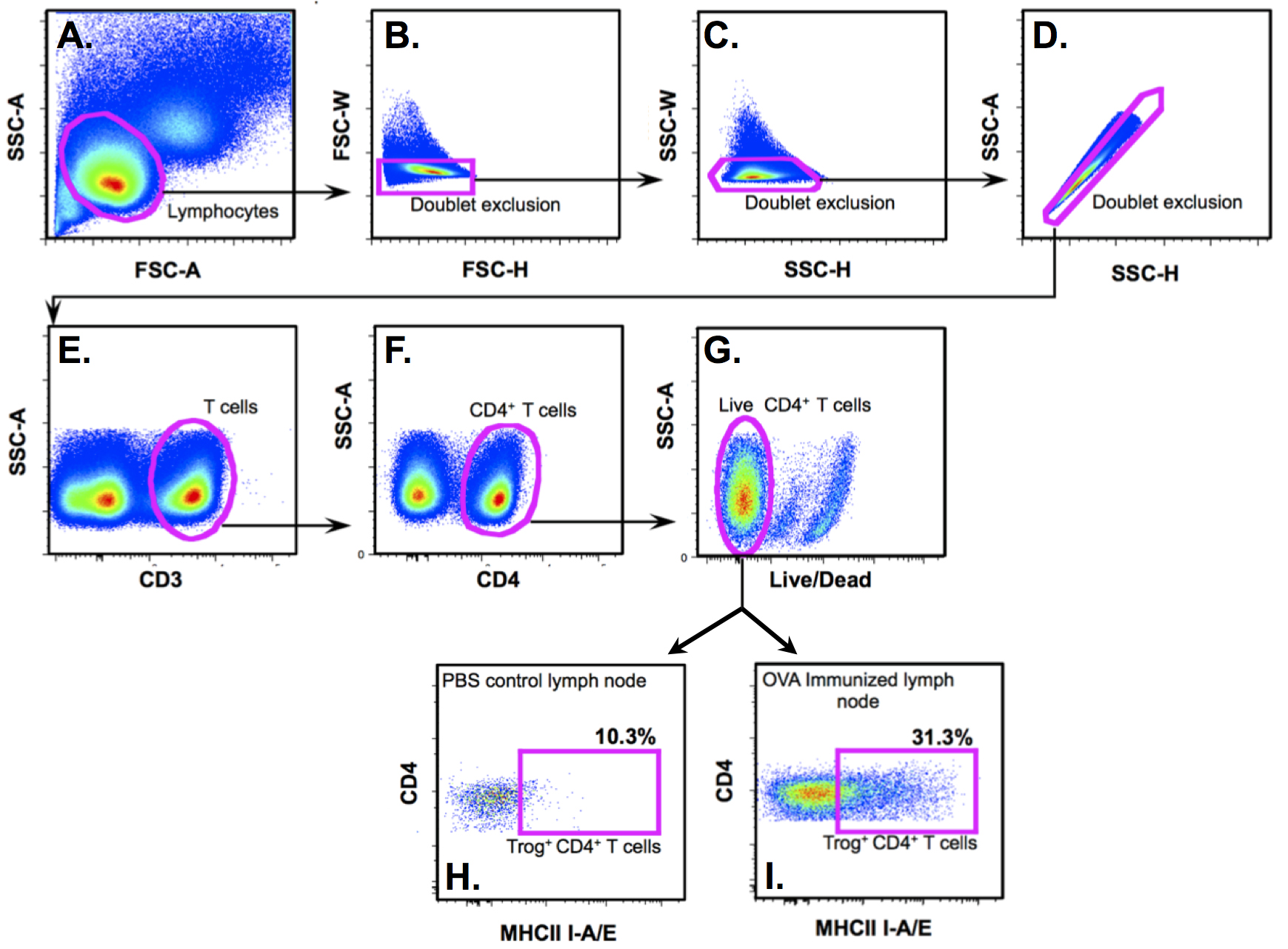
Figure 2. Gating strategy to identify trog+ cells generated in vivo. A. Lymphocyte population identified in Forward Scatter Area (FSC-A) vs. Side Scatter Area (SSC-A) plot. B-D. This if followed by rigorous doublet discrimination using SSC and FSC area and width plots. E. CD3+ singlet cells are gated followed by F. CD4+ gating. G. After staining with a live/dead indicator dye, live CD3+ CD4+ cells are then analyzed for the presence of trogocytosed MHC Class II (I-A/1-E.). H-I. Cells in the box are trogocytosis-positive CD4+ cells from antigen immunized (I) and PBS control (H) animals.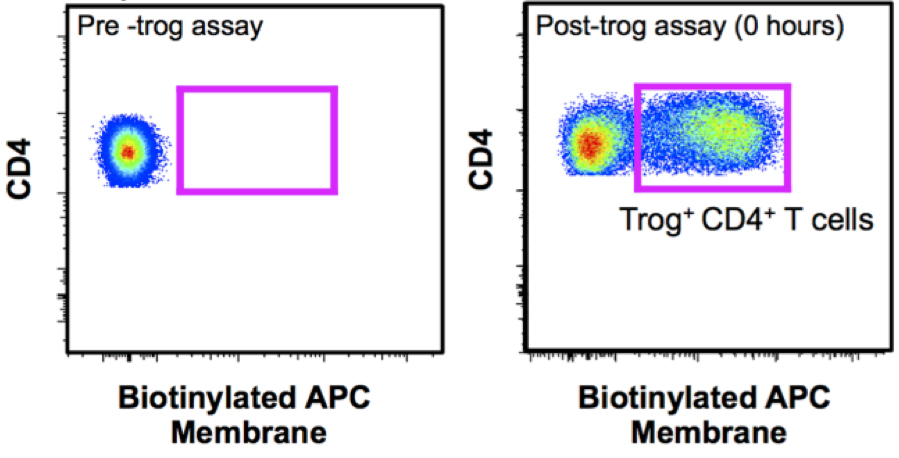
Figure 3. Measurement of biotinylated APC membrane to identify trog+ CD4+ cells. The presence of surface biotin on T cells before and immediately after T cell recovery from a 90 min in vitro trog assay.
Recipes
- Gey’s solution A
35 g NH4Cl
0.85 g KCl
0.595 g anhydrous Na2HPO4
0.12 g KH2PO4
5 g Glucose
50 mg Phenol Red
1 L Ultrapure water
Sterile filter and store at 4 °C - Gey’s solution B
1 g MgCl2·6H2O
0.35 g MgSO4·7H2O
0.85 g CaCl2 (anhydrous)
250 ml Ultrapure water
Sterile filter and store at 4 °C - Gey’s solution C
5.63 g NaHCO3
250 ml Ultrapure water
Sterile filter and store at 4 °C - 1x Gey’s hypotonic solution (RBC Lysis buffer)
200 ml Solution A
50 ml Solution B
50 ml Solution C
700 ml Ultrapure water
Sterile filter and store at 4 °C - Spinner minimum essential medium eagle with Joklik modification (S-MEM)
1 bottle MEM-Joklik
1,000 ml Ultrapure H2O
a.Dissolve 1 bottle (g) in 800 ml H2O. Stir ingredients until dissolved
b.Adjust pH to 7.0-7.1 with NaOH pellets or 10 N HCl. Add remaining water to bring total volume to 1 L
c.Sterile filter with 0.22 µm filter and store in the dark at 4 °C - Complete media supplement
630 ml S-MEM
7.5g D-Glucose
75 ml MEM Non-Essential Amino Acids
140ml MEM Essential Amino Acids
