- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Individual RNA in Fixed Cells and Tissues by Chromogenic ISH
Published: Vol 10, Iss 3, Feb 5, 2020 DOI: 10.21769/BioProtoc.3510 Views: 5223
Reviewed by: Imre GáspárNidhi SharmaShalini Low-Nam

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Polysome Fractionation to Analyze mRNA Distribution Profiles
Amaresh C. Panda [...] Myriam Gorospe
Feb 5, 2017 31880 Views

In situ Hybridization (ISH) and Quantum Dots (QD) of miRNAs
Sajni Josson [...] Leland W.K. Chung
Feb 20, 2017 11652 Views

miRNA Characterization from the Extracellular Vesicles
Sajni Josson [...] Leland W.K. Chung
Feb 20, 2017 9044 Views
Abstract
Visualization of RNA molecules in situ helps to better understand the functions of expressed genes. Currently, most conventional in situ hybridization methods for visualization of individual RNAs are based on fluorescence detection. Herein we present a chromogenic in situ hybridization protocol for visualization of single RNA molecules in fixed cells and tissues. The protocol is based on padlock probing and rolling circle amplification to generate detectable chromogenic signal from single RNA molecules. Chromogenic signal can avoid background autofluorescence and can be preserved for a longer period than fluorescence signal.
Keywords: Chromogenic in situ hybridizationBackground
The abundance and spatial location of expressed RNA molecules indicates the physiological and pathological status of cells and tissues. Therefore, there is an increasing interest for in situ RNA detection. Compared to conventional in situ hybridization methods, novel methods that can detect RNA at individual molecule level are more sensitive and specific (Crosetto et al., 2015). These methods include single molecule fluorescence in situ hybridization (smFISH) (Femino et al., 1998), methods based on rolling circle amplification (Larsson et al., 2010), hybridization chain reaction (HCR) (Shah et al., 2016), and branched DNA (bDNA) technology (Wang et al., 2012; Battich et al., 2013). Current methods for individual RNA in situ detection assays are normally readout as fluorescence signal, which offers good sensitivity and is easy to multiplex. Chromogenic readout is often used in conventional RNA ISH assays, providing stable signal that can be stored for long period of time, and is not affected by autofluorescence or photobleaching. Therefore, we introduce the single molecule chromogenic in situ hybridization (smCISH) assay that enables sensitive and specific detection of individual RNA in fixed cells and tissues (Jiang et al., 2019). Our protocol is based on padlock probing and rolling circle amplification (Nilsson et al., 1994; Banér et al., 1998). First, padlock probe is designed to directly bind to target RNA specifically. After padlock probe hybridization, probe circularization is performed using SplintR DNA ligase. The closed circle is RNA-templated ligation of its two ends. Next, an RCA primer is hybridized to the circularized padlock probe to initiate rolling circle amplification reaction. The padlock probe is then amplified into its complementary concatenated form to generate RCP. HRP labeled detection probes are then hybridized to the RCP. Finally, chromogenic signal is developed using H2O2 and 3,3'-Diaminobenzidine (DAB) to generate brown insoluble dots from individual RCPs, which corresponds to single RNA molecules. Our results show that single molecule chromogenic in situ hybridization assay can count and localize individual RNA molecules in fixed cells and tissue samples.
Materials and Reagents
- Secure-Seal hybridization chamber, 9 mm diameter, 0.8 mm deep (Containing seal tabs, Thermo Scientific, catalog number: S24732)
- EasYFlask 25 cm2 (Thermo Scientific Nunc, catalog number: 156340-ROS)
- Serological Pipette (Thermo Scientific Nunc, 2 ml, 5 ml, 10 ml, 50 ml)
- Cell culture Dish, 150 mm x 20 mm (Thermo Scientific Nunc, catalog number: 168381-ROS)
- Adhesion Microscope Slides (Citotest, catalog number: 188105)
- 15 ml Conical Centrifuge Tubes (Thermo Scientific Nunc, catalog number: 339650-SPL)
- 50 ml Conical Centrifuge Tubes (Thermo Scientific Nunc, catalog number: 339652-SPL)
- Filtered Pipette Tip (QSP, 0.1-10 µl, 2-20 µl, 10-100 µl, 20-200 µl, 100-1,000 µl)
- 0.2 ml PCR Tubes (AXYGEN, catalog number: PCR-02-C)
- 1.5 ml Microtubes (AXYGEN, catalog number: MCT-150-C)
- 0.22 μm membrane filter (Millipore, catalog number: SLGP033RS)
- HER2 padlock probe (sequence: 5′-ATTACTTGCAGGTTCTTTCCTTTTACGACCTCAATGCTGCTGCTGTACTACTCTTCAGAATTCGTCCCCGG, underline: target hybridization sequences andd detection probe complementary part, bold italics: RCA primer binding part) (Synbio Tech, China)
