- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measuring cAMP Specific Phosphodiesterase Activity: A Two-step Radioassay
(*contributed equally to this work) Published: Vol 10, Iss 7, Apr 5, 2020 DOI: 10.21769/BioProtoc.3581 Views: 3799
Reviewed by: Antoine de MorreeSaswata Sankar SarkarZijian Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In-Gel Activity Assay of Mammalian Mitochondrial and Cytosolic Aconitases, Surrogate Markers of Compartment-Specific Oxidative Stress and Iron Status
Wing-Hang Tong and Tracey A. Rouault
Dec 5, 2024 2200 Views

Assessment of SREBP Activation Using a Microsomal Vesicle Budding Assay
Mingfeng Xia [...] Shunxing Rong
Dec 20, 2024 1542 Views

Ub-POD: A Ubiquitin-Specific Proximity-Dependent Labeling Technique to Identify E3 Ubiquitin Ligase Substrates in Human Cells
Urbi Mukhopadhyay [...] Sagar Bhogaraju
Jun 20, 2025 2435 Views
Abstract
Cyclic nucleotide degrading phosphodiesterase (PDE) enzymes are crucial to the fine tuning of cAMP signaling responses, playing a pivotal role in regulating the temporal and spatial characteristics of discrete cAMP nanodomains and hence the activity of cAMP-effector proteins. As a consequence of orchestrating cAMP homeostasis, dysfunctional PDE activity plays a central role in disease pathogenesis. This highlights the need for developing methods that can be used to further understand PDE function and assess the effectiveness of potentially novel PDE therapeutics. Here we describe such an approach, where PDE activity is indirectly measured through the direct quantification of radioactively tagged cAMP (pmol/min-1/mg-1). This method provides a highly sensitive tool for investigating PDE functionality.
Keywords: PDEBackground
Cyclic 3’,5’-adenosine monophosphate (cAMP) is a ubiquitously expressed second messenger, synthesised by adenylate cyclase enzymes (ACs), that acts as a master regulator of a broad spectrum of intracellular signalling pathways (Hayes and Brunton, 1982; Beavo and Brunton, 2002). Homeostatic regulation of cAMP is fine-tuned by phosphodiesterase enzymes (PDEs), rapidly degrading cAMP into 5’AMP (Baillie, 2009). PDEs consist of 11 families (PDE1-11) spanning over 100 isoenzymes (e.g., PDE4A1-PDE4A5), all with the ability to rapidly hydrolyse cyclic nucleotides (Conti and Beavo, 2007). Of these, PDE1-4, 7, 8, 10 and 11 possess the ability to catalyse the hydrolysis of cAMP. Compartmentalisation of specific PDE isoforms within discrete signalosomes is crucial to the spatio-temporal control of cAMP nanodomains, tightly regulating cAMP-effector protein activity (Blair and Baillie, 2019). In light of this, it is no surprise that aberrant PDE activity plays a central role in disease pathogenesis, with many PDEs being identified as a driving force in cardiovascular disease (Bobin et al., 2016), respiratory disease (Zuo et al., 2019), neurological disease (Knott et al., 2017) and cancers (Peng et al., 2018). Thus, research and development into novel and effective therapies targeting PDE activity remains a highly competitive area in drug discovery (Maurice et al., 2014; Baillie et al., 2019). This demonstrates a clear need for the development of assays with the ability to measure PDE activity, not only as a tool to investigate PDE activity/function in cellular signaling, but as a means of assessing and developing therapies targeting PDE activity.
