- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Delayed Alternation Task for the Study of Spatial Working and Long Term Memory in Rats
Published: Vol 10, Iss 5, Mar 5, 2020 DOI: 10.21769/BioProtoc.3549 Views: 5457
Reviewed by: Fanny EhretAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1770 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 80 Views
Abstract
Memory systems can hold previously presented information for several seconds, bridging gaps between discontinuous events. It has been previously demonstrated that the hippocampus and the medial entorhinal cortex (mEC) are necessary for memory retention over delay intervals in alternation tasks. Here we describe the delayed alternation task, a spatial working memory (WM) task in which animals need to alternate between left and right sides of a figure-8 maze on a trial-by-trial basis to receive a reward. On each trial of this task, the rat has to remember the last episode and turn in the opposite direction compared to the previous trial. We manipulated the WM load by introducing delays of various lengths (10 s and 60 s) between trials. While other alternation task protocols use short delay intervals between trials, our protocol introduces a longer delay condition that requires animals to use long-term memory resources that are not necessarily supported by sequential neuronal firing patterns (i.e., time cells) as are seen with shorter delay intervals.
Keywords: HippocampusBackground
A main function of the hippocampus and entorhinal cortex is to connect events separated by delay intervals (Eichenbaum, 2017; Robinson et al., 2017; Ainge et al., 2007; Sabariego et al., 2019). It has been proposed that retention of memory for these events is accomplished by cells that fire at successive moments in temporally structured experiences, known as time cells (Eichenbaum, 2017). However, while most studies have used alternation task protocols with short delay intervals that did not exceed 15 s (Ainge et al., 2007; Ito et al., 2015; Pastalkova et al., 2008), recent data suggest that time cell firing begins to disappear after approximately the first 20 s of the delay interval (Sabariego et al., 2019). Therefore, it is important to explore both behavioral performance and neuronal firing during delays that exceed these shorter time intervals. Consequently, the delayed alternation task protocol described here uses longer delays of 60 s, which require animals to use long term memory resources, likely due to a WM overload (Kim et al., 2013). This protocol represents a valuable tool for exploring the discrete serial firing patterns observed during shorter delay periods and the mechanisms involved in supporting WM and long-term memory maintenance sustained by other mechanisms, like local synaptic plasticity in hippocampus, sharp-wave ripples, or by activity patterns elsewhere in the brain. Moreover, and because it also includes a shorter delay condition (10 s delay), where sequential firing occurs, it provides an opportunity for the study of these discrete serial firing patterns observed during shorter delay periods.
Materials and Reagents
Note: All materials and reagents listed are examples based on what has been used successfully using the protocol provided; however, using different rat strains/sex/weights, and cereals could also be successful.
- Paper towels
- Chocolate sprinkles (i.e., Cocoa PebblesTM)
- Weigh boats (Chemglass, catalog number CLS-1815-002 )
- Pencil
- Eraser
- Behavioral sheets (see Appendix 1)
- Experimentally naive, male Long-Evans rats with weighing between 300 g-350 g (Charles Rivers Laboratories)
- Ethanol 70% (Fisher Scientific, Decon Laboratories, catalog number: 04-355-223 )
Equipment
- Figure-8-maze