100 ml NaPyruvate
8.5 g NaHCO3
600 mg Penicillin G
500 mg gentamicin sulfate
1 g streptomycin sulfate
34 µl 50 mM β-Mercaptoethanol
a.Stir ingredients until dissolved (DO NOT USE HEAT)
b.Adjust pH to 7.0-7.1 with NaOH pellets or 10 N HCl. Add remaining water to bring total volume to 1 L
c.Sterile filter with 0.22 µm filter and store in the dark at 4 °C - Complete RPMI (cRPMI)
1 bottle Powdered RPMI 1640 (without L-glutamine or NaHCO3)
2 g NaHCO3
100 ml Fetal Bovine Serum (Atlanta Biologicals, Flowery Branch, GA)
100 ml Complete Media Supplement
10 ml 100x L-glutamine
800 ml Ultrapure water H2O
a.Add 700 ml H2O into 2 L beaker along with a clean stir bar
b.While slowly stirring water, empty bottle containing powdered media into beaker
c.Rinse the bottle with water to remove all powder and add to the beaker
d.Stir until dissolved (DO NOT USE HEAT)
e.Add NaHCO3, L-glutamine, complete media supplement and FBS
f.Adjust pH to 7.0-7.1 with NaOH pellets or 10 N HCl. Add remaining water to bring total volume to 1 L
g.Sterile filter with 0.22 µm filter and store in the dark at 4 °C - Complete DMEM (cDMEM)
1 bottle Powdered RPMI 1640 (without L-glutamine or NaHCO3)
1 g NaHCO3
100 ml Fetal Bovine Serum
100 ml Complete Media Supplement
10 ml 100x L-glutamine
800 ml Ultrapure water H2O
a.Add 700 ml H2O into 2 L beaker along with a clean stir bar
b.While slowly stirring water, empty bottle containing powdered media into beaker
c.Rinse the bottle with water to remove all powder and add to the beaker
d.Stir until dissolved (DO NOT USE HEAT)
e.Add NaHCO3, L-glutamine, complete media supplement and FBS
f.Adjust pH to 7.0-7.1 with NaOH pellets or 10 N HCl. Add remaining water to bring total volume to 1 L
g.Sterile filter with 0.22 µm filter and store in the dark at 4 °C - 10x Phosphate Buffered Saline (PBS)
400 g NaCl
57.3 g Na2HPO4
10 g KCl
10 g KH2PO4
5 L Ultrapure H2O
