- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fractionation of Ovarian Follicles and in vitro Oocyte Maturation and Ovulation Assay of Ciona intestinalis Type A
Published: Vol 10, Iss 7, Apr 5, 2020 DOI: 10.21769/BioProtoc.3577 Views: 4673
Reviewed by: Gal HaimovichDemosthenis ChronisAyush Ranawade

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2411 Views

An Ex Vivo Lung Histoculture Model for Studying Pulmonary Infection and Immune Response With SARS-CoV-2 as an Example of RNA Virus
Elena V. Maryukhnich [...] Elena J. Vasilieva
Dec 20, 2025 740 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 211 Views
Abstract
Ascidians are the closest living relatives of vertebrates (Delsuc et al., 2006; Satoh et al., 2014) and are important for the evolutionary study of the ovarian follicle development including oocyte maturation and ovulation. However, neither the endogenous factors nor the molecular mechanisms underlying the oocyte maturation and ovulation had been elucidated mainly due to the lack of efficient procedure for isolating ovarian follicles. Here, we present the protocol for the effective fractionation and isolation of the ovarian follicle of Ciona intestinalis type A using stainless steel sieves with various particle size-meshes, and the simple incubation method of Ciona follicles for evaluating oocyte maturation and ovulation. Combined with the RNA-seq data from each fraction, the current methods lead us to investigate ovarian follicle development including oocyte maturation and ovulation in a stage-specific manner.
Keywords: Ciona intestinalis Type ABackground
Ciona ovarian follicles are classified into four major developmental stages: stage I (pre-vitellogenic), stage II (vitellogenic), stage III (post-vitellogenic, pre-GVBD), and stage IV (post-GVBD, ovulated) follicles (Figure 1; Prodon et al., 2006; Silvestre et al., 2009). The ovary of adult ascidians contains numerous follicles at entire developmental stages, and thus, isolation of individual follicles at specific stage is time-consuming and troublesome. This hindered functional analyses in follicular development and suggests requirement for efficient method of follicle isolation. Here we provide an effective fractionation method that a mixture of every developmental stage of follicles can be fractionated using a series of stainless steel sieves with various particle-size meshes (20-150 μm). The current fractionation method enables us to isolate the entire developmental stages of follicles easily and quickly. Moreover, oocyte maturation and ovulation can be evaluated by germinal vesicle breakdown (i.e. GVBD) and rupture of follicular cell layer, respectively (Figure 1). Therefore, a simple incubation method of immature/pre-ovulatory follicle is useful for elucidating the endogenous regulator and the underlying molecular mechanisms oocyte maturation and ovulation as in Matsubara et al., 2019. Furthermore, transcriptomic data of the entire developmental stages of Ciona follicles, combined with fractionation of respective developmental-stage follicles, lead to the comprehensive understanding of not only oocyte maturation and ovulation but also the entire developmental mechanisms of Ciona follicles. The present fractionation and culture systems are also expected to be applicable to follicles of other aquatic animals including jellyfish, starfish, sea urchin and fish, and will contribute a great deal to advances in the study on oocyte maturation and ovulation in various organisms.