- ACTB padlock probe (sequence: 5′-CTGTGCTCGCGGGGCGTTCCTTTTACGACCTCAATGCTGCTGCTGTACTACTCTTGGCAAAGGCGAGGCT, underline: target hybridization sequences and detection probe complementary part, bold italics: RCA primer binding part) (Synbio Tech, China)
- RCA primer (sequence: 5′-ACAGCAGCAGCATTGAGGTC) (Synbio Tech, China)
- HRP labeled detection probe (sequence: 5′ HRP-CCTCAATGCTGCTGCTGTACTAC) (TaKaRa)
- Pepsin (Sigma-Aldrich, catalog number: P7012-1G), store at -20 °C
- Fetal Bovine Serum (Hyclone, catalog number: 10270-106), store at -20 °C
- Trypsin (Hyclone, catalog number: SH30042.01), store at -20 °C
- ATP, 100 mM Solution (Thermo Scientific, catalog number: R0441), store at -20 °C
- T4 Polynucleotide Kinase, 10 U/µl (Thermo Scientific, catalog number: EK0032, containing PNK buffer A), store at -20 °C
- dNTP Set, 100 mM Solutions (Thermo Scientific, catalog number: R0182), store at -20 °C
- SplintR Ligase, 25 U/µl (NEB, catalog number: M0375L), store at -20 °C
- phi29 DNA Polymerase, 10 U/µl (Thermo Scientific, catalog number: EP0094), store at -20 °C
- BSA, 20 mg/ml (NEB, catalog number: B9000S), store at -20 °C
- RiboLock RNase Inhibitor, 40 U/µl (Thermo Scientific, catalog number: EO0384), store at -20 °C
- Tween-20 (Sigma-Aldrich, catalog number: P9416-100ML), store at room temperature (RT)
- Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ML), store at RT
- NaOH (Sigma-Aldrich, catalog number: 795429-500G), store at RT
- KCl (Sigma-Aldrich, catalog number: P9541-500G), store at RT
- NaCl (Sigma-Aldrich, catalog number: S3014-1kG), store at RT
- Na2HPO4 (BBI LIFE SCIENCES, catalog number: A610404-0500), store at RT
- KH2PO4 (Sangon Biotech, catalog number: A100781-0500), store at RT
- Sodium iodate (BBI LIFE SCIENCES, catalog number: A600859), store at RT
- Aluminum potassium sulfate dodecahydrate (Sangon Biotech, catalog number: A500755), store at RT
- Glycerol (Sigma-Aldrich, catalog number: G9012-100ML), store at RT
- Hematoxylin (Sigma-Aldrich, catalog number: H3136-25G), store at RT
- Neutral balsam mounting medium (BBI LIFE SCIENCES, catalog number: E675007), store at RT
- Xylene (XILONG SCIENTIFIC, catalog number: 33535-500ML), store at RT
- Ethanol (Macklin, catalog number: E809061-500 ML), store at RT
- Acetate (Macklin, catalog number: A801295-500 ML), store at RT
- Hydrochloric acid (SCR, catalog number: 10011018), store at RT
- DEPC (Sigma-Aldrich, catalog number: D5758-25ML), store at 4 °C
- Paraformaldehyde (Sigma-Aldrich, catalog number: 16005-1KG-R), store at 4 °C
- Formamide (Sigma-Aldrich, catalog number: F9037-100ML), store at 4 °C
- 20x SSC buffer (Sigma-Aldrich, catalog number: S6639), store at 4 °C
- 1x Phosphate Buffered Saline (Hyclone, catalog number: SH30256.01), store at 4 °C
- DNase/RNase-Free Water (Solarbio, catalog number: R1600), store at 4 °C
- Hydrogen peroxide solution, 30 wt.% in H2O (Macklin, catalog number: H811240-500 ml), store at 4 °C
- DAB Immunohistochemistry Color Development Kit (BBI LIFE SCIENCES, catalog number: EP670033), store at -20 °C
- EDTA (Sigma-Aldrich, catalog number: RDD017-500G), store at RT
- Serum-free medium, DMEM/HIGH GLUCOSE (Hyclone, catalog number: SH30022.01), store at 4 °C
- Penicillin-Streptomycin (Hyclone, catalog number: SV30010), store at -20 °C
- Culture media (50 ml) (see Recipes)
- 10x PBS (500 ml), pH 6.8 (see Recipes)
- 1x DEPC-PBS (1 L), pH 7.4 (see Recipes)
- 1x DEPC-PBST (1 L) (see Recipes)
- 4% (w/v) PFA (500 ml) (see Recipes)
- 1x TE buffer (100 ml), pH 8.0 (see Recipes)
- 0.5%(v/v) Triton X-100 (5 ml) (see Recipes)
- 3% (w/v) H2O2 solution (50 μl) (see Recipes)
- 2x hybridization buffer (5 ml) (see Recipes)
- Washing buffer (15 ml) (see Recipes)
- 2x detection buffer (5 ml) (see Recipes)
- Staining buffer (50 μl) (see Recipes)
- 0.1% hydrochloric acid-ethanol (5 ml) (see Recipes)
- Hematoxylin Staining Solution (50 ml) (see Recipes)
- 25 mM dNTPs (100 μl) (see Recipes)
Equipment
- Vortex Mixers (MIULAB, model: MIX-25P)
- Microcentrifuge (MIULAB, model: Mini-6K)
- Magnetic stirrer (IKA, RCT basic)
- pH meter (OHAUS, model: starter3100)
- PCR machine (BIOER, model: TC-XP)
- Analytical balance (OHAUS, model: AS220.R2)
- Pipette (Eppendorf AG, Research plus)
- Cell culture incubator (Esco, model: CCL-170B-8)
- Biosafety cabinet (Esco, model: AC2-4S1)
- Ultra-low temperature freezer (Thermo, model: DW-86L338)
- Freezer (Siemens, model: KG32EV2S0C)
- Electro-heating standing-temperature cultivator (ENXIN, model: DRP-9052)
- Microscope (Leica, model: DM6B, equipped with a DFC7000T camera)
Software
- CellProfiler
- GraphPad Prism
Procedure
- Cell culture and sample preparation
- Pre-warm 2 ml 1x DEPC-PBS, 23 ml culture media and 0.8 ml 0.25% (w/v) trypsin-EDTA to 37 °C.
