- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Implanting and Recycling Neuropixels Probes for Recordings in Freely Moving Mice
Published: Vol 10, Iss 3, Feb 5, 2020 DOI: 10.21769/BioProtoc.3503 Views: 7141
Reviewed by: Edgar Soria-GomezGeoffrey C. Y. LauPavel Rueda

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 80 Views
Abstract
Recording neural activity in unrestricted animals is necessary to unravel the neural basis of ethological behaviors. Recently, Neuropixels probes have made important strides in improving yield and lowering noise, but have limited use cases in freely moving animals. Although there are a number of studies demonstrating the use of these probes in headfixed mice, there are not established protocols for the use and reuse of them in a freely moving mouse. We therefore designed a novel device (the AMIE) that maximizes the potential value of these powerful probes. Here, we provide the technical drawings for the AMIE and detail its preparation, implantation, and explantation. With our approach, researchers can record hundreds of neurons during freely moving behavior across weeks of experiments, and then recycle valuable probes for future use.
Keywords: ElectrophysiologyBackground
Understanding the brain during movement has proven crucial for investigating mechanisms of behaviors such as memory, fear, and exploration (e.g., O’Keefe and Dostrovsky, 1971; Hafting et al., 2005; Stujenske et al., 2014; Kerekes et al., 2017). Many researchers have investigated such behaviors by pioneering methods to record neural activity in freely moving rodents, including static electrode arrays or microdrives (Vandecasteele et al., 2012; Voigts et al., 2013; Okun et al., 2016). However, these techniques are not at pace with recent advancements in the spatial and temporal resolution of recording devices such as Neuropixels probes (Jun et al., 2017). Although previous studies have demonstrated the use of silicon probes in freely moving animals (Okun et al., 2016), we sought to design a device that would also enable the recycling of valuable Neuropixels probes. Our device, the AMIE, can be used to implant Neuropixels probes for experiments that record neural activity over weeks of behavior. After experiments, the AMIE can be explanted and used in additional experiments (Juavinett et al., 2019). Importantly, the AMIE can also be adapted for similar recording devices and for recording from various parts of the brain. As we have previously demonstrated, this device can empower researchers to use and reuse these probes to simultaneously record hundreds of neurons in freely moving animals (Juavinett et al., 2019). Here, we provide a detailed protocol for other researchers to do the same.