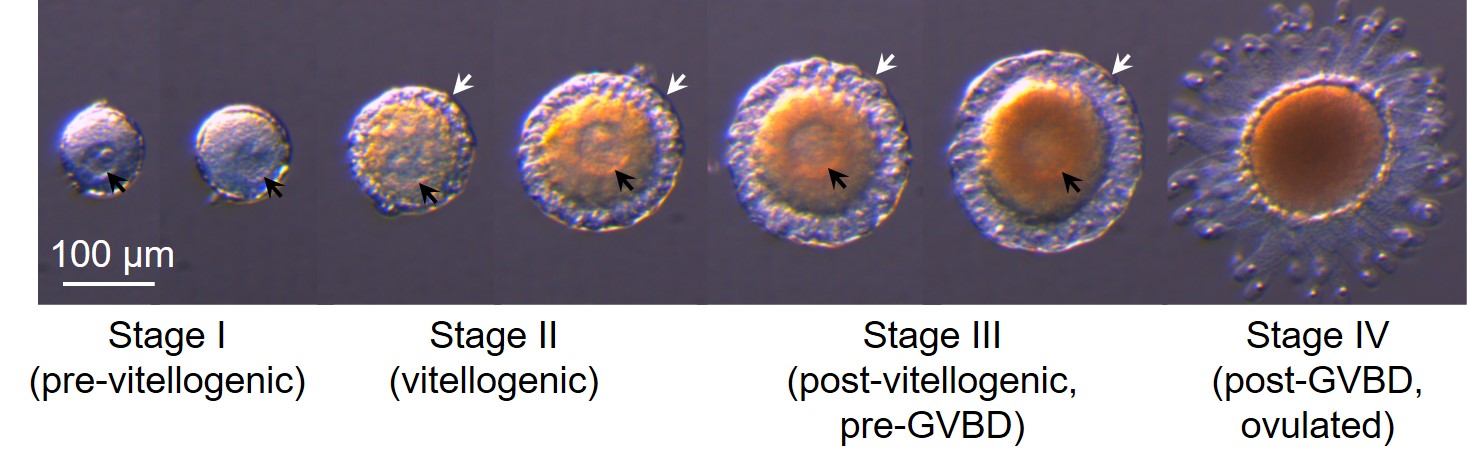
Figure 1. Developmental stages of Ciona ovarian follicles. Immature/pre-ovulatory (stage I-III) follicles show a germinal vesicle (GV, black arrow) and are enveloped by follicular cells and outer follicular cell (OFc) layer (white arrow). Oocytes mature via GV breakdown (GVBD) and ovulate via rupture of OFc layer. The ovary of adult ascidians contains numerous follicles of the entire developmental stages.
Materials and Reagents
- 50-ml tube (IWAKI AGC TECHNO GLASS, catalog number: 2345-050)
- 1.5-ml tube (Eppendorf, catalog number: 0030125215)
- 60-mm dish (Corning, catalog number: 353002 for follicles before fractionation or Greiner Bio-One, catalog number: 628979 for follicles after fractionation)
- 35-mm dish (IWAKI AGC TECHNO GLASS, catalog number: 1000-035)
- Pipette tips:
1,000-μl tips (Thermo Scientific, catalog number: H-111-R100NS-Q )
10-μl tips (Greiner Bio-One, catalog number: 771251) - 1-L filter (Corning, catalog number: 431098 )
- 1-L storage bottle (Corning, catalog number: 430518)
- 50-ml pipette (AS ONE, catalog number: 2-4132-06)
- Adult ascidians (Ciona intestinalis Type A) cultivated in Maizuru Fisheries Research Station of Kyoto University or Misaki Marine Biological Station of the University of Tokyo
- Positive factors of oocyte maturation and ovulation (e.g., Synthetic Ciona vasopressin (CiVP) peptide (Sequence: CFFRDCSNMDWYR, disulfide bridge: 1-6, Kawada et al., 2008)
- Negative factors of oocyte maturation and ovulation, e.g.,
U0126 (Promega, catalog number: V1121 )
Ro-3306 (Abcam, catalog number: ab141491)
MMP-2/MMP-9 inhibitor II (Calbiochem, catalog number: 444249) - DMSO (Nacalai tesque, catalog number: 13407-45)
- REI-SEA marine powder (REI-SEA IWAKI, catalog number: 274513)
- Artificial sea water (ASW) (see Recipes)
- 1 mM CiVP (see Recipes)
- 10 mM U0126 (see Recipes)
- 10 mM Ro-3306 (see Recipes)
- 10 mM MMP-2/MMP-9 inhibitor II (see Recipes)
Equipment
- 1-L beaker (IWAKI AGC TECHNO GLASS, catalog number: 0988-01-01-66)
- Water tank (width 75 cm, depth 45 cm, height 45 cm)
- Water pump (REI-SEA IWAKI, model: RMD-151, maximum flow rate: 19 L/min)
- Air pump (TECHNO TAKATSUKI, model: HP-30, maximum flow rate: 30 L/min)
- Portable pipet controller (Drummond, catalog number: 4-040-101-J )
- Forceps (Rubis, catalog numbers: 2-5149-08, 2-5149-12)
- Scissors (AS ONE, catalog number: 2-539-11)
- Stainless steel sieves (TOKYO SCREEN Co., LTD., catalog numbers: TS-50-20-39 (Aperture: 180 μm); TS-50-20-41 (Aperture: 150 μm); TS-50-20-45 (Aperture: 90 μm); TS-50-20-47 (Aperture: 63 μm); TS-50-20-50 (Aperture: 38 μm); TS-50-20-53 (Aperture: 20 μm), https://www.tokyo-screen.com/.en/)
- Stereo microscopes (ZEISS, model: SteREO Discovery V8 for dissecting of the ovary and isolating follicles; Leica Microsystems, model: Leica M205 FA equipped with Leica DFC 310 FX camera for recording follicles before and after incubation)
- Microscale slide (WATSON Bio Lab, catalog number: 177-401C)
Software
- Leica Aplication Suite Advanced Fluorescence (LAS-AF)
- Fiji software (http://fiji.sc/; Schindelin et al., 2012)
- Microsoft Excel (Microsoft Corporation)
Procedure
- Isolation of Ciona follicles from the ovary
- Keep the adult ascidians (20-60 individuals) in a water tank with constant lighting, ASW circulating (19 L/min), and air bubbling (moderate level) at 18 °C for four or five days.