Marchmont and Houslay (1980) previously described a method of measuring PDE activity, modified from a two-step procedure developed by Thompson and Appleman (1971). This radioassay directly quantifies the formation of radioactively tagged 8-[3H] adenosine, formed as a result of cAMP hydrolysis by PDE within a given sample, allowing for the rate of cAMP hydrolysis to be determined. This highly sensitive assay has proven to be an invaluable tool in the understanding of unique PDE functionality and in assessing the effectiveness of novel PDE therapies (example studies utilising this method: Schafer et al., 2010; Moretto et al., 2015; Omar et al., 2019; Houslay et al., 2019). Here we present a straight-forward and effective procedure for indirectly measuring the activity of cAMP hydrolysing PDEs (Figure 1).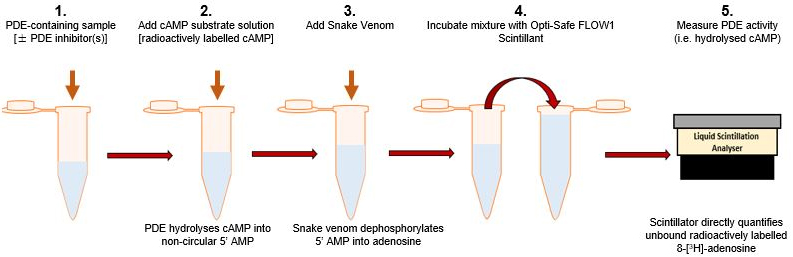
Figure 1. Simple schematic representing Phosphodiesterase Assay procedure
Materials and Reagents
- Eppendorf® tubes (1.5 ml, clear tubes) (Greiner Bio-one, catalog number: 616-201)
- Magnetic stir plate
- pH paper
- Tris Base (20 mM, pH 7.4 with HCl) (Fisher Scientific, catalog number: 77-86-1 )
- MgCl2 (VWR, catalog number: 25108.260)
- Dowex (Sigma-Aldrich, catalog number: 44340 )
- Absolute Ethanol (VWR, catalog number: 20821.33 0)
- Deionized H2O (Milli-Q Pure)
- Adenosine 3’5’-cyclic monophosphate, sodium salt (Sigma-Aldrich, catalog number: A6885 , storage -20 °C)
- 8-[3H]-cAMP substrate (Amersham Biosciences, catalog number: TRK304-1mCi, storage -20 °C)
- Snake venom (Opiophagus Hannah) (Sigma, catalog number: V0376 , storage -20 °C)
- Opti-Flow SAFE 1 scintillant (Fisher Scientific (chemicals), catalog number: SC/9255/21, storage: room temperature)
- NaOH (Fisher Scientific, catalog number: 4920/53)
- HCl (Fisher Scientific, catalog number: H/1200/PB17)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- Dowex Anion Exchange Resin (see Recipes)
- Snake Venom (see Recipes)
- cAMP Substrate Solution (see Recipes)
Equipment
- TRI-CARB 2900TR Liquid Scintillation Analyzer (Packard) (Figure 2)
More up-to-date models exist (e.g., TRI-CARB 4910TR 110V Liquid Scintillation Counter, Packard)
Figure 2. TRI-CARB 2900TR Liquid Scintillation Analyzer (Packard)
Software
- Microsoft Excel
Procedure
- Activating Dowex 1x8-400 Anion Exchange Resin.
Prepare large batches of Dowex resin:- Dissolve 400 g Dowex resin in 4 L 1 M NaOH.
- Mix solution at room temperature for 15 min.
Note: To mix, a magnetic stir plate was used. - Allow resin to settle by gravity and remove supernatant.
- Wash resin with 4 L of deionized H2O until pH 7.0, using pH paper to measure.
Note: This may take up to 30 x washes. - At pH 7.0, allow resin to settle and remove supernatant.
- Suspend resin in 4 L 1 M HCl and mix for 15 min at room temperature.
- Allow resin to settle and remove supernatant.
- Finally, wash resin in 4 L of deionized H2O 3x until pH 3.0, using pH paper to measure.
- Stop mixing of solution after each wash to allow resin to settle before carefully removing deionized H2O and refilling with fresh deionized H2O.
- Store resin in deionized H2O at 4 °C.
- Add ethanol and deionized H2O to resin at a 1:1:1 ratio (dowex:ethanol:dH2O) prior to use in assay.
- Label triplicate 1.5 ml Eppendorf® tubes per sample for assay (i.e., three technical replicates), including blanks. Incubate on ice.
- Thaw all stock solutions being used.
- Heat water-bath to 30 °C.
- Prepare sample(s) to be assayed.
- Incubate appropriate volume/concentration of PDE containing sample in 50 µl Buffer A (Recipe 1).
Note: Pilot studies should be carried out to assess optimal concentration(s) of PDE containing sample being used. Typical concentration range of purified protein / PDE overexpressing cellular lysates: 1-10 μg/μl in a total volume of 50 μl Buffer A. - If investigating effect of inhibitor(s), make sample up in 40 µl Buffer A and 10 µl of appropriately diluted inhibitor.