Materials and Reagents
For neuropixel casing assembly
- 2-56A Screws (Amazon, catalog number: B00F34U238)
- 30AWG silver wire (WPI, catalog number: AGW1010)
- Medical-grade clear silicon adhesive (Mastersil, catalog number: 912MED)
- Loctite Instant Adhesive 495 (ULINE, catalog number: S-17190)
- Kapton Tape (ULINE, catalog number: S-7595)
- Bleach
For Neuropixels assembly protective case
- M6 Cap Screw (Thorlabs, catalog number: SH6MS20)
- M6 Nut (Thorlabs, part in kit, catalog number: HW-KIT2/M)
Surgical equipment
- Kimwipes (Kimtech, catalog number: 34120)
- Silicone Gel Kit (Dow Corning, catalog number: 3-4860)
- Isoflurane (Isothesia; Allivet, catalog number: 50562)
- MediGel CPF (Clear, catalog number: H20 74-05-5022)
- C&B Metabond Radiopaque L-Powder (Parkell, catalog number: S396)
- C&B Metabond 'B' Quick Base (Parkell, catalog number: S398)
- C&B Metabond 'C' Universal TBB Catalyst (Parkell, catalog number: S371)
- OptiBond Solo Plus (Kerr, catalog number: 31514)
- Vetbond (Santa Cruz Biotechnology, catalog number: sc-361931)
- Eye ointment (Rugby, catalog number: 370435)
- Mouse anesthesia system with isoflurane box (Parkland Scientific, catalog number: V3000PK)
- DMSO
- Metacam
- Meloxicam (1.5 mg/ml) (see Recipes)
- DiI Stain (see Recipes)
Equipment
For neuropixel casing assembly
- Neuropixel Probe
Probes can be ordered at http://www.neuropixels.org/order. We recommend using the ‘silicon spacer’ version for this protocol–the metal cap can make it difficult to attach the probe to the internal mount. - 3D Printed Internal Mount and External Casing (Figure 1; https://github.com/churchlandlab/ChronicNeuropixels)
Note: We printed and tested in VeroWhite material using a Stratasys Eden 260VS PolyJet 3D Printer with 16 µm resolution. - Stereotax Adaptor (machined from Aluminum or Stainless Steel, designs found here: https://github.com/churchlandlab/ChronicNeuropixels)
For Neuropixels assembly protective case
- 4” post holder with thumbscrew (Thorlabs, catalog number: PH4)
- Slim right angle bracket (Thorlabs, catalog number: AB90B)
- Aluminum Breadboard (Thorlabs, catalog number: MB624)