- Isolate the ovaries as in Figure 2 from 6-8 ascidians and collect them into a 50-ml tube with ASW.
Note: All the procedures should be performed at room temperature (20 °C).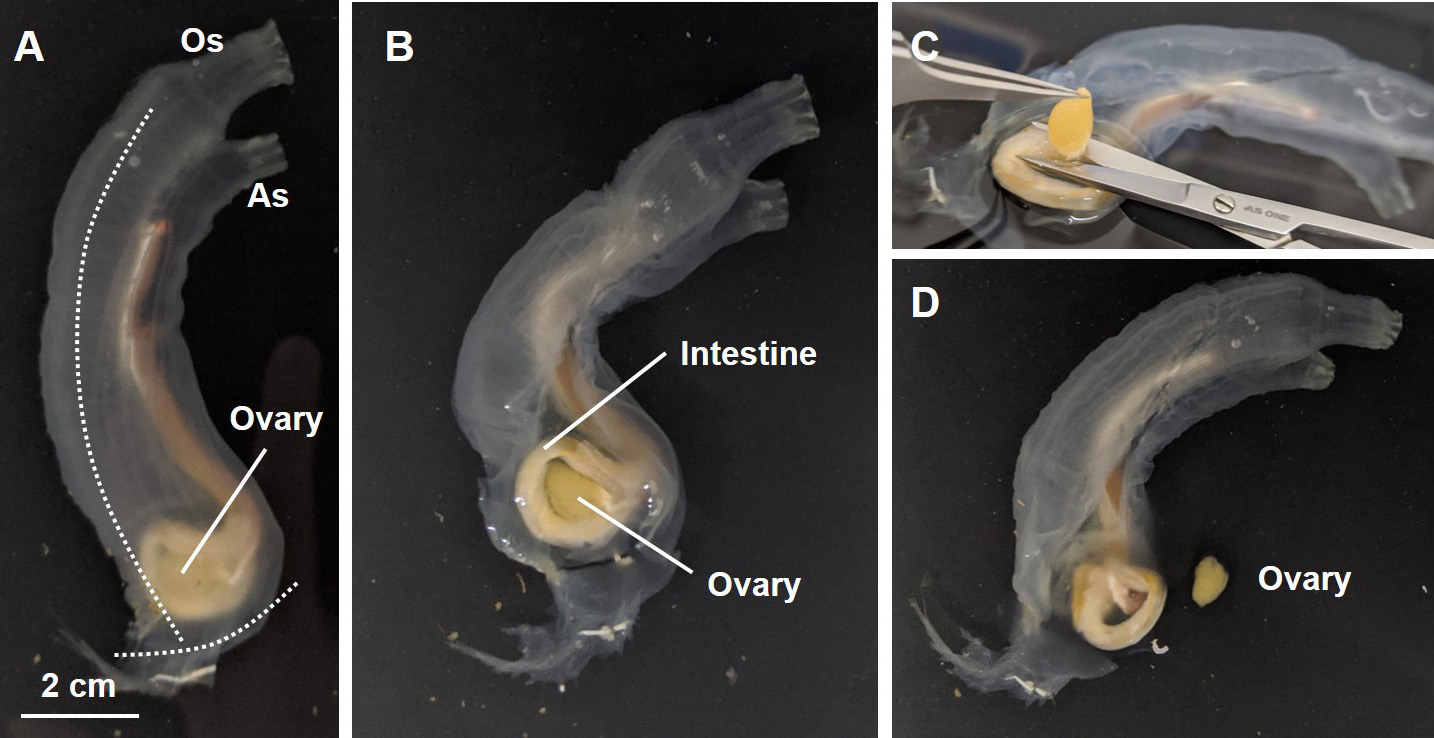
Figure 2. Enucleation of the ovary from an ascidian. A. Overview of an adult ascidian. Os, oral siphon; As, atrial siphon. B. Incise the transparent tunic [dashed line in (A)] with scissors and open the tunic with forceps. C. Pick up the ovary with forceps and isolate the ovary with scissors. D. Isolated ovary and the remaining tissues. - Wash the ovaries three times each with 45 ml of ASW by inverting tube several times and gravity fall of the ovary.