Dimensions: 101 cm long crosspiece connected to a center arm to the right and left arms at the top and bottom of the maze. Side pieces were 101 cm long as well. The top and bottom pieces were 150 cm long. All runways were 10 cm wide x 2 cm tall. The maze was elevated 50 cm from the ground (Figure 1). Weigh boats containing reward pieces (i.e., Cocoa PebblesTM) were attached underneath the reward locations and served as non visible odor cues to prevent subjects from using odor when taking their decision.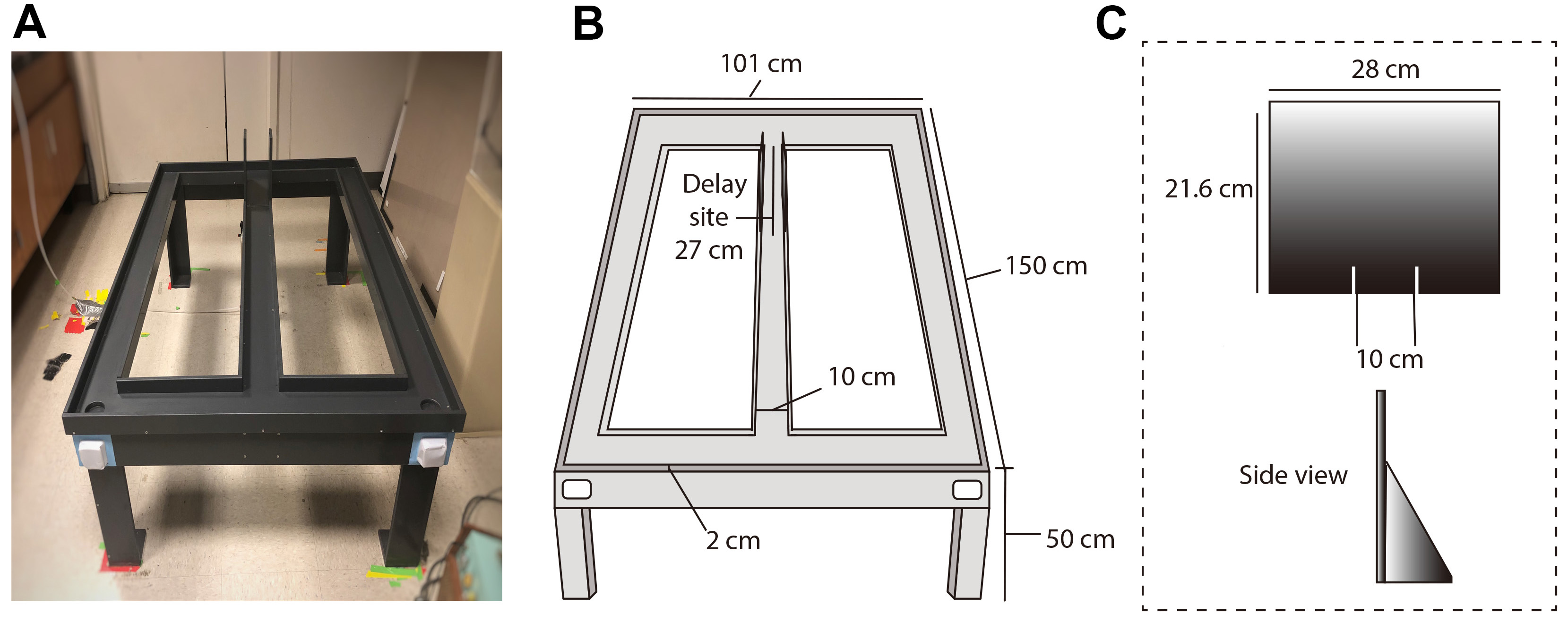
Figure 1. The Figure-8-maze apparatus used for behavioral training and testing. A. Photography of the maze. Side barriers in the center stem indicate the delay site. Odor cues were provided in both sides of the maze, below the reward sites, in order to prevent a bias in choosing a particular arm (white weigh boats). B. Illustration of the maze indicating measurements of each section. C. Removable card-board barriers (hand crafted). - Card-board barriers (hand crafted, dimensions: 21.6 cm x 28 cm)
- Stop-watch (Fisher Scientific, catalog number: ISO 17025 )
- Infrared camera (such as Acazon Conch-shape Infrared LEDs Security Camera, https://amzn.to/37XHKyG)
- Scale (Kent Scientific, catalog number SCL-1015 )
- Dim light source (i.e., desk lamp)
Software
Notes:
- Software listed below describes what has been used successfully using the protocol provided; however, using alternative software that allows for scoring of alternation could also be successful.
- For electrophysiological recordings a 4SX 64 channel Neuralynx system was used. We do not discuss electrophysiological procedures in this protocol but any high-density, low-noise electrophysiology system could be successful.
- Microsoft Excel
- Statistical package (i.e., SPSS, GraphPad prism, R)
Procedure
- General Notes
Note: All behavioral sessions were performed during the dark phase (reversed light cycle: between 08:00 and 16:00) under dimly lit conditions. Performance of this task during the light phase could also be successful.- Rats should be housed individually.
- Keep rats on a reversed 12 h light/dark cycle.
- Behavioral testing should take place during the dark cycle.
- Before behavioral training, rats should gradually be food deprived until reaching 85% of their ad libitum weight and they should be maintained at this weight throughout the experiment.
- Water should always be kept available.
- Weigh the rats daily, right before the behavioral session.
- Feed the rats in the end of the behavioral session.
- The order in which rats run the task should change daily so that the first rat running the task each day is different from the one that ran first the previous day.
- The experimenter/s should avoid any kind of unnecessary interference.
- Odor cues (food reward used as a reinforcer) should be provided to prevent the rat from making its decision based on the smell of the reward (Figure 1).
- Salient visual cues should be available in the room and they should be kept constant.
- Add some chocolate sprinkles (Cocoa PebblesTM or the cereal you will use as a reward) to the rats’ home-cages daily for 3 days before the habituation day to prevent neophobia.
- Handle the rats (~5 min each) daily for 3 days before the habituation day.
- Habituation
- On the fourth day, bring the rats (one by one) to the room where behavioral testing will take place and after weighting the rat, allow it to familiarize with the room and the apparatus by letting it freely explore the maze for 10 min with chocolate sprinkles spread all over the maze.
- Clean the maze with a 70% ethanol solution and paper towels at the end of the session and between animals. Make sure the maze is dry before the next rat is placed on the apparatus.
- Make sure the homecage bedding is kept clean to avoid rats from consuming their waste. This could interfere with the food deprivation procedure/weight loss.
- Feed all the rats at the end of the behavioral session according to their weights.
- Continue adding pieces of the food reward into the animal’s homecage to avoid neophobia.