- Custom acrylic box with dimensions: L24” x W6” x H8”
For surgery
- Charisma A1 Syringe (Net32, catalog number: 66000085)
- Lidocaine (injectable or cream; e.g., Actavis, catalog number: NDC 0591-2070-30)
- Oxygen cylinders (Airgas, catalog number: OX USP300)
- Dental drill (Osada, model: EXL-M40)
- 0.9-mm burrs for micro drill (Fine Science Tools, catalog number: 19007-09)
- T/Pump Warm Water Recirculator (Kent Scientific, catalog number: TP-700) with warming pad (Kent Scientific, catalog number: TPZ)
- Cotton applicators (Fisher Scientific, catalog number: 19-062-616)
- Dental Cement
- DiI (ThermoFisher Scientific, catalog number: D282)
- Surgical Spears (Braintree Scientific Inc., catalog number: SP 40815)
- Small rodent stereotax fitted with anesthesia mask (Narishige, catalog number: SG-4N)
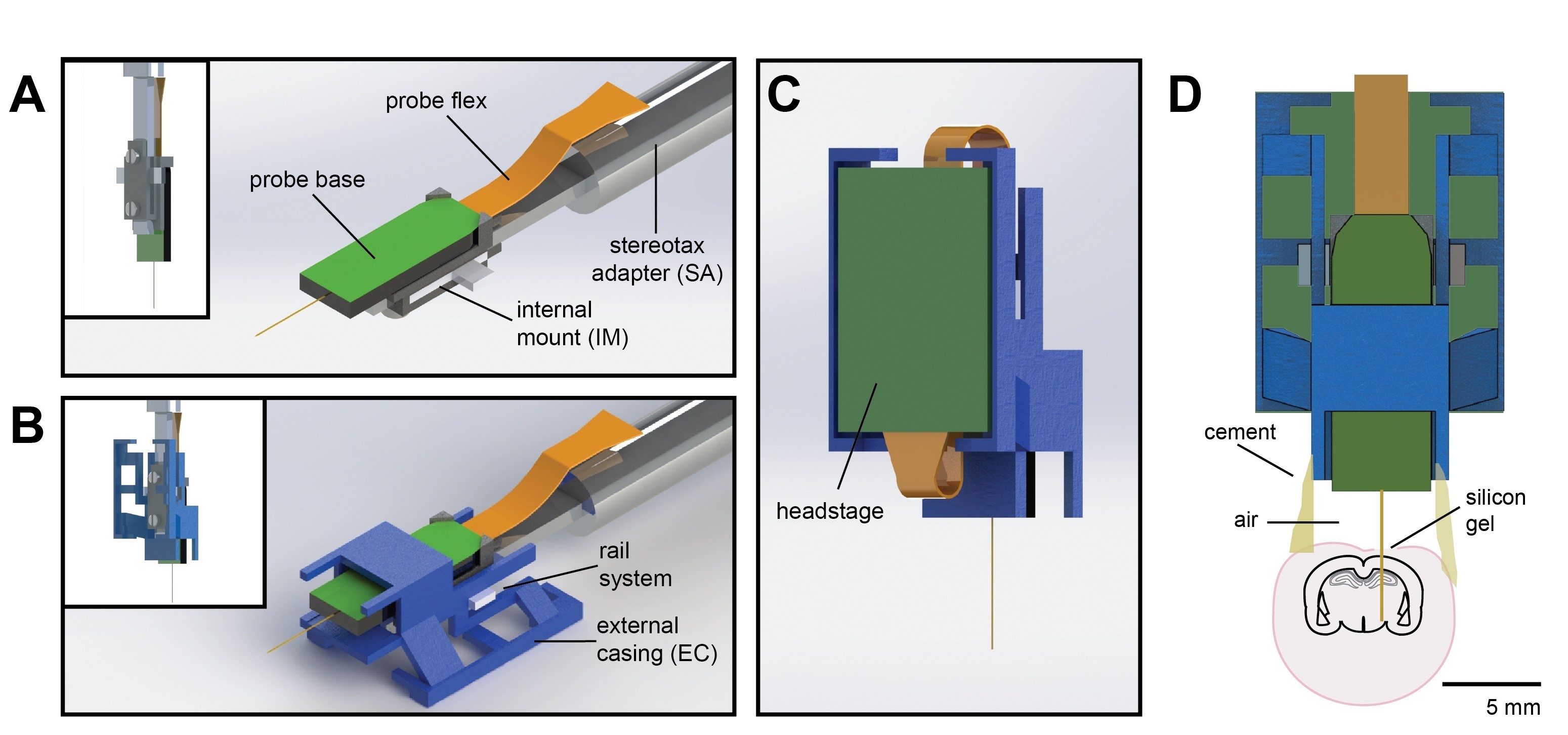
Figure 1. Schematic of Neuropixels AMIE. A. Probe base mounted onto 3D printed internal mount (IM) and attached to machined metal stereotax adapter (SA). Inset: Rear view, with screws that attach the IM to the SA. B. Entire assembly in a. within 3D printed external casing (EC). Inset: Rear view. C. The headstage is positioned on the back of the encasing, with the flex wrapped in an "S" shape. D. Entire assembly in relation to size of mouse brain and skull. The EC is attached to the skull with cement. Silicon gel is used to as an artificial dura to protect the open craniotomy.
Procedure
Note: We recommend performing the entire process of preparing and implanting the probe using a dummy probe for practice.
- Making AgCl wire
- Remove any insulation from silver wire using forceps or a razor blade.
- Leave silver wire in 100% bleach for 30 min or until black.
- Assembling Neuropixels holder and storage box (not shown)
Before mounting the probe, ensure that you have a secure location to store it. We’ve designed a display case for mounted Neuropixels storage:- Attach a Thorlabs slim right angle bracket to an aluminum breadboard.
- Mount 4” post holder with thumb screw to the right angle bracket.
- Cover breadboard with custom acrylic box.
- Mounting the probe
- Hand-tap the internal mount (IM) screw holes with a #2-56 thread. The screw holes on the (SA) should be tapped during the machining process as this is a metal part.
- Mount the SA in its holder on the breadboard.
- Slide the IM onto the SA. Align holes and screw into place (Figure 2A).