- Collect the ovaries into the normal 60-mm dish (Corning) with 5-6 ml of ASW (Figure 3A).
- Incise the ovaries using two forceps under a stereo microscope (ZEISS, Video 1).
Note: If you do not need stage IV (matured and ovulated) follicles (Figures 3B-3C), remove them by gently shaking the ovaries using a forceps.Video 1. Incising the Ciona ovary. The Ciona ovary is carefully incised and gently shaken using two forceps. - Transfer the ovaries to another 60-mm dish with 5-6 ml of new ASW.
- Rip the ovaries at many sites using two forceps and release the immature/pre-ovulatory follicles by shaking the ovaries gently.
- Repeat Steps A6-A7 five or six times (Figures 3D-3H) and collect all of the isolated follicles (Figure 3I) into the first sieve (see below, Figure 4).

Figure 3. Dissection of the Ciona ovary and collection of follicles. A. An isolated ovary from an ascidian. B. The ovary was incised using two forceps. Dark dots represent stage IV follicles (dashed line). C. Enlarged image of stage IV follicles in (B). D-H. Series of dissecting the ovary. I. Resulting collected follicles at stage I-IV.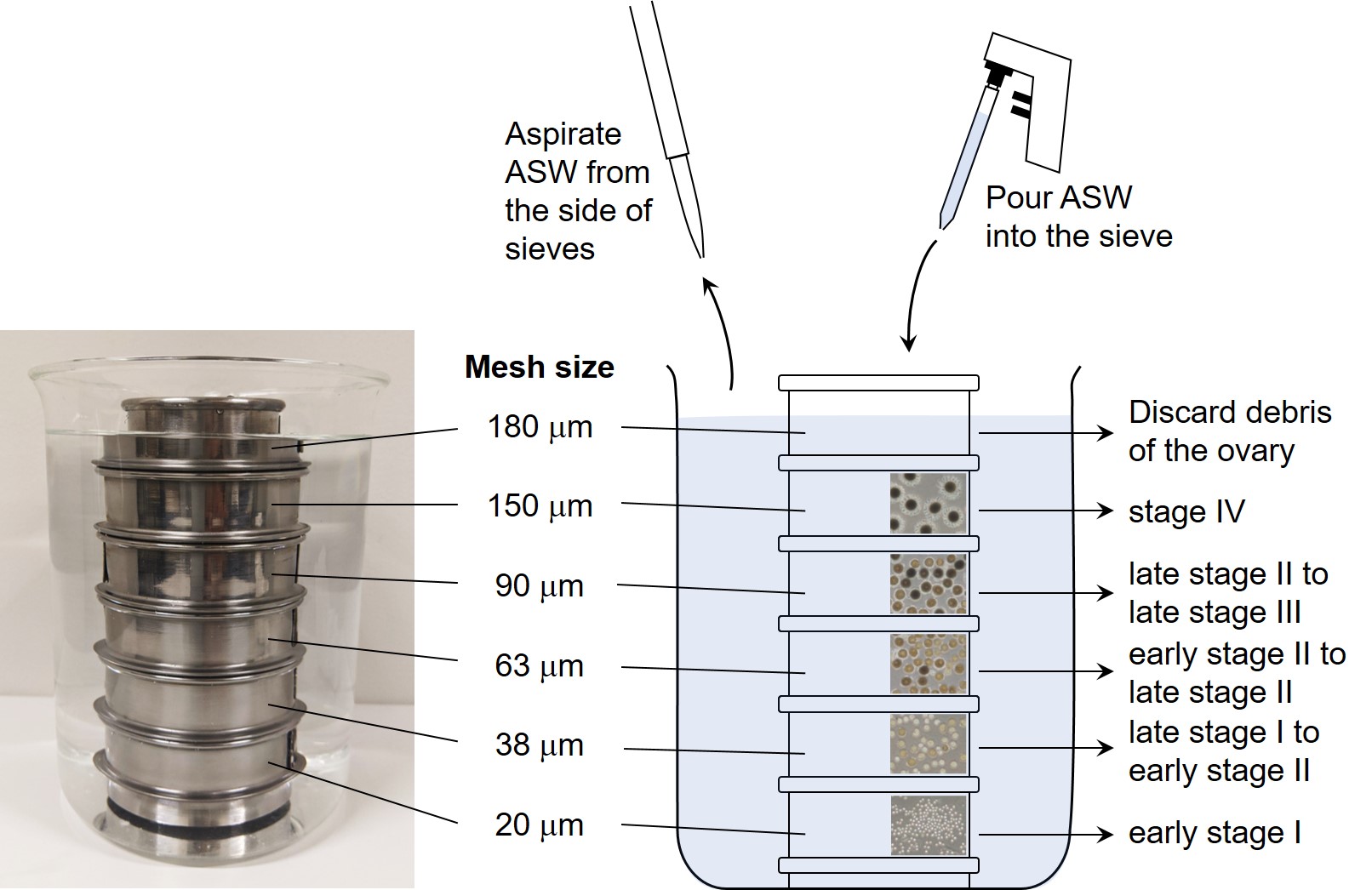
Figure 4. Series of stainless steel sieves in ASW. The series of sieves were stacked in ASW without any bubbles in order from the lowest one (20-μm mesh sieve). Fractionated follicles were washed by pouring and aspirating ASW, and collected at each sieve.
- Fractionation and isolation of Ciona follicles
- Prepare a series of stainless steel sieves in a 1-L beaker with ASW (Figure 4).
Note: Do not get air bubbles between the meshes. - Transfer all of the collected follicles (Figure 3I) into the upper sieve (Figure 4, 180 μm).