- First Stage–Training
- On day five (or the fifth day), weigh the subject. Start placing the rat at the base of the center arm of the maze (the most distal point from the reward locations, see Figure 2).
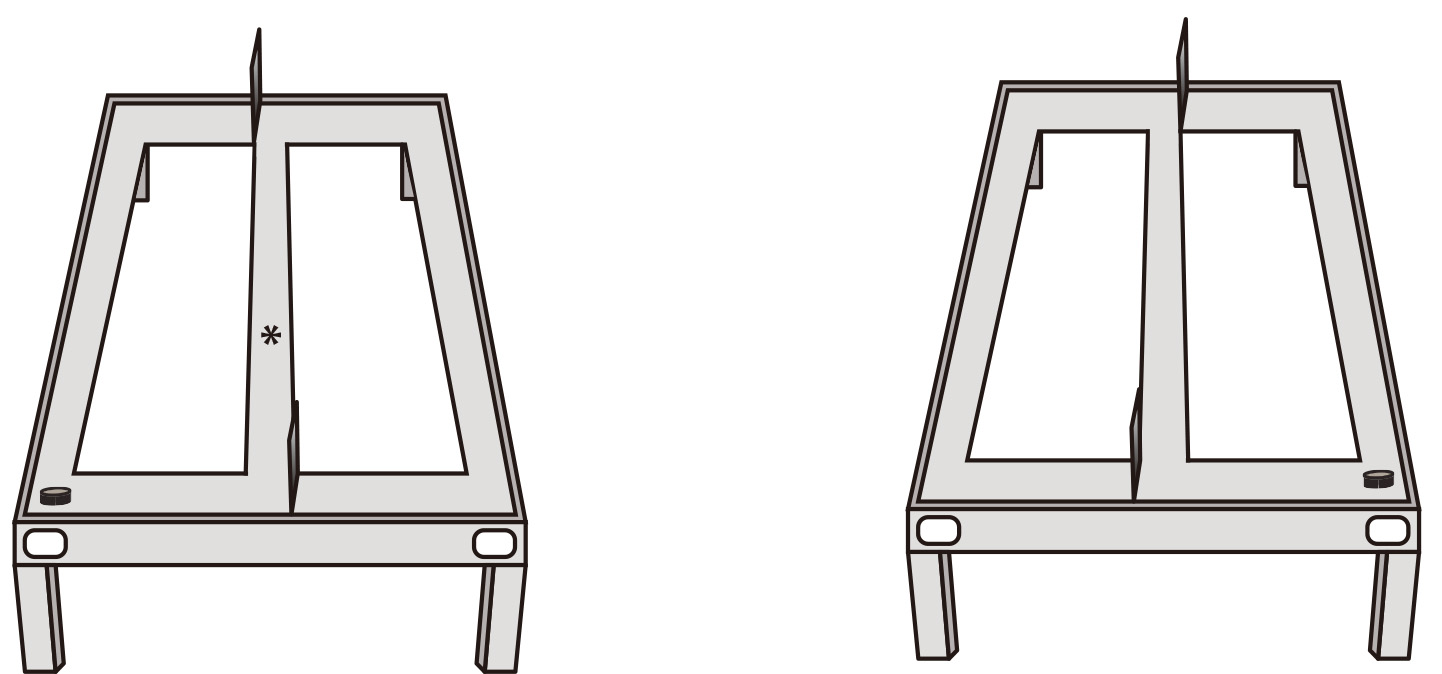
Figure 2. Illustration of the first stage of the training protocol. Each rat is placed at the base of the central stem of the apparatus, facing the choice arms (the asterisk notes the point where the animal is placed). Barriers are placed to force the rat to enter one of the choice arms. After it enters one of the arms, a reward will be delivered. - Place a barrier to force the rat to enter one of the side arms where the reward (1 cocoa pebble) is located.
- After the rat consumes the reward, use the barriers to gently guide the rat to return to the base of the center arm. Then, switch the barrier at the far end of the central arm to force the rat to turn down the opposite choice arm for the next reward. It is crucial to not allow the rat to retrace its route at any point.
- Each session should last 20 min or 30 trials, whichever comes first.
- Repeat this procedure, alternating the path the rat can follow by using the barrier and the alternating arms until the rat is able to follow the pattern and run consistently, without trying to retrace its steps, during two consecutive days (criterion).
- Feed all the rats at the end of the behavioral session according to their weights.
- On day five (or the fifth day), weigh the subject. Start placing the rat at the base of the center arm of the maze (the most distal point from the reward locations, see Figure 2).
- Second Stage–Training
Note: Before this stage rats should have reached the criterion in stage 1.- Weigh the subject.
- The use of barriers should be phased out as the rat is now allowed to enter either arm each time it reaches the end of the stem (see Figure 3).