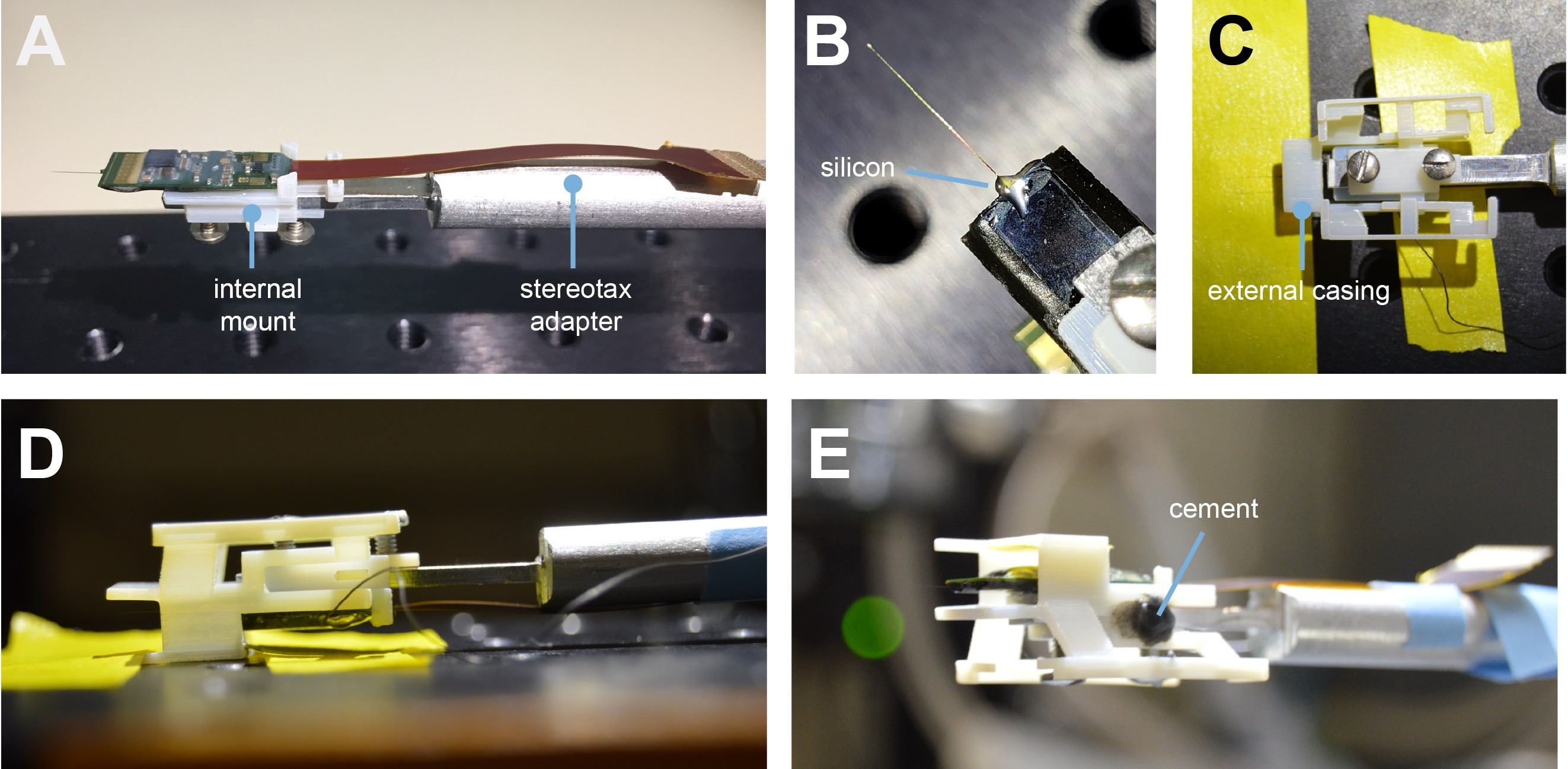
Figure 2. Mounting Neuropixels probe into the encasing. A. The internal mount (IM) is attached to the stereotax adapter with two screws, and probe is attached to the internal mount using an epoxy. B. The external case (EC) is attached to a breadboard, and the IM+probe assembly is carefully guided into the internal compartment of the EC (top view). C. Medical-grade silicon is added to the base of the shank to add extra support. D. Same as (B) but sideview. E. After cementing the IM to the EC, the entire assembly is ready to be implanted - Mount the Neuropixels to the IM by applying a small drop of cyanoacrylate to the face of the IM. Using one hand, gently lay the probe into place (Figure 2A).
CRITICAL STEP: There are two angled spokes on the face of the IM which allows for proper alignment (Figure 1A). The silver part on the PCB probe is the part that should be aligned with the axis of the shank. Therefore, this silver part is where the probe should be glued to the IM. The flex should fit snugly between the angled spokes and can be taped to the SA to keep it out of the way. Allow the adhesive to dry. - Solder the reference and ground sites on the Neuropixels (see https://github.com/cortex-lab/neuropixels/wiki/Referencing for additional information).
- Solder a 10 cm piece of the prepared AgCl wire to the soldered reference/ground sites.
Note: If you’re re-using a previously implanted probe, and need to attach a new ground wire, you may choose to solder it to the PCB board, or to the end of the old ground wire. - Using a needle or wire, apply a tiny drop of the MasterSil 912 Med to the interface of the shank and the PCB board. This will reinforce the shank and, in our hands, seemed to improve the chances of a successful explant (Figure 2B).