- Wash the follicles by pouring 50-60 ml of ASW into the stainless sieve gently, and aspirate ASW from the side of sieves (Figure 4).
Note: Keep the surface of ASW at about 1-cm height from the mesh not to dry the follicles. - Repeat Step B3 four or five times and collect the follicles from the sieve to the 60-mm cell-repellent dish (Greiner Bio-One) by pipetting with 1,000 μl of pipette tip.
Note: Do not use the normal 60-mm dish (Corning), given that pre-ovulatory follicles stick to it. - Aspirate ASW to the 1-cm height from the mesh of the lower sieve and remove the upper sieve.
Note: The 180-μm sieve mainly includes debris of the ovary. - Repeat Steps B3-B5 and collect developmental-stage follicles of interest.
Note: Representative images of entire developmental stages of fractionated follicles (including stages I-IV) are shown in Figure 2A in Matsubara et al. (2019). - Isolate the immature/pre-ovulatory follicles (approximately 20 follicles per sample) using a 10-μm pipette tip (Greiner Bio-One) under the stereo microscope (ZEISS).
Note: Immature/pre-ovulatory follicles can be isolated from the 150-μm or 90-μm fraction of stage IV-removed samples, when follicles are used for maturation and ovulation (see Figure 2B in Matsubara et al., 2019). - Transfer the 20 follicles to the center of a new 35-mm dish with 2 ml of ASW, and record the position of every follicle (Figure 5, left).
Note: Do not use a 60-mm cell-repellent dish (Greiner Bio-One). Movement of follicles makes it difficult to distinguish individual follicles. - Take images of each follicle (Figure 5, right) and microscale slide using the stereo microscope (Leica) and LAS-AF software at the maximum magnification (160x).