Dissolve in reagents in 5 L ultrapure H2O and adjust pH to 6.9 - 1x Sterile Phosphate Buffered Saline, pH 7.4
100 ml 10 PBS
900 ml Ulturapure H2O
a.Add 100 ml 10x PBS and 850 ml Ultrapure H2O to 2 L beaker
b.Stir and adjust pH to 7.0-7.1 with NaOH pellets or 10 N HCl. Add remaining water to bring total volume to 1 L
c.Sterile filter with 0.22 µm filter and store at RT or at 4 °C - 1x Sterile phosphate buffered saline, pH 8.0
100 ml 10 PBS
900 ml Ulturapure H2O
a.Add 100 ml 10x PBS and 850 ml Ultrapure H2O to 2 L beaker
b.Stir and adjust pH to 7.6-7.9 with NaOH pellets or 10N HCl. Add remaining water to bring total volume to 1 L
c.Sterile filter with 0.22 µm filter and store at RT or at 4 °C - Flow cytometry staining buffer
20 g BSA Fraction V
10 ml 10% NaN3 Solution
990 ml 1x PBS pH 7.4
a.Add ingredients together and stir to dissolve BSA
b.Once in solution, store at 4 °C protected from the dark - 10% Sodium azide solution (NaN3)
10 g NaN3
100 ml 1x PBS, pH 7.4
Dissolve NaN3 in PBS while stirring. Once in solution, transfer to a 100 ml bottle and store at room temperature - 4% Paraformaldehyde
4 g Paraformaldehyde
100 ml 1x PBS, pH 7.4
a.Add paraformaldehyde and 90 ml PBS together in a 100 ml bottle
b.In a fume hood, warm solution to approximately 50 °C while stirring to get paraformaldehyde into solution. This can take 30-60 min
c.Once the cloudy solution becomes clear, remove from heat and add remaining PBS
d.Store at 4 °C protected from light for up to 1 month
Acknowledgments
Work funded by R03AI122167 and University of Montana Small Grant program (to S.A.W). Fluorescence Cytometry and Molecular Histology and Florescence imaging core facilities used to perform studies are supported by P30RR033379. The protocols outlined here were originally described in several papers from the Wetzel lab (Wetzel et al., 2005; Osborne and Wetzel, 2012; Reed and Wetzel, 2019).