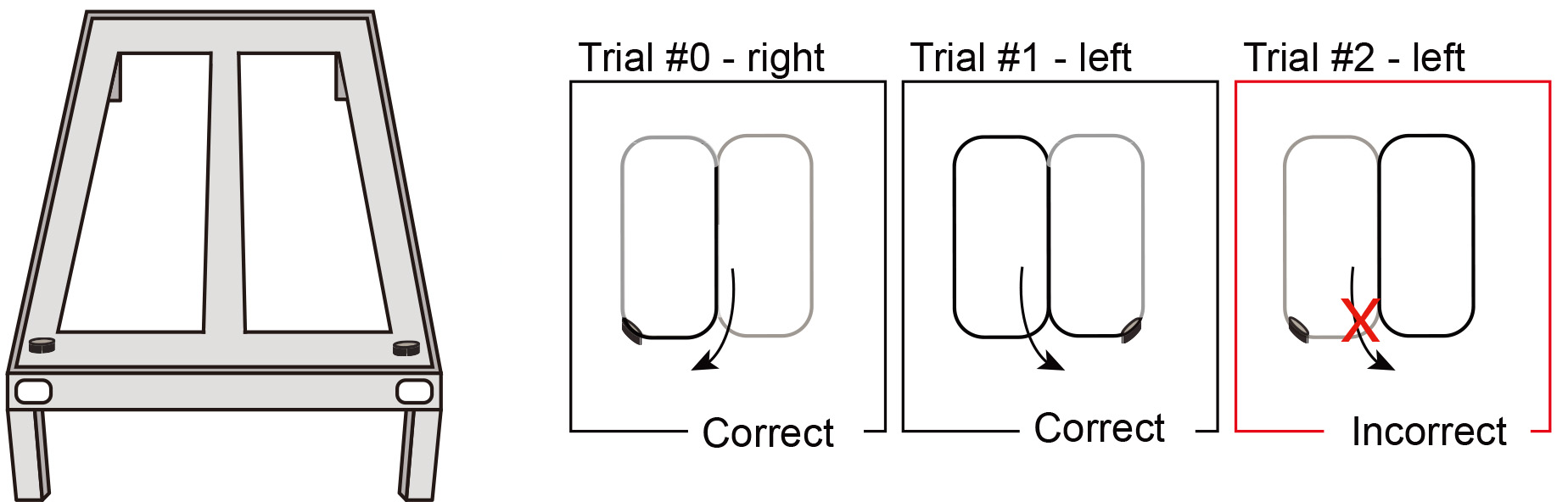
Figure 3. Illustration of the second stage of the training protocol. The use of barriers at the choice point should be phased out; each time the rat reaches the end of the stem it will be able to enter either arm, but it will be rewarded only for alternating arm entries in a “figure 8”-like pattern and they will be prevented from retracing their steps at any point. - The first trial will be considered “trial 0” and the rat will always be rewarded.
- After this trial, rats will be rewarded only when running in alternating arm entries.
- Prevent the rats from retracing their steps at any point.
- This procedure should take 20 min or 30 trials (not including the initial run) to be accomplished, whichever comes first. If the animal is not improving its performance, increase the number of trials per day (up to 60).
- For each trial, the arm chosen will be scored as either correct (alternation) or incorrect (repeat entry into the previously chosen arm, see Appendix 1).
- Rats should be trained to a criterion performance of at least (90%) correct trials on 2 out of 3 consecutive days.
- Feed the animals at the end of the behavioral session according to their weights.
- Third Stage–Testing
Note: Before this stage rats should have reached the criterion in stage 2. This third stage includes trials with delays.- Weigh the subject.
- Rats should receive 30 trials daily, divided into 3 blocks of 10 trials each (no delay, 10-s delay and 60-s delay).
- The order of the trial blocks should be randomized every day. Depending on the questions the experimenters are trying to address, trial blocks can be doubled up to 60 so that each block is repeated, e.g., no delay, 10-s delay, 60-s delay, no delay, 10-s delay, 60-s delay). This structure would be beneficial for example if electrophysiological recordings are being performed simultaneously since a comparison between blocks of trials of the same type could be done.
- For delay trials, once the rat returns to the base of the stem, after the last trial of the previous block, confine the rat by placing two barriers at the base of the center stem (the delay site should be approximately 25 cm long). See Figure 4 and Video 1.