- Place IM/SA/probe assembly in a micromanipulator or stereotax. Secure (with tape or wax) the EC so that it is aligned with the IC/SA/probe assembly. Slowly position the IM/SA assembly so the IM’s wings slide into the ECs rail system (Figures 2C-2E). It is imperative to verify the Neuropixels shank isn’t coming in contact with the ECs walling. The IC wing should be equidistant from each EC rail (Figures 2C and 2D).
- Apply a small drop of epoxy or cement to each wing/rail system interface and let dry.
Note: It is perilous applying anything directly to the shank; work with caution. - Using a stereotax or micromanipulator, lower the Neuropixels shank in saline solution and test the acquisition.
- Implanting procedure
Note: Mice should be given an NSAID at least one day prior to surgery. We recommend oral Carprofen (Medigel) to avoid handling mice after the procedure. Injectables such as Metacam (5 mg/kg) also work well.
- Anesthetize the mouse with isoflurane (2% in oxygen for induction, 1.5% during surgery).
- Prepare the mouse for surgery: apply eye lubricant to the eyes, and secure the mouse in a stereotax on a heat pad. Remove hair from the scalp and apply lidocaine.
Note: To ensure proper targeting of the probe, be sure that the mouse’s skull is centered and level in the stereotax. - Apply Vetbond to the edges of the incision to secure the skin to the skull.
- Draw a mark on the skull for the desired implant site. It is useful to put a “dummy” pipette into the stereotax in order to identify the target coordinates.
- Draw a second mark for the ground screw site–we have successfully placed the ground screw over contralateral cortex (Figure 3A), as well as over the cerebellum.
Note: After marking the implant and ground skull sites, one can affix the headbar. - (Optional) Using the Metabond cement, affix the headbar to the skull.
- Apply a thin layer of OptiBond to the skull.
CRITICAL STEP: Avoid the craniotomy site and animals skin. Perform three UV curing sessions, each lasting thirty seconds and spaced apart thirty seconds. - Apply a ring of Charisma adhesive on top of the OptiBond, avoiding the skin and ground screw.
CRITICAL STEP: The Charisma will provide a protective barrier around your craniotomy and electrode site.