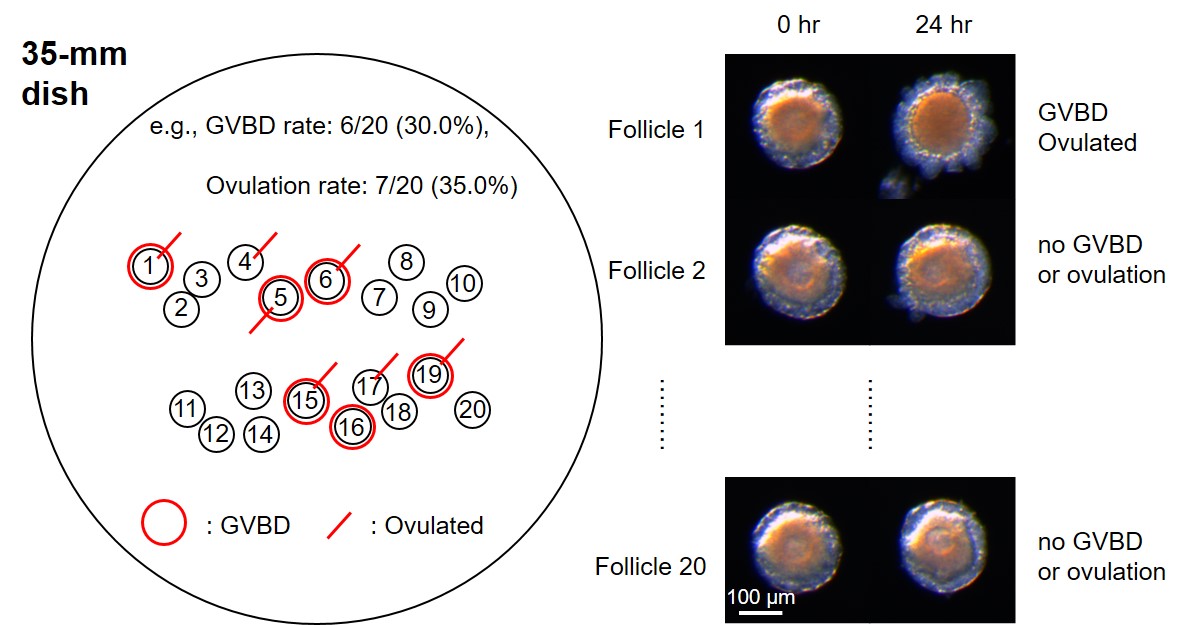
Figure 5. Recording follicle positions and states of GVBD and ovulation. Isolated follicles were attached to the 35-mm dish, and their positions are recorded (left). Images of individual follicle were taken before and after incubation (right). GVBD- and/or ovulated-follicles were marked and the ratios were calculated (left).
- Prepare a series of stainless steel sieves in a 1-L beaker with ASW (Figure 4).
- Induction of Ciona oocyte maturation and ovulation by synthetic CiVP
- Isolate the approximately 40 of late stage II follicles (20 each for Control and CiVP).
- Mix 15 μl of the synthetic CiVP (1 mM) peptide or sterile MilliQ in 1 ml of ASW in a 1.5-ml tube.
- Add the peptide or sterile MilliQ to the 35-mm dish of follicles and mix well gently.
- Incubate the follicles for 24 h at 20 °C.
- Take images of follicles with a microscale slide using a stereo microscope (Leica) at the maximum magnification (x160) and record the position of every follicle (Figure 5).
- Evaluate the GVBD and/or ovulation state of each follicle.
Note: GVBD and ovulation were evaluated by disappearance of germinal vesicle and rupturing of the outer follicular cell layer followed by release of the ovulated follicle, respectively.
- Inhibition of Ciona oocyte maturation and ovulation by chemicals (e.g., MEK inhibitor, U0126)
- Isolate the approximately 40 early stage III follicles (20 each for Control and U0126).
Note: Isolate late stage III follicles for inhibition of oocyte maturation and ovulation using 10 mM Ro-3306 and 10 mM MMP-2/MMP-9 inhibitor II, respectively. - Mix 3 μl of U0126 (10 mM) or DMSO in 1 ml of ASW in a 1.5-ml tube.