- Add 50 µl of Buffer A only for blanks.
- Incubate appropriate volume/concentration of PDE containing sample in 50 µl Buffer A (Recipe 1).
- Add 50 µl of cAMP substrate solution (Recipe 5) to each PDE containing sample, including blanks, and vortex. Radioactively tagged cAMP in mix will be hydrolyzed to 5’-AMP in samples containing PDEs.
- Incubate samples in a water bath at 30 °C for 10 min.
- To terminate reaction (i.e., inactivate PDEs in sample), boil samples at 100 °C for 2 min.
- Cool samples on ice for 15 min.
- Add 1 mg/ml snake venom to each sample (i.e., 0.2 mg/ml final concentration), including blanks, and mix.
Note: Snake venom dephosphorylates the 5’-AMP to adenosine, preventing 5’-AMP from recircularising into cAMP. - Incubate samples in a water bath at 30 °C for a further 10 min.
- Add 400 µl Dowex:ethanol:dH2O mix (aka Dowex anion exchange resin) to each sample and vortex. Ensure Dowex anion exchange resin is thoroughly suspended before adding to sample(s). Dowex anion exchange resin separates negatively charged cAMP from uncharged adenosine.
- Cool samples on ice for a further 15 min.
- Vortex samples thoroughly and centrifuge at 10,000 x g for 3 min at 4 °C in a refrigerated bench-top centrifuge.
- Concurrently, add 1 ml of Opti-Flow SAFE 1 scintillant to freshly labeled 1.5 ml Eppendorf® tubes.
- Remove 150 µl of clear supernatant from PDE containing reaction samples (see Step 14) and add to a appropriate scintillant tube containing 1 ml of Opti-Flow SAFE 1 scintillation fluid.