Let Charisma dry for ~10 min (Figure 3A).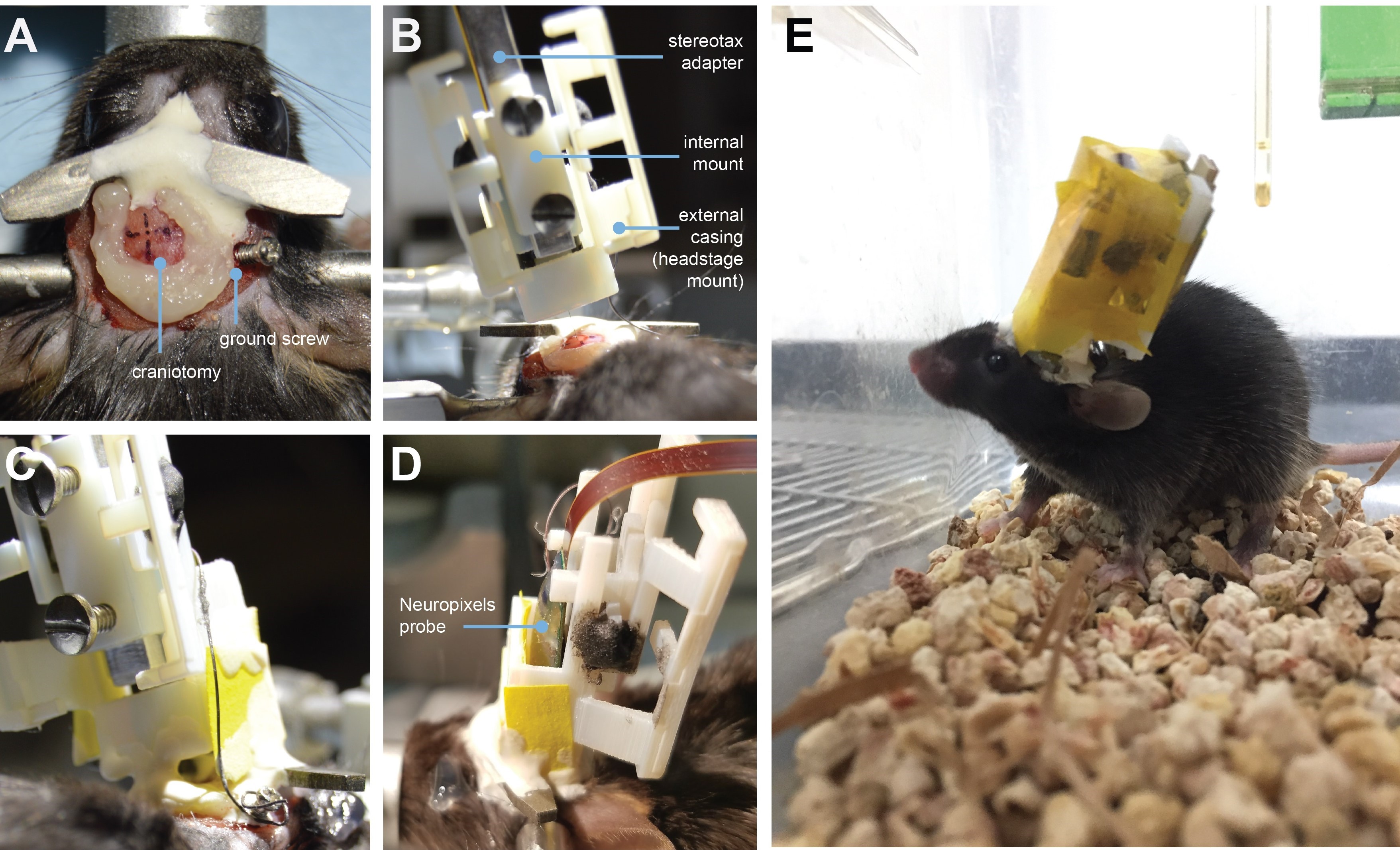
Figure 3. Implantation procedure. A. Implant site, with headbar already affixed. Target coordinates are marked and surrounded by a ring of Charisma. The ground screw is implanted posterior to the target site (it can also be implanted over the cerebellum). B. Lowering Neuropixel into the brain at a ~16 degree angle. C. The entire encasing is attached to the headbar and skull using Metabond (rearview). Tape is added where necessary to add protection between the encasing and the skull. The ground wire extends down the side of the implant and is attached to the ground screw. The stereotax adapter is removed after this support structure is dry. D. Same as (C), after stereotax adapter is removed, sideview. After the implant is cemented in place, the entire assembly is wrapped with Kapton tape. E. The animal fully recovered with the implant. - Perform a small craniotomy for the ground screw and slowly rotate the screw into place. The skull screw should be lowered 0.2-0.35 mm so it is in contact with the surface of the brain.
CRITICAL STEP: Make sure the ground screw will not interfere with placement of the Neuropixels casing. - Carefully drill a 1-2 mm circular craniotomy at the implant site. Gently apply saline to clean the craniotomy area.
- Attach SA/IM/EC assembly to stereotax and position above the brain (Figure 3B).
- Carefully apply DiI solution to the Neuropixels shank.
CRITICAL STEP: The DiI solution will attract the Neuropixels shank, possibly pulling it away. We recommend doing this step by pushing out a drop of the DiI solution from a syringe needle, and slowly moving the drop along the length of the shank. - Remove excess saline and blood at the craniotomy site using surgical spears and Kimwipes.
- Zero the “Z” axis on the stereotax (if relevant) and slowly lower the shank into the brain (Figure 3B).
- CRITICAL STEP: Monitor the shank for bending during implantation. Watch for bleeding and absorb with surgical spears as necessary. Stop lowering at the halfway point so the craniotomy site is still visible.
- TROUBLESHOOTING: If the probe will not enter the brain without significant bending, try a slightly different entry location. You may also try to dry the dura just slightly before insertion.