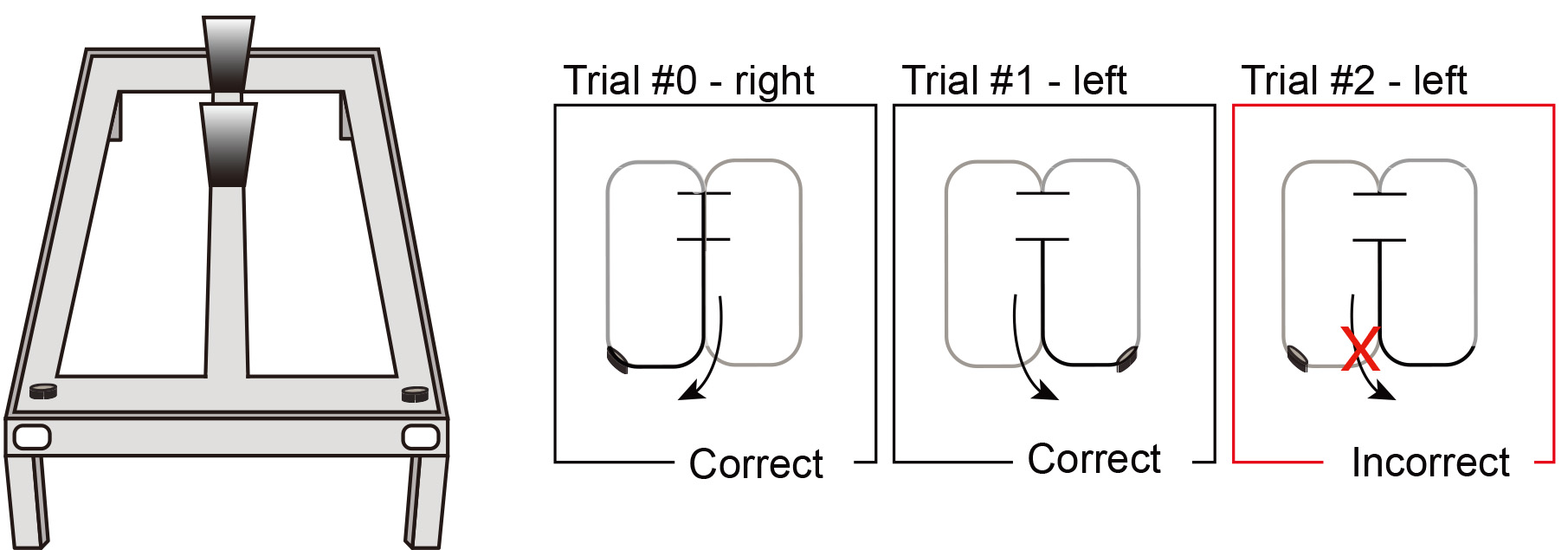
Figure 4. Illustration of the third stage (testing). Delay testing will start once the rat has reached the criterion in stage 2. In each daily session, rats will receive 30 trials, grouped into three blocks of 10 trials. The order of the 3 blocks of delays will be pseudorandomized every day.Video 1. Example video of a 60-s delay trial during the third stage of behavioral testing. (All experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of California, San Diego (protocol number S08276, 3-year approval since 4/19/2017).) - Start a stop-watch to keep track of the delay duration.
Note: The stop-watch should be in silent mode to avoid any auditory cues. - At the end of the delay interval, remove the barrier that blocks the rat from accessing the central arm of the figure-8-maze, so that it can transverse freely into the stem and make its next choice.
- After the rat makes a choice and consumes the reward (if applicable), let it return to the delay zone on the center arm for the next trial to occur.
- Repeat this stage for at least 6 continuous days.
Note: The duration of this stage can be variable depending on the question that the experimenters are trying to address. - Clean the maze with a 70% ethanol solution and paper towels after running each rat. Make sure the maze is dry before the next rat is placed on it.
- Feed the animals at the end of the behavioral session according to their weights.
Data analysis
Note: Use Microsoft Excel or a similar software to record the behavioral data for each stage of the experiment.
- For the second stage enter data into an Excel spreadsheet with the following columns (see Figure 5):