Competing interests
There are no competing interests, financial and non-financial, for the authors.
Ethics
All procedures were supervised and in accordance with the University of Montana Institutional Animal Care and Use Committee under protocol 069-17, which is active 12/17-12/20.
References
- Adamopoulou, E., Diekmann, J., Tolosa, E., Kuntz, G., Einsele, H., Rammensee, H. G. and Topp, M. S. (2007). Human CD4+ T cells displaying viral epitopes elicit a functional virus-specific memory CD8+ T cell response. J Immunol 178(9): 5465-5472.
- Ambudkar, S. V., Sauna, Z. E., Gottesman, M. M. and Szakacs, G. (2005). A novel way to spread drug resistance in tumor cells: functional intercellular transfer of P-glycoprotein (ABCB1). Trends Pharmacol Sci 26(8): 385-387.
- Aucher, A., Magdeleine, E., Joly, E. and Hudrisier, D. (2008). Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 111(12): 5621-5628.
- Baba, E., Takahashi, Y., Lichtenfeld, J., Tanaka, R., Yoshida, A., Sugamura, K., Yamamoto, N. and Tanaka, Y. (2001). Functional CD4 T cells after intercellular molecular transfer of 0X40 ligand. J Immunol 167(2): 875-883.
- Bonaccorsi, I., Morandi, B., Antsiferova, O., Costa, G., Oliveri, D., Conte, R., Pezzino, G., Vermiglio, G., Anastasi, G. P., Navarra, G., Munz, C., Di Carlo, E., Mingari, M. C. and Ferlazzo, G. (2014). Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol (2): 824-832.
- Carlin, L. M., Eleme, K., McCann, F. E. and Davis, D. M. (2001). Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med 194(10): 1507-1517.
- Daubeuf, S., Lindorfer, M. A., Taylor, R. P., Joly, E. and Hudrisier, D. (2010). The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J Immunol 184(4): 1897-1908.
- Dhainaut, M. and Moser, M. (2014). Regulation of immune reactivity by intercellular transfer. Front Immunol 5: 112.
- Doherty, M., Osborne, D. G., Browning, D. L., Parker, D. C. and Wetzel, S. A. (2010). Anergic CD4+ T cells form mature immunological synapses with enhanced accumulation of c-Cbl and Cbl-b. J Immunol 184(7): 3598-3608.
- Espinosa, E., Tabiasco, J., Hudrisier, D. and Fournie, J. J. (2002). Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J Immunol 168(12): 6336-6343.
- Ford McIntyre, M. S., Young, K. J., Gao, J., Joe, B. and Zhang, L. (2008). Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol 181(4): 2271-2275.
- Gardell, J. L. and Parker, D. C. (2017). CD40L is transferred to antigen-presenting B cells during delivery of T-cell help. Eur J Immunol 47(1): 41-50.
- Gary, R., Voelkl, S., Palmisano, R., Ullrich, E., Bosch, J. J. and Mackensen, A. (2012). Antigen-specific transfer of functional programmed death ligand 1 from human APCs onto CD8+ T cells via trogocytosis. J Immunol 188(2): 744-752.
- Gertner, J., Wiedemann, A., Poupot, M. and Fournie, J. J. (2007). Human gammadelta T lymphocytes strip and kill tumor cells simultaneously. Immunol Lett 110(1): 42-53.