- Remove the used culture media in the cell culture flask (take 25 cm2 flask for example).
- Wash briefly with 2 ml 1x DEPC-PBS. Aspirate PBS.
- Add 0.8 ml 0.25% (w/v) trypsin-EDTA into flask and cover the cells.
- Check the cells with inverted microscope to be sure that most of the cells are rounded up, but still attached to the flask. Carefully remove trypsin.
- Add 3 ml culture media to the flask, suspend the cells from attached cell layer and mix them well by pipetting.
- Put 5 slides into a Petri dish and add 20 ml culture media into it to cover the slides.
- Seed the resuspended cells on slides.
- Rest for at least 10 min until resuspended cells subside on the slides.
- Transfer the Petri dish into the cell culture incubator (37 °C, 5% CO2).
- Culture the cells until they reach 80% confluence (12-48 h).
- After 12-48 h incubation, remove and discard the culture media.
- Wash 3 times with 25 ml 1x DEPC-PBS for 3 min each.
- Add 25 ml 4% (w/v) PFA into the Petri dish to cover the slides for 30 min at RT.
- Wash twice with 25 ml 1x DEPC-PBS for 3 min each.
- Perform dehydration using an ethanol series of 70%, 85%, and ethanol absolute for 5 min each. Air dry.
- Pretreatments
- Phosphorylate padlock probes before use. Prepare 50 μl mix containing 38 μl DEPC-H2O, 1 μl padlock probes (100 μM), 5 μl PNK buffer A (10x), 5 μl ATP (10 mM), 1 μl T4 polynucleotide kinase (10 U/μl) in the 0.2 ml PCR Tubes.
- Incubate the above mixture at 37 °C for 30 min and 65 °C for 10 min in the PCR machine.
- Bake the slide at 60 °C for 30 min.
- Put the slide into the xylene for 15 min and another fresh xylene for 10 min, respectively.
- Put the slide into ethanol absolute twice, 95% ethanol twice, and 70% ethanol twice for 2 min each time.
- Treat the slide with DEPC-H2O for 5 min to complete rehydration.
- Wash with DEPC-PBS for 2 min.
- Perform the fixation procedure with 4% (w/v) PFA in DEPC-PBS for 10 min.
- Wash with DEPC-PBS for 2 min.
- Put the slide into the solution of 0.1 mg/ml pepsin in 0.1 M HCl for 30 min at 37 °C.
- Wash with DEPC-H2O for 5 min.
- Wash with DEPC-PBS for 2 min.
- Dehydrate the slide by an an ethanol series of 70%, 85%, and ethanol absolute for 1 min each.
- Air dry.
- Assemble the 9 mm diameter and 0.8 mm depth Secure-Seal hybridization chamber to the surface of the slide on the side where the samples are (Figure 1). All the following incubation step take place in the chamber with a volume of 50 μl.