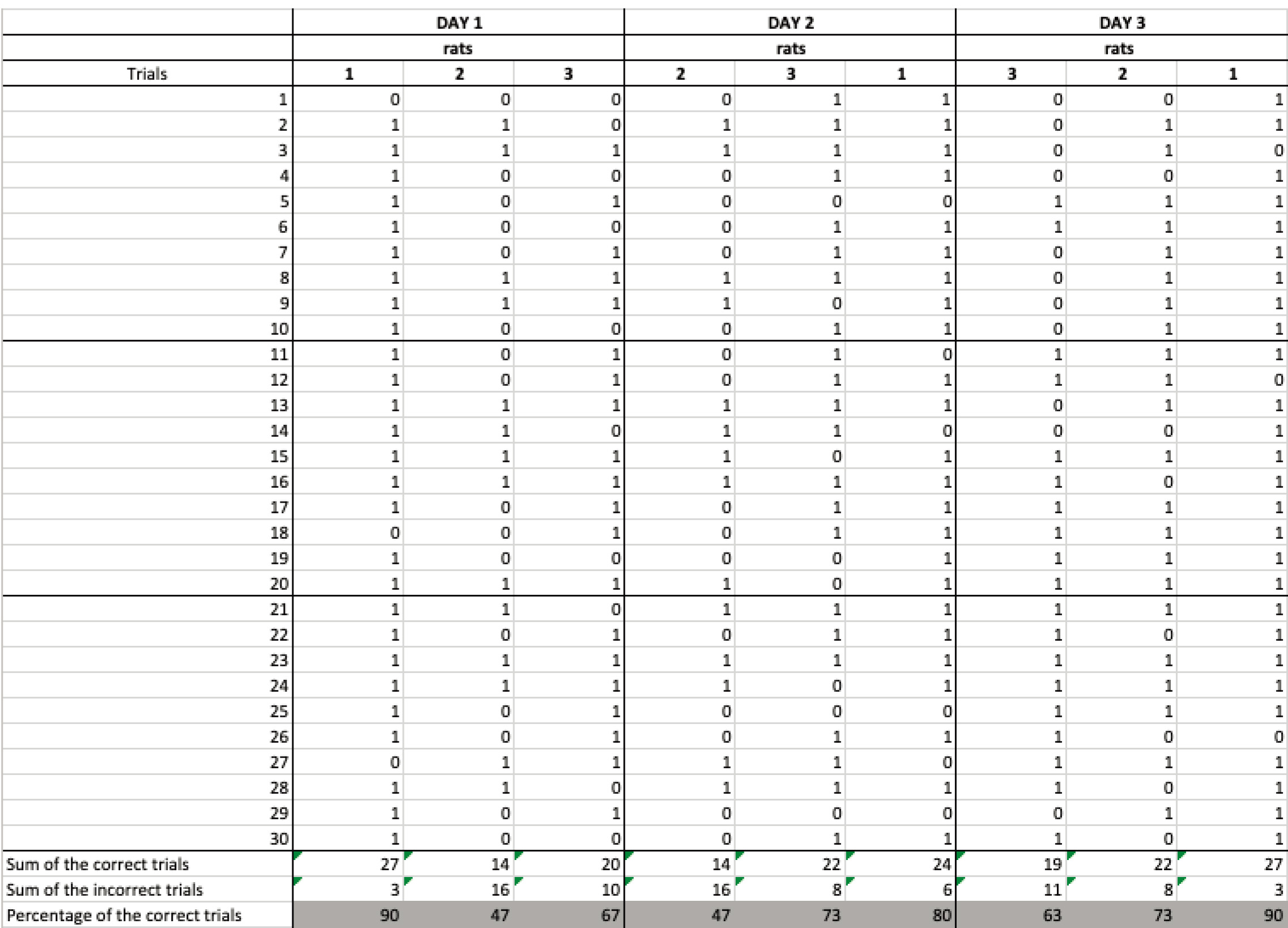
Figure 5. Excel spreadsheet showing the recorded data for the second stage and a summary table (bottom) of the results with the sum of the correct trials, incorrect trials, and the percentage of correct trials. Numbers 1, 2, 3 represent the rats. Columns with data recorded from rat 1 show an example of a rat meeting the criterion performance of 90 % correct trials on 2 out of 3 consecutive days, ready to move to the next stage.- Number of trials.
- Day the experiment takes place (e.g., day 1, day 2, day 3).
- Separate the day sections into further columns with numbers that identify the rats (e.g., 1, 2, 3).
- For each trial the rat completes correctly enter 1, otherwise 0.
- At the end create a table that summarizes:
The sum of the correct trials
The sum of the incorrect trials
The calculated percentage of correct trials - For the third stage data follow the same instructions as for stage two with the addition of the randomized order of the delay blocks (no delay, 10 s, 60 s delays)
Create a summary table of the correct, incorrect and percentage of the correct trials for each block (see Figure 6).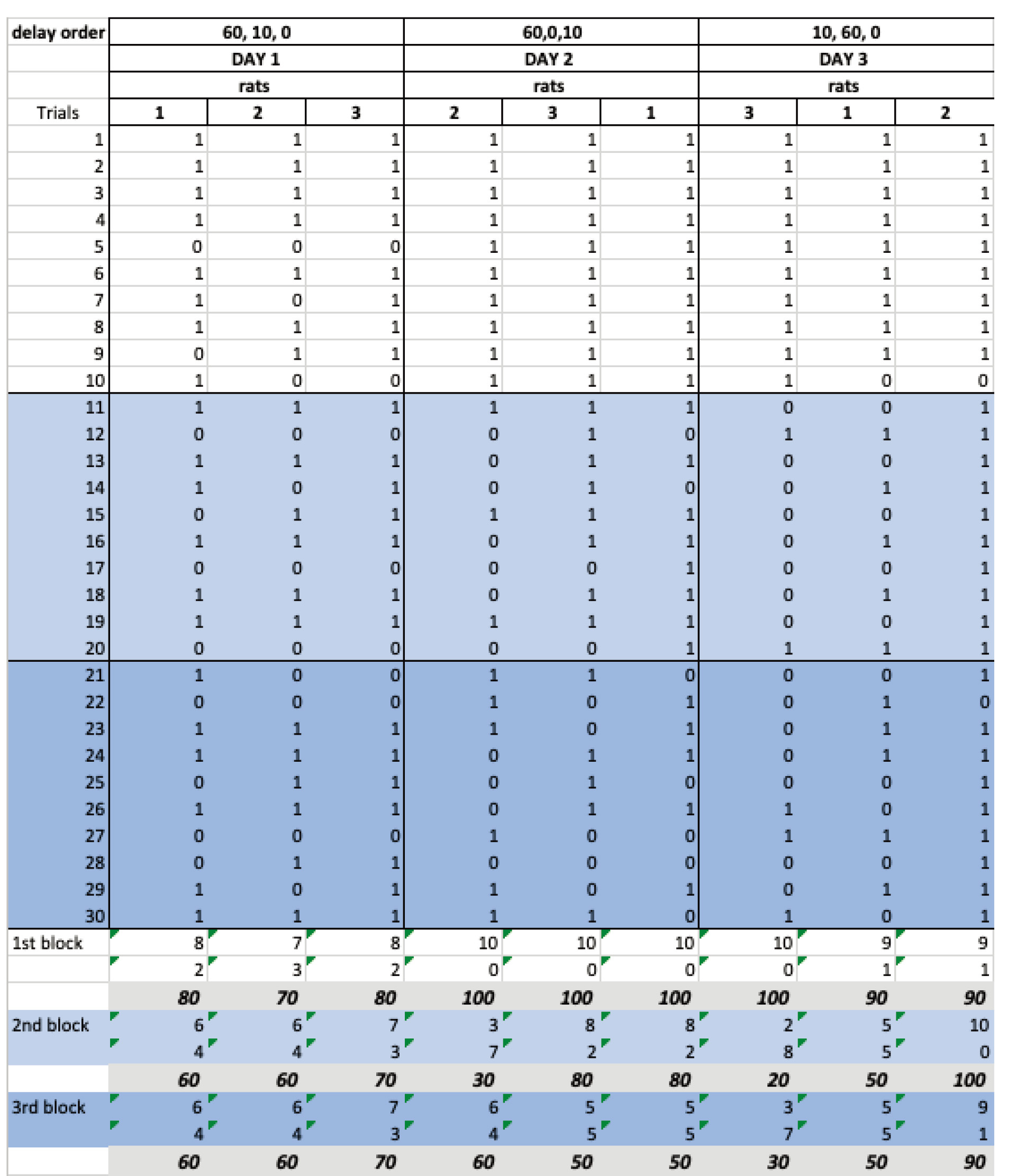
Figure 6. Excel spreadsheet showing the recorded data for the third stage with the randomized delay orders, no delay (white), 10 s delay (light blue), 60 s delay (dark blue). Below is a summary of the correct, incorrect and percentage of the correct trials for each block of trials. Note that each day a the different block of trials (1st, 2nd and 3rd) will correspond to a different type of delay (no delay, 10 s delay and 60 s delay). Experimenters should consider this to organize their data before analysis. One