- Helft, J., Jacquet, A., Joncker, N. T., Grandjean, I., Dorothee, G., Kissenpfennig, A., Malissen, B., Matzinger, P. and Lantz, O. (2008). Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood 112(4): 1249-1258.
- Hudrisier, D., Aucher, A., Puaux, A. L., Bordier, C. and Joly, E. (2007). Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol 178(6): 3637-3647.
- Hudrisier, D., Riond, J., Garidou, L., Duthoit, C. and Joly, E. (2005). T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol 35(8): 2284-2294.
- Hudrisier, D., Riond, J., Mazarguil, H., Gairin, J. E. and Joly, E. (2001). CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol 166(6): 3645-3649.
- Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S. and Steinman, R. M. (1992). Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176(6): 1693-1702.
- Inaba, K., Swiggard, W. J., Steinman, R. M., Romani, N., Schuler, G. and Brinster, C. (2009). Isolation of dendritic cells. Curr Protoc Immunol. Chapter 3: Unit 3.7.
- Kennedy, R., Undale, A. H., Kieper, W. C., Block, M. S., Pease, L. R. and Celis, E. (2005). Direct cross-priming by th lymphocytes generates memory cytotoxic responses. J Immunology 174(7): 3967-3977.
- LeMaoult, J., Caumartin, J., Daouya, M., Favier, B., Le Rond, S., Gonzalez, A. and Carosella, E. D. (2007). Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 109(5): 2040-2048.
- LeMaoult, J., Caumartin, J., Daouya, M., Switala, M., Rebmann, V., Arnulf, B. and Carosella, E. D. (2015). Trogocytic intercellular membrane exchanges among hematological tumors. J Hematol Oncol 8: 24-33.
- Li, G., Bethune, M. T., Wong, S., Joglekar, A. V., Leonard, M. T., Wang, J. K., Kim, J. T., Cheng, D., Peng, S., Zaretsky, J. M., Su, Y., Luo, Y., Heath, J. R., Ribas, A., Witte, O. N. and Baltimore, D. (2019). T cell antigen discovery via trogocytosis. Nat Methods 16(2): 183-190.
- Li, K. J., Wu, C. H., Shen, C. Y., Kuo, Y. M., Yu, C. L. and Hsieh, S. C. (2016). Membrane transfer from mononuclear cells to polymorphonuclear neutrophils transduces cell survival and activation signals in the recipient cells via anti-extrinsic apoptotic and MAP kinase signaling pathways. PLoS One 11(6): e0156262.
- Lis, R., Capdet, J., Mirshahi, P., Lacroix-Triki, M., Dagonnet, F., Klein, C., Mirshahi, M., Fournie, J. J., Rafii, A. and Poupot, M. (2010). Oncologic trogocytosis with Hospicells induces the expression of N-cadherin by breast cancer cells. Int J Oncol 37(6): 1453-1461.
- Mercer, F., Ng, S. H., Brown, T. M., Boatman, G. and Johnson, P. J. (2018). Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol 16(2): e2003885.
- Miner, C. A., Giri, T. K., Meyer, C. E., Shabsovich, M. and Tripathy, S. K. (2015). Acquisition of activation receptor ligand by trogocytosis renders NK cells hyporesponsive. J Immunol 194(4): 1945-1953.
- Miyake, K., Shiozawa, N., Nagao, T., Yoshikawa, S., Yamanishi, Y. and Karasuyama, H. (2017). Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A 114(5): 1111-1116.
- Nakayama, M., Takeda, K., Kawano, M., Takai, T., Ishii, N. and Ogasawara, K. (2011). Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci U S A 108(45): 18360-18365.
- Osborne, D. G. and Wetzel, S. A. (2012). Trogocytosis results in sustained intracellular signaling in CD4+ T cells. J Immunol 189(10): 4728-4739.
- Poupot, M., Fournie, J. J. and Poupot, R. (2008). Trogocytosis and killing of IL-4-polarized monocytes by autologous NK cells. J Leukoc Biol 84(5): 1298-1305.