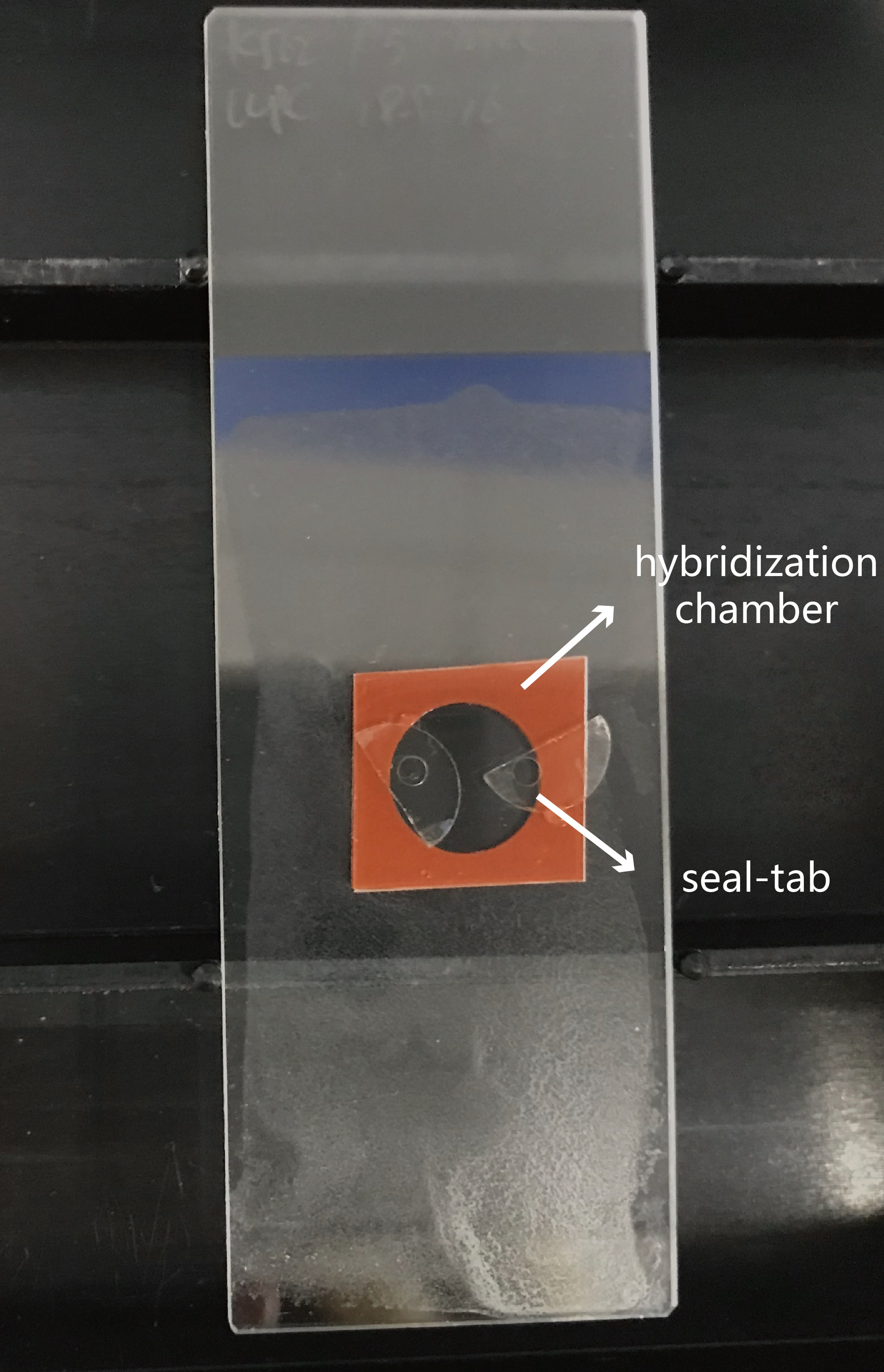
Figure 1. Assemble hybridization chamber to the surface of slide where the samples are. Place a seal-tab over each filling port to prevent evaporation of the solutions and the drying out of the specimen. - Rinse the cell sample with DEPC-PBS with 0.05% Tween-20 (DEPC-PBST).
- Add 50 μl 0.5%(v/v) Triton X-100 (in DEPC-PBS) into the chamber to permeabilize the cells for 10 min at RT.
- Wash 3 times with 1x DEPC-PBST.
- Apply 50 μl 3% (w/v) H2O2 solution to the sample for 15 min at RT to block endogenous peroxidases.
- Wash 3 times with DEPC-PBST.
- Padlock probe hybridization
- Prepare 50 μl padlock probe hybridization mix containing 22.5 μl DEPC-H2O, 25 μl 2x hybridization buffer and 2.5 μl phosphorylated padlock probe (2 μM, from B2).
- Add the mix into the chamber and incubate for 2 h at 37 °C.
- Wash 3 times with washing buffer for 5 min each.
- Wash 3 times with DEPC-PBST.
- Padlock probe ligation
- Prepare 50 μl padlock probe ligation mix containing 6.5 μl DEPC-H2O, 31.25 μl 80% (v/v) glycerol, 5 μl BSA (2 mg/ml), 1.25 μl RiboLock RNase Inhibitor (40 U/μl), 5 μl SplintR ligase reaction buffer (10x) and 1 μl SplintR ligase (25 U/μl).
- Add the mix into the chamber and incubate for 1 h at 37 °C.
- Wash 3 times with washing buffer for 5 min each.
- RCA primer hybridization
- Prepare 50 μl RCA primer hybridization mix containing 20 μl DEPC-H2O, 25 μl 2x hybridization buffer and 5 μl RCA primer (10 μM).
- Add the mix into the chamber and incubate for 30 min at 37 °C
- Wash 3 times with washing buffer for 5 min each.
- Wash 3 times with DEPC-PBST.
- RCA
- Prepare 50 μl RCA mix containing 26.75 μl DEPC-H2O, 5 μl 50% (v/v) glycerol, 2 μl dNTP (25 mM), 5 μl BSA (2 mg/ml), 1.25 μl RiboLock RNase Inhibitor (40 U/μl), 5 μl phi29 DNA polymerase buffer (10x) and 5 μl phi29 polymerase (10 U/μl).
- Add the mix into the chamber and incubate for 16-18 h at RT.
- Wash 3 times with washing buffer for 5 min each.
- Wash 3 times with DEPC-PBST.