option would be to enter the data always following a particular order (e.g., no delay, 10 s, 60 s delay) even if this is not the real order the animal follows that day. - Use a statistical package (i.e., SPSS, GraphPad prism, R) to perform two-way anova inferences to analyze the differences between the groups of animals and the types of delays (see Figure 7 adapted from Sabariego et al. (2019) for a representative way to represent and analyze the data). Multiple comparisons of the significant interactions can be analyzed using a post hoc test like the Tukey’s test.
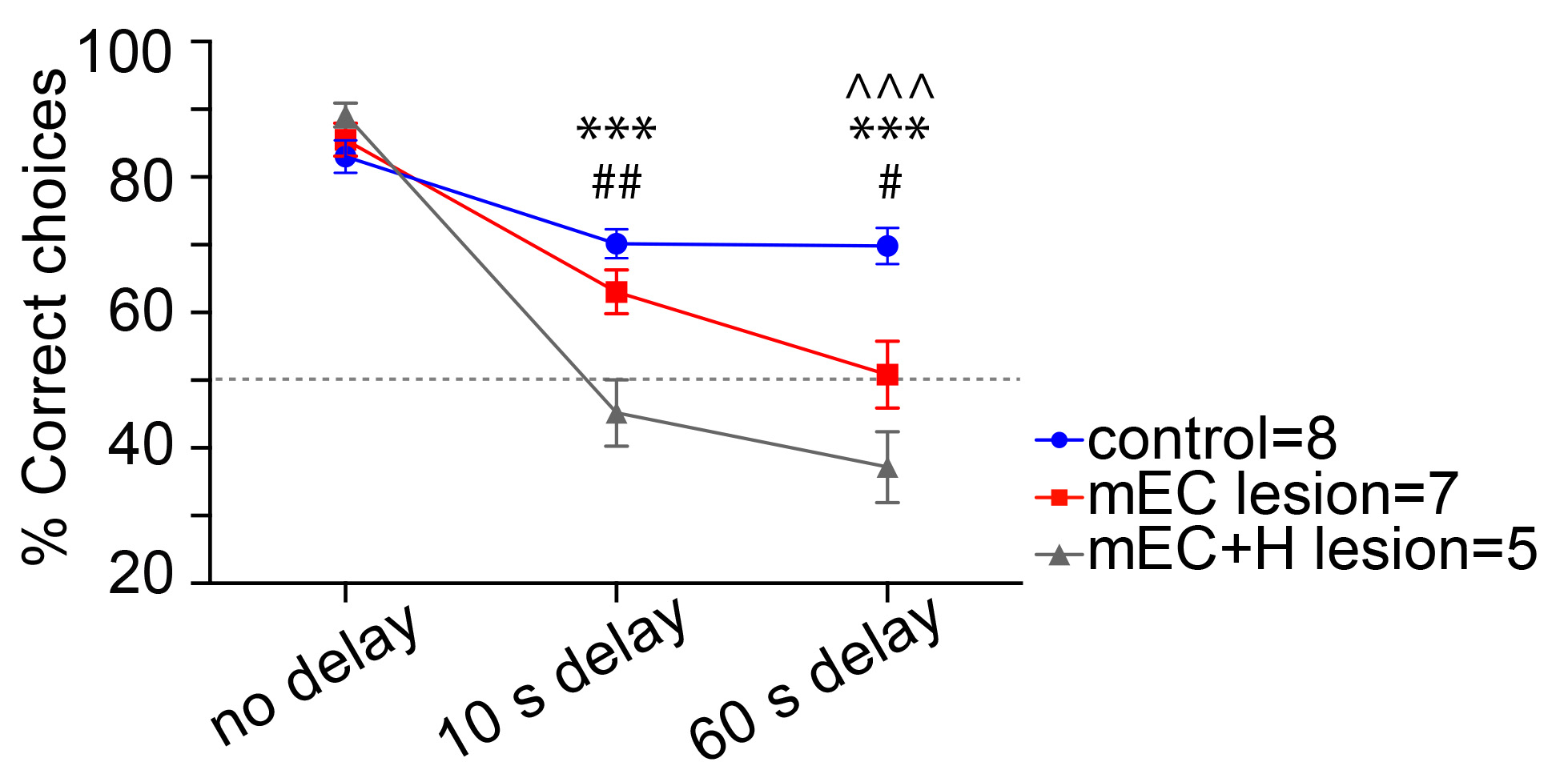
Figure 7. Representative example of data plotting and analysis on a lesion experiment in the alternation task. Lesioned rats were impaired in the delayed versions but not in the continuous version of the task. The 7 days of testing were analyzed with a two-way ANOVAs (Group x Delay) that revealed main effects of Lesion and Delay and a Delay x Lesion interaction (P-value = 0.0001). Tukey’s post hoc tests: ^^^ P < 0.001 for control versus mEC lesion group comparison; *** P < 0.001 for control versus mEC+H lesion group comparison (lesions of mEC and hippocampus; ## P < 0.01 for mEC versus mEC+H lesion group comparisons (adapted from Sabariego et al., 2019).
Acknowledgments
This protocol was adapted from Sabariego et al. (2019). Recent work has been supported by Mount Holyoke College, Program of Neuroscience and Behavior.
Competing interests
The authors have no conflicts of interest.
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of California, San Diego (protocol number S08276, 3-year approval since 4/19/2017).
References