- Vortex all samples thoroughly.
- Using a TRI-CARB 2900TR Liquid Scintillation Analyzer (Figure 2), measure/count unbound 8-[3H]-adenosine. This determines the rate of cAMP hydrolysis and thus PDE activity.
- Analyse data (i.e., determine PDE activity. See Tables 1 and 2 for example set up/data analyses.)
Table 1. Example experimental set up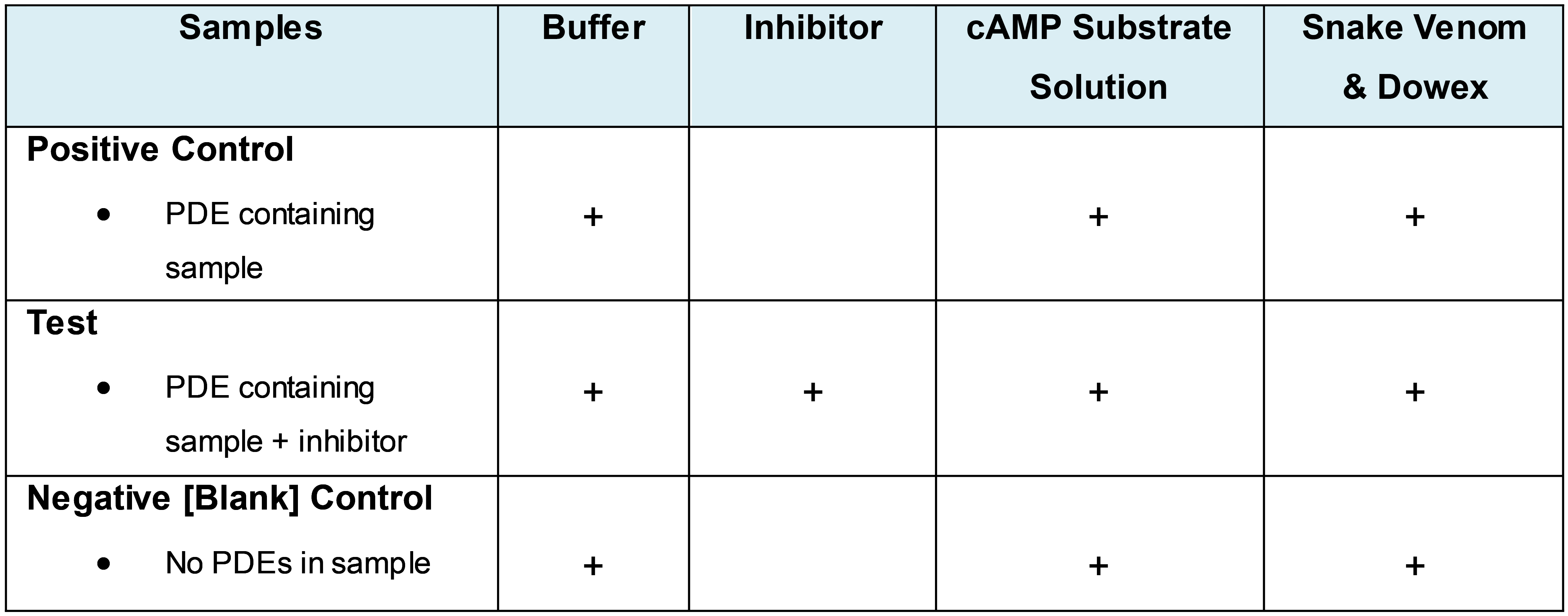
Data analysis
- Analysing scintillation counter results (see Table 2)
(Example results: Omar et al., 2019)- Correct each sample to the background appropriately using blank reaction samples containing no PDEs.
- Use background corrected counts to determine the initial rate of reaction (i.e., cAMP hydrolyzed/min).
- To express results in cAMP hydrolyzed in pM/min-1/mg-1 protein, pre-determine protein concentration of samples used in assay through a chosen standard method (e.g., Bradford assay, BCA assay, etc.).
- Thus, use the following formula to determine the activity of a specific PDE within sample:
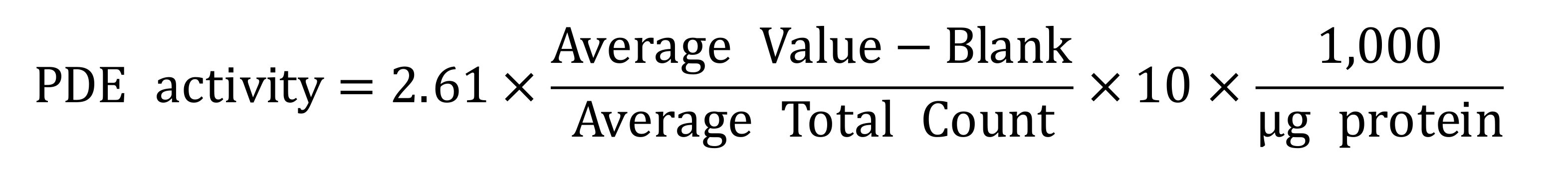
•PDE activity = cAMP hydrolyzed in pM/min-1/mg-1
•2.61 stands for the Dowex practical concentration
•10 represents the concentration of cAMP (in pM) hydrolyzed in 100 µl in 10 min - To determine the effects of a selected PDE inhibitor, directly compare uninhibited control reactions to those that were inhibited and expressed as a percentage of the uninhibited control.