- RCA products detection
- Prepare 50 μl RCA products detection mix containing 10 μl DEPC-H2O, 25 μl 2x detection buffer, 12.5 μl 80% (v/v) glycerol and 2.5 μl HRP-labeled detection probe (2 μM).
- Add the mix into the chamber and incubate for 30 min at RT.
- Wash 3 times with DEPC-PBST.
- Staining and mounting
- Incubate the sample with staining solution for 3 to 8 min depending on the color development.
- Wash 3 times with DEPC-H2O.
- Stain the nuclei in Hematoxylin staining solution for 1 min.
- Wash 3 times with DEPC-H2O.
- Differentiate with 0.1% hydrochloric acid-ethanol for 1 min.
- Wash 3 times with DEPC-H2O.
- Place slide in 1x DEPC-PBS for 1 min.
- Wash 3 times with DEPC-H2O.
- Remove the chamber and rinse in running tap water for 5 min.
- Dehydrate the slide by an ethanol series of 70%, 85%, and ethanol absolute for 1 min each.
- Mount the slide with 15-20 μl neutral balsam mounting medium.
- Image acquisition
- Acquire images with bright-filed microscope using the 40x objective.
- In images, brown dots are detected individual mRNA molecules and nuclei are shown in blue (Figures 2 and 3).
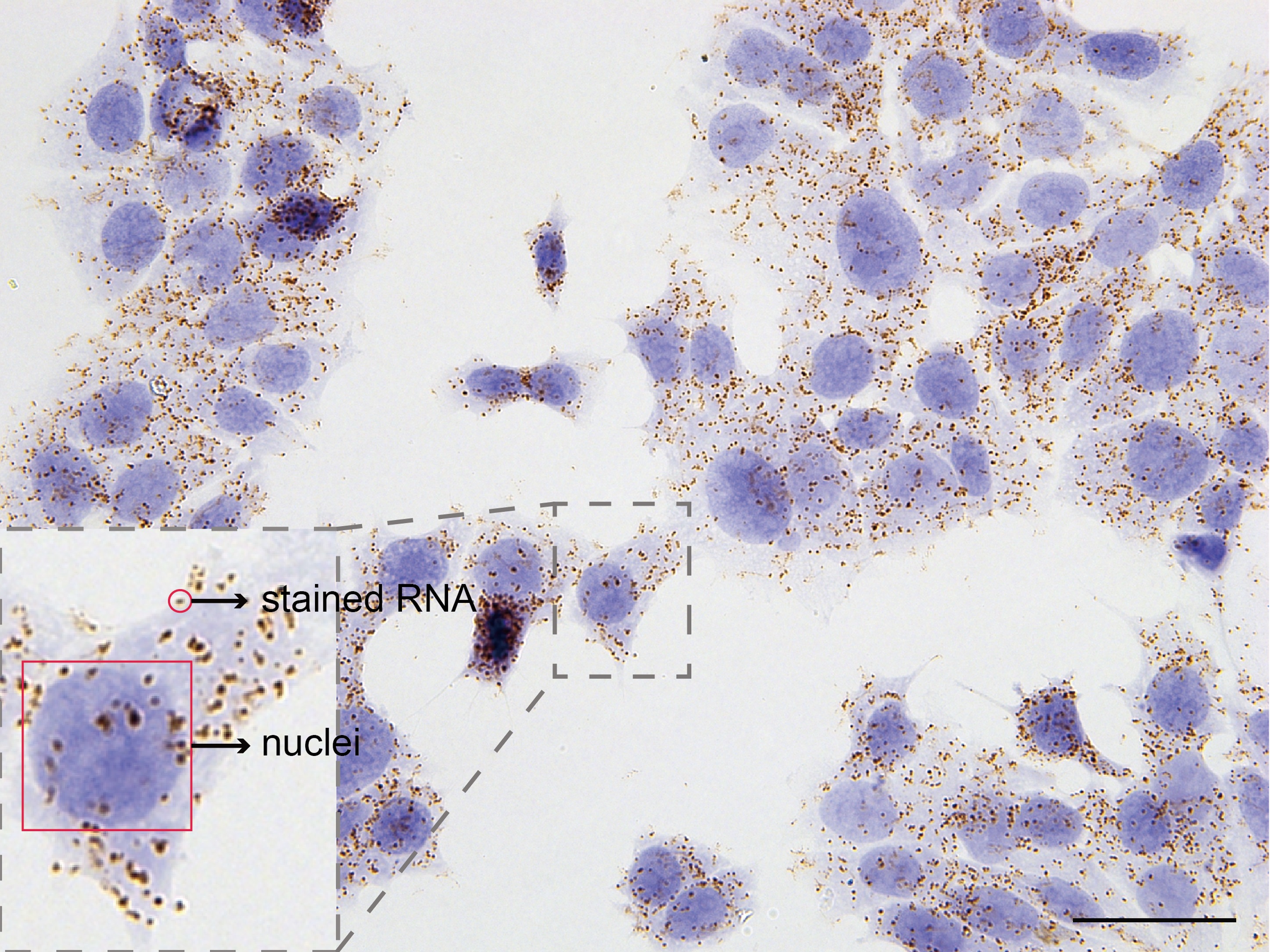