- Ainge, J. A., van der Meer, M. A., Langston, R. F. and Wood, E. R. (2007). Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus 17(10): 988-1002.
- Eichenbaum, H. (2017). Time (and space) in the hippocampus. Curr Opin Behav Sci 17: 65-70.
- Ito, H. T., Zhang, S. J., Witter, M. P., Moser, E. I. and Moser, M. B. (2015). A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature 522(7554): 50-55.
- Kim, S., Sapiurka, M., Clark, R. E. and Squire, L. R. (2013). Contrasting effects on path integration after hippocampal damage in humans and rats. Proc Natl Acad Sci U S A 110(12): 4732-4737.
- Pastalkova, E., Itskov, V., Amarasingham, A. and Buzsaki, G. (2008). Internally generated cell assembly sequences in the rat hippocampus. Science 321(5894): 1322-1327.
- Robinson, N. T. M., Priestley, J. B., Rueckemann, J. W., Garcia, A. D., Smeglin, V. A., Marino, F. A. and Eichenbaum, H. (2017). Medial entorhinal cortex selectively supports temporal coding by hippocampal neurons. Neuron 94(3): 677-688 e676.
- Sabariego, M., Schonwald, A., Boublil, B. L., Zimmerman, D. T., Ahmadi, S., Gonzalez, N., Leibold, C., Clark, R. E., Leutgeb, J. K. and Leutgeb, S. (2019). Time cells in the hippocampus are neither dependent on medial entorhinal cortex inputs nor necessary for spatial working memory. Neuron 102(6): 1235-1248 e1235.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hoxha, M. and Sabariego, M. (2020). Delayed Alternation Task for the Study of Spatial Working and Long Term Memory in Rats. Bio-protocol 10(5): e3549. DOI: 10.21769/BioProtoc.3549.
Category
Neuroscience > Behavioral neuroscience > Animal model
Systems Biology > Genomics > Functional genomics
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