Table 2. Example Data Set. PDE containing samples contained 5 μg total purified PDE protein in 100 μl sample.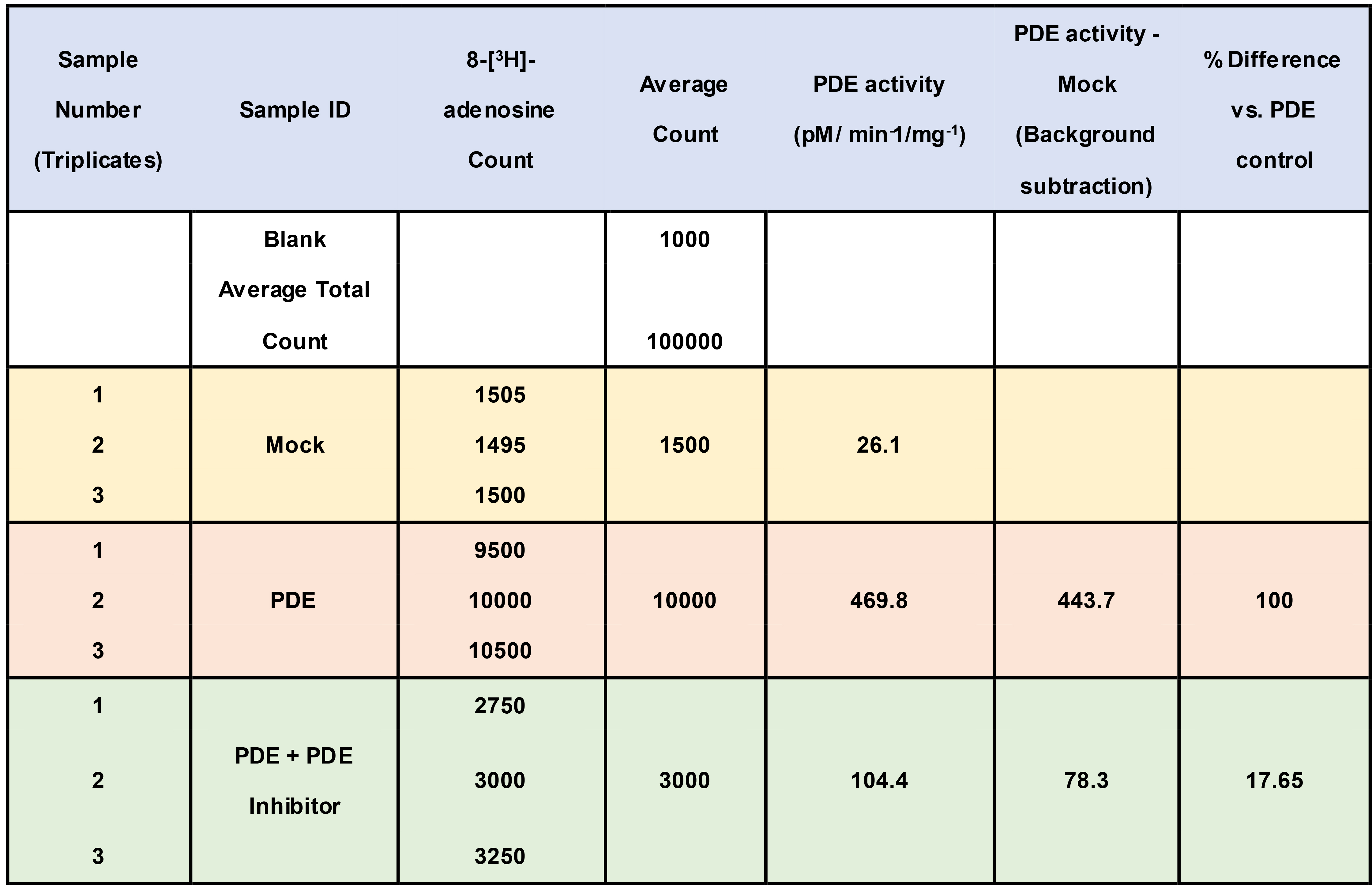
Notes
- 3H1 (Tritium)
Half Life: 12.3 Years
Specific activity: 3.59E + 14 Bq.g-1
Recipes
- Buffer A
20 mM Tris-HCl (pH 7.4)
Store at 4 °C - Buffer B
20 mM Tris-HCl (pH 7.4)
10 mM MgCl2
Store at 4 °C - Dowex anion exchange resin
Dowex:ethanol:deionized H2O [1:1:1 ratio]
Store at 4 °C - Snake venom (10mg/ml stock; derived from Ophiophagus Hannah)
Dilute to 1 mg/ml in Buffer A
Store at -20 °C - cAMP substrate solution
2 µl ‘cold’ cAMP (i.e., unlabeled) [1 mM cAMP]
3 µl ‘hot’ cAMP (i.e., 8-[3H]-labeled) [1 µCi/µl]
995 µl Buffer B
Store at -20 °C
Acknowledgments
Connor Blair is funded by an Industrial PhD studentship that is supported by Portage Glasgow Ltd and the College of Medical, Veterinary and Life Sciences, University of Glasgow.
Competing interests
The authors have no competing interests.
References
- Baillie, G. S. (2009). Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276(7): 1790-1799.
- Baillie, G. S., Tejeda, G. S. and Kelly, M. P. (2019). Therapeutic targeting of 3',5'-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discov 18(10): 770-796.