Figure 2. Detection of ACTB mRNA molecules in MCF-7 cells. Scale bar = 40 μm.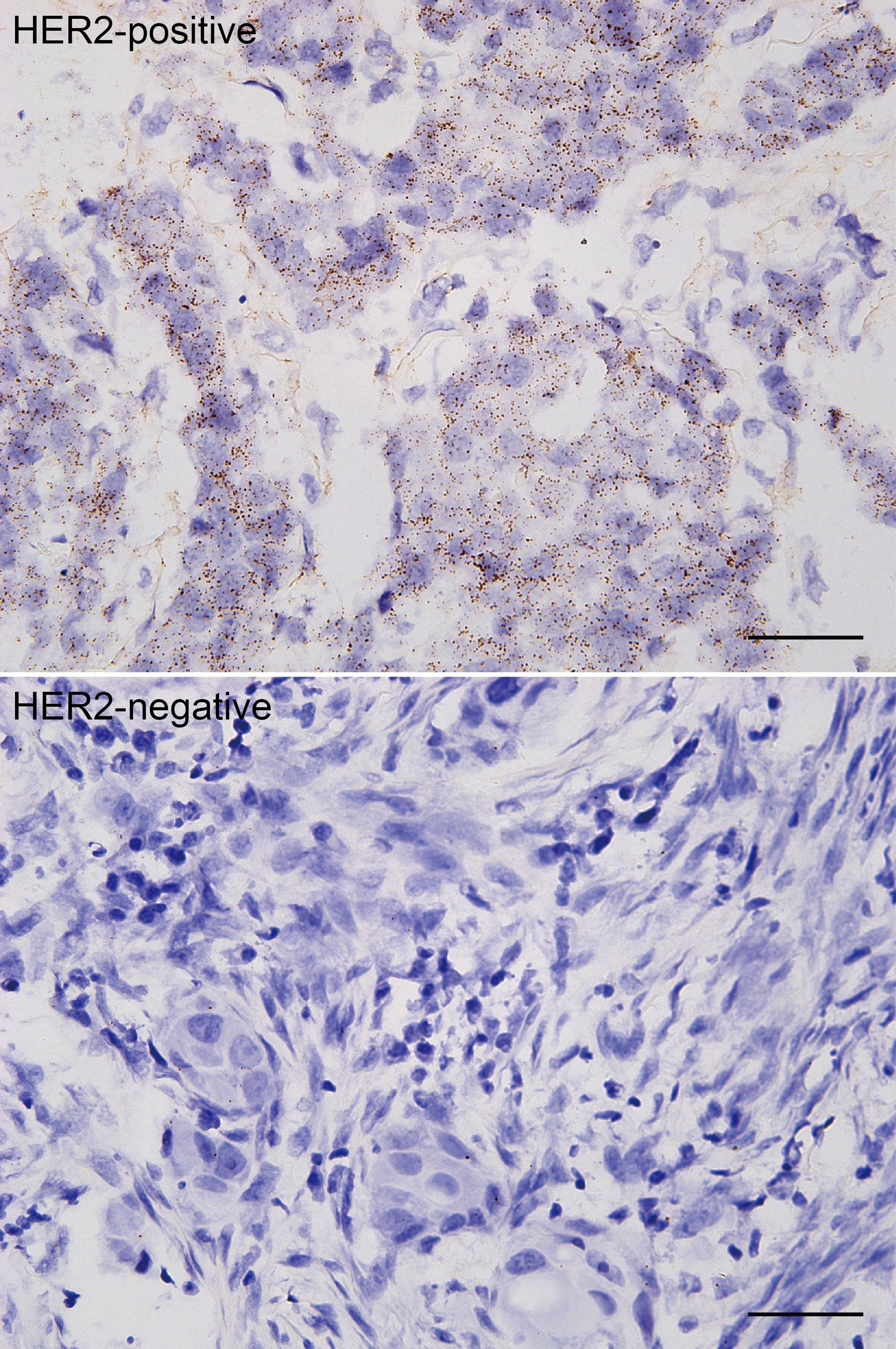
Figure 3. Detection of HER2 mRNA molecules in HER2-positive and HER2-negative breast cancer FFPE tissue sections. Scale bars = 40 μm.
Data analysis
Quantitative analysis of detected RNA molecules is achieved by using CellProfiler image analysis software. The images are processed with “Unmixcolors”, “EnhanceOrSuppressFeatures”, “IndentifyPrimaryObjects”, “IndentifySecondaryObjects”, “IndentifyPrimaryObjects” and “RelateObjects” modules in turn. To remove the background, the “Unmixcolors” module is used to split into hematoxylin and DAB two channels. The “EnhanceOrSuppressFeatures” module is used to highlight DAB staining spots. Finally, the relation of signals with their host cells is identified with the “RelateObjects” module. Then the number of RCPs per cell was listed. Statistical plotting was performed using GraphPad Prism.
Notes
- For FFPE tissue sections and fresh frozen tissue sections, the pretreatment is different due to different kinds of tissue, including permeabilization and fixation.