- Puaux, A. L., Campanaud, J., Salles, A., Preville, X., Timmerman, B., Joly, E. and Hudrisier, D. (2006). A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. Eur J Immunol 36(3): 779-788.
- Reed, J. and Wetzel, S. A. (2019). Trogocytosis-mediated intracellular signaling in CD4+ T cells drives TH2-associated effector cytokine production and differentiation. J Immunol 202(10): 2873-2887.
- Riond, J., Elhmouzi, J., Hudrisier, D. and Gairin, J. E. (2007). Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8+ T cells. Scand J Immunol 66(4): 441-450.
- Romagnoli, P. A., Premenko-Lanier, M. F., Loria, G. D. and Altman, J. D. (2013). CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS One 8(2): e56999.
- Rosenits, K., Keppler, S. J., Vucikuja, S. and Aichele, P. (2010). T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur J Immunol 40(12): 3450-3457.
- Sarvari, A. K., Doan-Xuan, Q. M., Bacso, Z., Csomos, I., Balajthy, Z. and Fesus, L. (2015). Interaction of differentiated human adipocytes with macrophages leads to trogocytosis and selective IL-6 secretion. Cell Death Dis 6: e1613.
- Shi, M., Hao, S., Chan, T. and Xiang, J. (2006). CD4+ T cells stimulate memory CD8+ T cell expansion via acquired pMHC I complexes and costimulatory molecules, and IL-2 secretion. J Leukoc Biol 80(6): 1354-1363.
- Smith, J. A., Tso, J. Y., Clark, M. R., Cole, M. S. and Bluestone, J. A. (1997). Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J Exp Med 185(8): 1413-1422.
- Thueson, L. E., Emmons, T. R., Browning, D. L., Kreitinger, J. M., Shepherd, D. M. and Wetzel, S. A. (2015). In vitro exposure to the herbicide atrazine inhibits T cell activation, proliferation, and cytokine production and significantly increases the frequency of Foxp3+ regulatory T cells. Toxicol Sci 143(2): 418-429.
- Umeshappa, C. S. and Xiang, J. (2010). Tumor-derived HLA-G1 acquisition by monocytes through trogocytosis: possible functional consequences. Cell Mol Life Sci 67(23): 4107-4108.
- Uzana, R., Eisenberg, G., Sagi, Y., Frankenburg, S., Merims, S., Amariglio, N., Yefenof, E., Peretz, T., Machlenkin, A. and Lotem, M. (2012). Trogocytosis is a gateway to characterize functional diversity in melanoma-specific CD8+ T cell clones. J Immunol 188(2): 632-640.
- Valgardsdottir, R., Cattaneo, I., Klein, C., Introna, M., Figliuzzi, M. and Golay, J. (2017). Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 129(19): 2636-2644.
- Wetzel, S. A., McKeithan, T. W. and Parker, D. C. (2002). Live-cell dynamics and the role of costimulation in immunological synapse formation. J Immunol 169(11): 6092-6101.
- Wetzel, S. A., McKeithan, T. W. and Parker, D. C. (2005). Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol 174(1): 80-89.
- Xiang, J., Huang, H. and Liu, Y. (2005). A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol 174(12): 7497-7505.
- Zhang, Q. J., Li, X. L., Wang, D., Huang, X. C., Mathis, J. M., Duan, W. M., Knight, D., Shi, R., Glass, J., Zhang, D. Q., Eisenbach, L. and Jefferies, W. A. (2008). Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS One 3(8): e3097.
- Zhou, G., Ding, Z. C., Fu, J. and Levitsky, H. I. (2011). Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response. J Immunol 186(4): 2148-2155.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Reed, J. and Wetzel, S. A. (2020). Assessing in vitro and in vivo Trogocytosis By Murine CD4+ T cells. Bio-protocol 10(9): e3607. DOI: 10.21769/BioProtoc.3607.
Category
Immunology > Immune cell function > Antigen-specific response
Immunology > Immune cell staining > Flow cytometry
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