- Beavo, J. A. and Brunton, L. L. (2002). Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol 3(9): 710-718.
- Blair, C. M. and Baillie, G. S. (2019). Reshaping cAMP nanodomains through targeted disruption of compartmentalised phosphodiesterase signalosomes. Biochem Soc Trans 47(5): 1405-1414.
- Bobin, P., Belacel-Ouari, M., Bedioune, I., Zhang, L., Leroy, J., Fischmeister, R. and Vandecasteele, G. (2016). Cyclic nucleotide phosphodiesterases in hear and vessels: A therapeutic perspective. Arch Cardiovasc Dis 109(6-7): 431-443.
- Conti, M. and Beavo, J. (2007). Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481-511.
- Hayes, J. S. and Brunton, L. L. (1982). Functional compartments in cyclic nucleotide action. J Cyclic Nucleotide Res 8(1): 1-16.
- Houslay, K. F., Fertig, B. A., Christian, F., Tibbo, A. J., Ling, J., Findlay, J. E., Houslay, M. D. and Baillie, G. S. (2019). Phosphorylation of PDE4A5 by MAPKAPK2 attenuates fibrin degradation via p75 signalling. J Biochem 166(1): 97-106.
- Knott, E. P., Assi, M., Rao, S. N. R., Ghosh, M. and Pearse, D. D. (2017). Phosphodiesterase Inhibitors as a Therapeutic Approach to Neuroprotection and Repair. Int J Mol Sci 18(4): E696
- Marchmont, R. J. and Houslay, M. D. (1980). A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J 187(2): 381-392.
- Maurice, D. H., Ke, H., Ahmad, F., Wang, Y., Chung, J. and Manganiello, V. C. (2014). Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13(4): 290-314.
- Moretto, N., Caruso, P., Bosco, R., Marchini, G., Pastore, F., Armani, E., Amari, G., Rizzi, A., Ghidini, E., De Fanti, R., Capaldi, C., Carzaniga, L., Hirsch, E., Buccellati, C., Sala, A., Carnini, C., Patacchini, R., Delcanale, M., Civelli, M., Villetti, G. and Facchinetti, F. (2015). CHF6001 I: a novel highly potent and selective phosphodiesterase 4 inhibitor with robust anti-inflammatory activity and suitable for topical pulmonary administration. J Pharmacol Exp Ther 352(3): 559-567.
- Omar, F., Findlay, J. E., Carfray, G., Allcock, R. W., Jiang, Z., Moore, C., Muir, A. L., Lannoy, M., Fertig, B. A., Mai, D., Day, J. P., Bolger, G., Baillie, G. S., Schwiebert, E., Klussmann, E., Pyne, N. J., Ong, A. C. M., Bowers, K., Adam, J. M., Adams, D. R., Houslay, M. D. and Henderson, D. J. P. (2019). Small-molecule allosteric activators of PDE4 long form cyclic AMP phosphodiesterases. Proc Natl Acad Sci U S A 116(27): 13320-13329.
- Peng, T., Gong, J., Jin, Y., Zhou, Y., Tong, R., Wei, X., Bai, L. and Shi, J. (2018). Inhibitors of phosphodiesterase as cancer therapeutics. Eur J Med Chem 150: 742-756.
- Schafer, P. H., Parton, A., Gandhi, A. K., Capone, L., Adams, M., Wu, L., Bartlett, J. B., Loveland, M. A., Gilhar, A., Cheung, Y. F., Baillie, G. S., Houslay, M. D., Man, H. W., Muller, G. W. and Stirling, D. I. (2010). Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 159(4): 842-855.
- Thompson, W. J. and Appleman, M. M. (1971). Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry 10(2): 311-316.
- Zuo, H., Cattani-Cavalieri, I., Musheshe, N., Nikolaev, V. O. and Schmidt, M. (2019). Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol Ther 197: 225-242.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Blair, C. M., Ling, J. and Baillie, G. S. (2020). Measuring cAMP Specific Phosphodiesterase Activity: A Two-step Radioassay. Bio-protocol 10(7): e3581. DOI: 10.21769/BioProtoc.3581.
Category
Molecular Biology > Protein > Targeted degradation
Biochemistry > Protein > Degradation
Molecular Biology > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