- Add the chemical or DMSO to follicles in the 35-mm dish and mix it well gently.
- Incubate the follicles for 24 h at 20 °C.
- Take images of follicles with a microscale slide using a stereo microscope (Leica) at the maximum magnification (x160) and record the position of every follicle (Figure 5).
- Evaluate the GVBD and/or ovulation state of each follicle.
- Isolate the approximately 40 early stage III follicles (20 each for Control and U0126).
Data analysis
Export every image of follicles and a microscale slide as JPEG or TIFF files and open it with Fiji software. Set a 100-μm scale of the microscale slide as a reference of distance using the line selection tool. Surround the follicle with the oval selection tool and measure the size of each follicle before incubation by the length of the major axis of oval fit (Figure 6A) and export the results to Microsoft Excel (Figure 6B). Analyze the follicle-size distribution between experimental groups (e.g., Control vs CiVP) by Student’s t-test and confirm no significant difference (Figure 6B). Annotate GVBD and/or ovulation of each follicle to the size information, calculate GVBD and ovulation rates (Figure 6B), and indicate the results as graph (Figure 6C). Repeat the experiments three to six times and perform Student’s t-test in Microsoft Excel (Matsubara et al., 2019).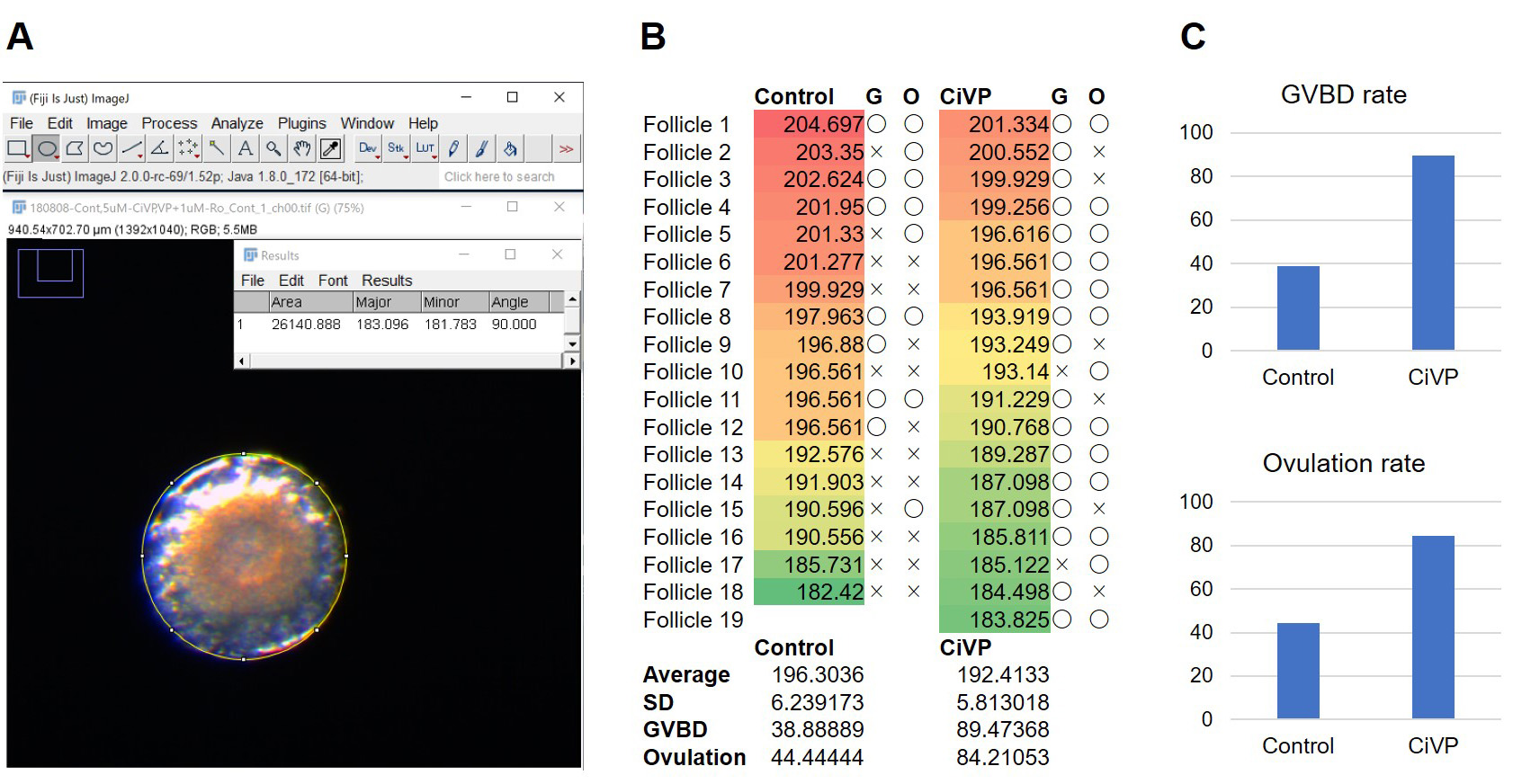
Figure 6. Data analysis of the in vitro oocyte maturation and ovulation assay. A. Size of each follicle was measured using Ellipse fit of Fiji software. B. Information of GVBD (G) and ovulation (O) of each follicle was annotated to the follicle number and size. C. GVBD and Ovulation rates were calculated and indicated by bar graphs.