- Oligonucleotides were dissolved and diluted with 1x TE buffer. The stock concentration of the padlock probe, RCA primer and detection probe were 100 μM, 10 μM and 2 μM respectively.
- According to the cell type, adjust the experimental conditions of nucleic counterstaining, such as staining time, concentration of stain fluid.
- DEPC is heat inactivated before using the solution.
- Aspirate the solutions from the previous steps before adding a new solution into the chamber.
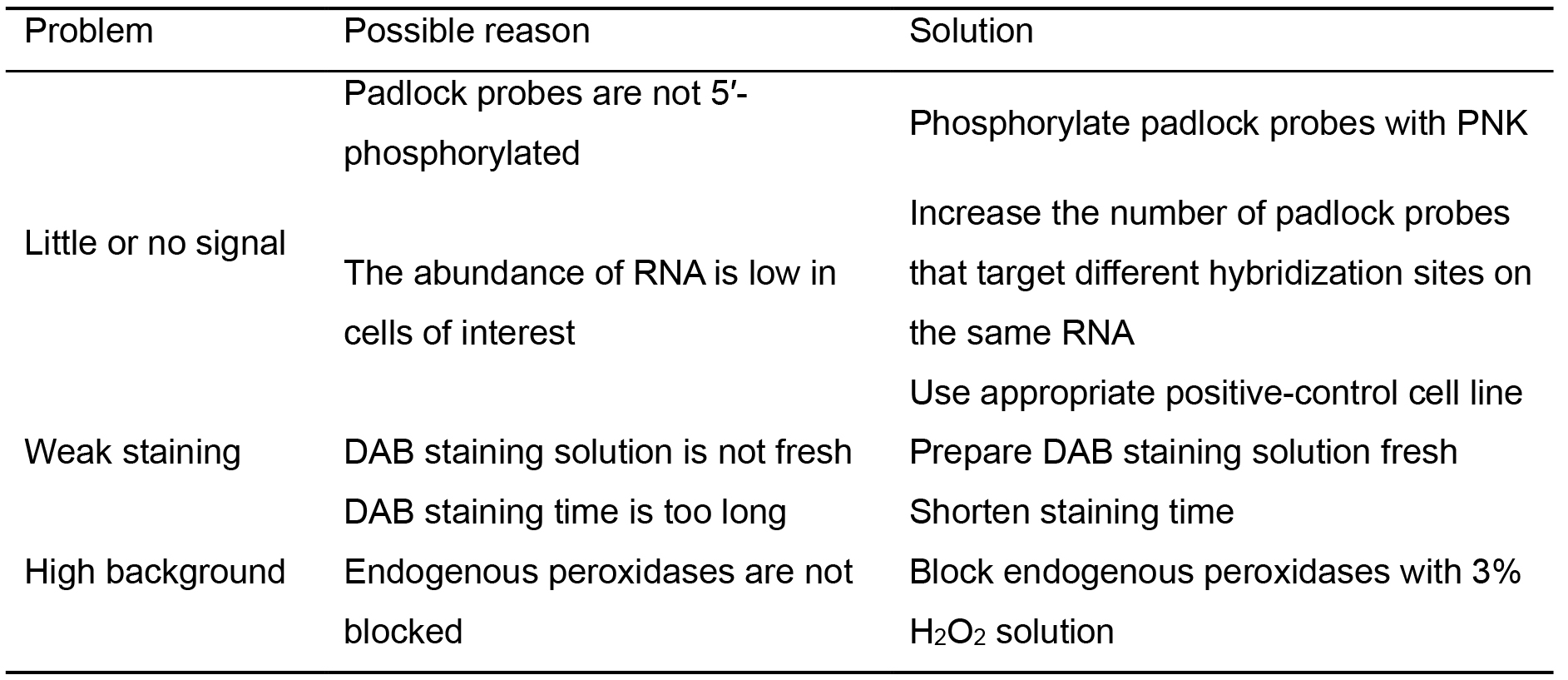
Table 1. Troubleshooting table
Recipes
- Culture media (50 ml)
45 ml serum-free medium
5 ml fetal bovine serum
0.5 ml Penicillin-Streptomycin Solution
Store at 4 °C - 10x PBS (500 ml), pH 6.8
40 g NaCl
1 g KCl
7.2 g Na2HPO4
1.2 g KH2PO4
500 ml ultrapure water
Filter the solution using 0.22 μm membrane filter
Prepare fresh - 1x DEPC-PBS (1 L), pH 7.4
100 ml 10x PBS
900 ml ultrapure water
1 ml DEPC
Store at 4 °C - 1x DEPC-PBST (1 L)
1 L DEPC-PBS
0.5 ml Tween-20
Store at 4 °C - 4% (w/v) PFA (500 ml)
For a 4% paraformaldehyde solution:- Add 20 g of EM grade paraformaldehyde to 480 ml of 1x DEPC-PBS
- Add 5 ml of 5 M NaOH and stir gently on a heating block at ~30 °C until the paraformaldehyde is dissolved
- Allow the mixture to cool to room temperature and adjust the pH to 7.4 with HCl
- Then adjust the final volume to 500 ml with 1x DEPC-PBS
- Filter the solution through a 0.22 μm membrane filter to remove any particulate matter
- Make the paraformaldehyde solution fresh prior to use, or store in aliquots at -20 °C for several months. Avoid repeated freeze/thawing
- 1x TE buffer (100 ml), pH 8.0
1 ml 1 M Tris-HCl (pH 8.0)
0.2 ml 0.5 M EDTA (pH 8.0)
98.8 ml DEPC-H2O
Filter the solution using 0.22 μm membrane filter
Store at 4 °C - 0.5%(v/v) Triton X-100 (5 ml)
25 μl Triton X-100
4.975 ml DEPC-H2O
Store at RT - 3% (w/v) H2O2 solution (50 μl)
5 μl 30% (w/v) H2O2
45 μl DEPC-H2O
Prepare fresh - 2x hybridization buffer (5 ml)
3 ml 20x SSC
1 ml Formamide
1 ml DEPC-H2O
Store at RT - Washing buffer (15 ml)
1.5 ml 20x SSC
3 ml Formamide
10.5 ml DEPC-H2O
Store at RT - 2x detection buffer (5 ml)
1 ml 20x SSC
5 μl Tween-20
0.2 ml 20 mg/ml BSA
3.975 ml DEPC-H2O
Store at 4 °C - Staining buffer (50 μl)
45 μl DAB buffer
2.5 μl 20x DAB substrate solution
2.5 μl 20x DAB chromogen solution
Prepare fresh - 0.1% hydrochloric acid-ethanol (5 ml)
50 μl hydrochloric acid
3.713 ml ethanol
1.237 ml DEPC-H2O
Store at RT - Hematoxylin Staining Solution (50 ml)
- Dissolve 0.1 g hematoxylin with 12.5 ml ethanol
- Dissolve 0.85 g aluminum potassium sulfate dodecahydrate with 37.5 ml DEPC-H2O
- Then mix the two solutions
- After hematoxylin is oxidized to a purplish red by adding 10 mg sodium iodate, add 0.5 ml acetate