- Mix the white and blue silicon gel (1:1) and carefully apply a thin layer to the craniotomy site. This silicon gel helps to protect the exposed brain after the implant.
- Slowly lower the second half of the shank and monitor for bending. Watch for bleeding and absorb with surgical spears. The bottom of the external assembly should be a few millimeters from the skull surface.
- Once the probe is fully inserted to your desired depth, wrap the ground wire around the ground screw.
- Apply Metabond to EC and skull to build up a support structure that will mount the assembly to the skull (Figures 3C and 3D). You may also need to use tape to protect any remaining openings between the EC and skull.
CRITICAL STEP: It is imperative that no adhesive enter the internal cavity of the EC otherwise this could bind to the Neuropixels shank or the IM, interfering with recording and explantation. Allow the Metabond to dry for ~15 min. - Remove the screws from the SA/IM assembly and slowly raise the SA from the IM/EC assembly.
CRITICAL STEP: Monitor each component of the assembly to ensure that the SA is the only component pulling away from the assembly. If there is any movement of the EC, pause and identify the source of friction.
- Explanting procedure
- Anesthetize the mouse with isoflurane (2% in oxygen for induction, 1.5% during surgery).
- Remove the mouse from isoflurane box, apply eye lubricant to the mouse's eyes, and secure the mouse in stereotax on a heat pad.
Note: It can be difficult to perfectly align the mouse’s head with the SA. It may be useful to have the mouse loosely positioned in the ear bars in order to make this easier. However, be sure that the mouse’s head is secure while raising the SA/IM assembly (Step E7 below). - Unravel the ground wire from the screw (if possible) or cut the ground wire (if necessary).
- Align the SA with the IM, and slowly lower until screw holes are aligned.
CRITICAL STEP: Carefully watch the SA as you’re lowering into the IM because poor alignment will cause tension during the lowering process. This will apply pressure to the skull which can cause bleeding. If you implanted the assembly at an angle, make sure the SA is being lowered at the same angle. - Screw the SA into place.
- Drill away dental cement interfacing the IM and EC’s rail system.
CRITICAL STEP: Be careful not to drill away any plastic on the IM or cause damage to the Neuropixels PCB board. - Once you are confident that there is no cement binding the EC to the IM, slowly raise the SA/IM assembly from the EC.
Note: There will likely be debris on the shank after removing it. Leave the shank in tergazyme and gently agitate in order to remove it.
Notes
There are a wide range of applications for Neuropixels. Each of these will call for a particular set of implant coordinates and probe angles depending on the brain regions researchers hope to study. To this end, we have included the original, modifiable design-files in our supplementary documentation. If it is possible, we recommend performing practice surgeries to confirm that the implant design will work for your experiment, and modifying these to your needs.
- Angle of implant. The surgery procedure detailed above can be modified for different angles of implants. We have had success with implants up to ~16 degrees. Researchers should determine an appropriate implant angle for the desired recording sites.
- Bottom of encasing. The EC is designed to allow researchers to monitor the shank location while implanting the probe. However, this means that these gaps on the side of the EC will need to be closed after implantation, either with cement or tape (Figures 2C and 2D). This gap will change depending on how deep the probe is implanted, and the angle of the implant. We recommend modifying the bottom of the encasing to best suit your needs.
- Addition of a headbar. Headbars are commonly used to restrain mice, and researchers may want to also affix a headbar for such experiments. The front of the external casing here is designed to accommodate room for a headbar in the front, just behind the eyes. Although the current protocol does not detail a headbar implant, we indicate where it would make sense to do this.
- Recording during implantation. If possible, it is useful to record from the probe during implantation. Of course, the researcher should take care to ground the surgery setup (especially the earbars) in order to do so.
Recipes
- Meloxicam (1.5 mg/ml)
Add 0.1 ml of Metacam (1.5 mg/ml) to 10 ml of saline - DiI Stain
Make a 0.5% dilution of DiI in DMSO
Note: Although DMSO is not officially approved by IMEC for use with the Neuropixels probe, it doesn’t cause any noticeable decay in the signal or probe quality.