Recipes
- ASW (Artificial sea water)
Dissolve 200-g REI-SEA marine powder in 5-L tap water
Adjust specific gravity to 1.026 and filter sterlize
Store in a 1-L storage bottle at 20 °C up to 1 month - 1 mM CiVP
Dissolve in sterile MilliQ
Store at -20 °C up to 6 months - 10 mM U0126
Dissolve in DMSO
Store at -20 °C in the dark up to 2 weeks - 10 mM Ro-3306
Dissolve in DMSO
Store at -20 °C up to 6 months - 10 mM MMP-2/MMP-9 inhibitor II
Dissolve in DMSO
Store at -20 °C for up to 3 months
Acknowledgments
We gratefully acknowledge the National Bio-Resource Project (Maizuru Fisheries Research Station of Kyoto University or Misaki Marine Biological Station of the University of Tokyo) for providing ascidians. This work was supported in part by grants from the Japan Society for the Promotion of Science to SM (JP16K18581 and JP17J10624) and HS (JP16KK07430).
Competing interests
The authors declare that no competing interests exist.
References
- Delsuc, F., Brinkmann, H., Chourrout, D. and Philippe, H. (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439(7079): 965-968.
- Kawada, T., Sekiguchi, T., Itoh, Y., Ogasawara, M. and Satake, H. (2008). Characterization of a novel vasopressin/oxytocin superfamily peptide and its receptor from an ascidian, Ciona intestinalis. Peptides 29(10): 1672-1678.
- Matsubara, S., Shiraishi, A., Osugi, T., Kawada, T. and Satake, H. (2019). The regulation of oocyte maturation and ovulation in the closest sister group of vertebrates. Elife 8: e49062.
- Prodon, F., Chenevert, J. and Sardet, C. (2006). Establishment of animal-vegetal polarity during maturation in ascidian oocytes. Dev Biol 290(2): 297-311.
- Satoh, N., Rokhsar, D. and Nishikawa, T. (2014). Chordate evolution and the three-phylum system. Proc Biol Sci 281(1794): 20141729.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P. and Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7): 676-682.
- Silvestre, F., Cuomo, A. and Tosti, E. (2009). Ion current activity and molecules modulating maturation and growth stages of ascidian (Ciona intestinalis) oocytes. Mol Reprod Dev 76(11): 1084-1093.
Article Information
Copyright
Matsubara et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Matsubara, S., Shiraishi, A., Osugi, T., Kawada, T. and Satake, H. (2020). Fractionation of Ovarian Follicles and in vitro Oocyte Maturation and Ovulation Assay of Ciona intestinalis Type A. Bio-protocol 10(7): e3577. DOI: 10.21769/BioProtoc.3577.
- Matsubara, S., Shiraishi, A., Osugi, T., Kawada, T. and Satake, H. (2019). The regulation of oocyte maturation and ovulation in the closest sister group of vertebrates. Elife 8: e49062.
Category
Cell Biology > Cell isolation and culture > Organ culture
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