- Filter the solution using 0.22 μm membrane filter. Store at RT
- Concentration of hematoxylin staining solution can be diluted with DEPC-H2O
- 25 mM dNTP (100 μl)
25 μl 100 mM ATP
25 μl 100 mM GTP
25 μl 100 mM CTP
25 μl 100 mM TTP
Store at -20 °C
Competing interests
Authors declare no conflicts of interest or competing interests.
Acknowledgments
This work was supported by the funds from the National Natural Science Foundation of China (31770927), the National Key Research and Development Program of China (2017YFA0106800), the Natural Science Foundation of Fujian Province, and the Scientific Research Funds of Huaqiao University.
References
- Banér, J., Nilsson, M., Mendel-Hartvig, M. and Landegren, U. (1998). Signal amplification of padlock probes by rolling circle replication. Nucleic Acids Res 26(22): 5073-5078.
- Battich, N., Stoeger, T. and Pelkmans, L. (2013). Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nat Methods 10(11): 1127-1133.
- Crosetto, N., Bienko, M. and van Oudenaarden, A. (2015). Spatially resolved transcriptomics and beyond. Nat Rev Genet 16(1): 57-66.
- Femino, A. M., Fay, F. S., Fogarty, K. and Singer, R. H. (1998). Visualization of single RNA transcripts in situ. Science 280(5363): 585-590.
- Jiang, M., Liu, L., Hong, C., Chen, D., Yao, X., Chen, X., Lin, C. and Ke, R. (2019). Single molecule chromogenic in situ hybridization assay for RNA visualization in fixed cells and tissues. RNA 25(8): 1038-1046.
- Larsson, C., Grundberg, I., Soderberg, O. and Nilsson, M. (2010). In situ detection and genotyping of individual mRNA molecules. Nat Methods 7(5): 395-397.
- Nilsson, M., Malmgren, H., Samiotaki, M., Kwiatkowski, M., Chowdhary, B. P. and Landegren, U. (1994). Padlock probes: circularizing oligonucleotides for localized DNA detection. Science 265(5181): 2085-2088.
- Shah, S., Lubeck, E., Schwarzkopf, M., He, T. F., Greenbaum, A., Sohn, C. H., Lignell, A., Choi, H. M., Gradinaru, V., Pierce, N. A. and Cai, L. (2016). Single-molecule RNA detection at depth by hybridization chain reaction and tissue hydrogel embedding and clearing. Development 143(15): 2862-2867.
- Wang, F., Flanagan, J., Su, N., Wang, L. C., Bui, S., Nielson, A., Wu, X., Vo, H. T., Ma, X. J. and Luo, Y. (2012). RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14(1): 22-29.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jiang, M., Lin, C. and Ke, R. (2020). Detection of Individual RNA in Fixed Cells and Tissues by Chromogenic ISH. Bio-protocol 10(3): e3510. DOI: 10.21769/BioProtoc.3510.
Category
Cancer Biology > General technique > Biochemical assays > RNA
Cell Biology > Cell imaging > Confocal microscopy
Molecular Biology > RNA > RNA labeling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