Acknowledgments
This work was originally published in eLife (Juavinett et al., 2019).
We would like to acknowledge Tim Harris for his leadership on the development of the Neuropixels probes and his constant encouragement of this project.
The UCL Neuropixels course, taught by Nick Steinmetz, Matteo Carandini, Andrew Peters, Adam Kampff, was imperative in getting this project off the ground (http://www.ucl.ac.uk/neuropixels/courses). In particular, we would like to thank Nick Steinmetz for his critically important feedback, code, and upkeep of the Neuropixels Wiki page (https://github.com/cortex-lab/neuropixels/wiki).
We would also like to acknowledge Claudia Boehm and Albert Lee (Janelia Research Campus) for allowing us to observe their rat Neuropixels implant. Their protocol served as an important starting point for the protocol we developed in mice.
We have also benefitted from troubleshooting help from many individuals, including Wade Sun, James Jun, Marius Bauza and Bill Karsh (SpikeGLX).
We are also grateful to the CSHL Undergraduate Research Program, which yearly provides a diverse group of students with funding and resources to complete invaluable research experiences at CSHL. This program funded G.B. for his initial summer in our lab.
We welcome feedback from the community regarding the diversity of methods used to implant and record with these probes.
Competing interests
None of the authors have competing interests.
Ethics
All surgical and behavioral procedures conformed to the guidelines established by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Cold Spring Harbor Laboratory, approval number 19-16-13-10-7, valid until June 7, 2020.
References
- Hafting, T., Fyhn, M., Molden, S., Moser, M. B. and Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436(7052): 801-806.
- Juavinett, A. L., Bekheet, G. and Churchland, A. K. (2019). Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. Elife 8: e47188.
- Jun, J. J., Steinmetz, N. A., Siegle, J. H., Denman, D. J., Bauza, M., Barbarits, B., Lee, A. K., Anastassiou, C. A., Andrei, A., Aydin, C., Barbic, M., Blanche, T. J., Bonin, V., Couto, J., Dutta, B., Gratiy, S. L., Gutnisky, D. A., Hausser, M., Karsh, B., Ledochowitsch, P., Lopez, C. M., Mitelut, C., Musa, S., Okun, M., Pachitariu, M., Putzeys, J., Rich, P. D., Rossant, C., Sun, W. L., Svoboda, K., Carandini, M., Harris, K. D., Koch, C., O'Keefe, J. and Harris, T. D. (2017). Fully integrated silicon probes for high-density recording of neural activity. Nature 551(7679): 232-236.
- Kerekes, P., Daret, A., Shulz, D. E. and Ego-Stengel, V. (2017). Bilateral discrimination of tactile patterns without whisking in freely running rats. J Neurosci 37(32): 7567-7579.
- O'Keefe, J. and Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34(1): 171-175.
- Okun, M., Lak, A., Carandini, M. and Harris, K. D. (2016). Long term recordings with immobile silicon probes in the mouse cortex. PLoS One 11(3): e0151180.
- Stujenske, J. M., Likhtik, E., Topiwala, M. A. and Gordon, J. A. (2014). Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83(4): 919-933.
- Voigts, J., Siegle, J. H., Pritchett, D. L. and Moore, C. I. (2013). The flexDrive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front Syst Neurosci 7: 8.
- Vandecasteele, M, M.S., Royer, S., Belluscio, M., Berényi, A., Diba, K., Fujisawa, S., Grosmark, A., Mao, D., Mizuseki, K., Patel, J., Stark, E., Sullivan, D., Watson, B. and Buzsáki, G. (2012). Large-scale recording of neurons by movable silicon probes in behaving rodents. J Vis Exp 4(61): e3568.
Article Information
Copyright
Juavinett et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Juavinett, A. L., Bekheet, G. and Churchland, A. K. (2020). Implanting and Recycling Neuropixels Probes for Recordings in Freely Moving Mice. Bio-protocol 10(3): e3503. DOI: 10.21769/BioProtoc.3503.
- Juavinett, A. L., Bekheet, G. and Churchland, A. K. (2019). Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. Elife 8: e47188.
Category
Neuroscience > Behavioral neuroscience > Animal model
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








