- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sulfatase Assay to Determine Influence of Plants on Microbial Activity in Soil
Published: Vol 10, Iss 2, Jan 20, 2020 DOI: 10.21769/BioProtoc.3490 Views: 5599
Reviewed by: Zhibing LaiYing FengShweta Panchal

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vitro Auto- and Substrate-Ubiquitination Assays
Hye Lin Park [...] Gyeong Mee Yoon
Apr 5, 2022 3551 Views

Measuring in vitro ATPase Activity with High Sensitivity Using Radiolabeled ATP
Sarina Veit and Thomas Günther Pomorski
May 20, 2023 2301 Views

A Semi-throughput Procedure for Assaying Plant NADP-malate Dehydrogenase Activity Using a Plate Reader
Kevin Baudry and Emmanuelle Issakidis-Bourguet
Aug 20, 2023 1506 Views
Abstract
Sulfatase activity is often used as a measure of the activity of soil microorganisms. It is thus a suitable tool to investigate the response of microbes to plants. Here we present a method to determine the influence of various Arabidopsis genotypes on the function of soil microbiota using the sulfatase as a quantitative measure. We grew the plants in soil/sand mix under control conditions and measured the sulfatase activity in soil using a spectrophotometric determination of the product. This protocol can be used to test the contribution of individual genes to control of microbiome assembly through analysis of mutants as well as the influence of environment on plant-microbe interactions.
Keywords: ArabidopsisBackground
Plants in their natural environment interact with plethora of microorganisms, pathogenic as well as beneficial. Many microorganisms are beneficial to plants, e.g., by improving their immunity or their nutrition (Kertesz and Mirleau, 2004; Jacoby et al., 2017; Stringlis et al., 2018). Indeed, the role of bacteria in plant sulfur nutrition has long been recognized (Kertesz and Mirleau, 2004). Sulfur is present in soil mainly bound to organic compounds and thus not available to plants. However, bacteria and fungi can metabolize such organosulfur compounds and release the sulfate group to the rhizosphere, where it can be utilized by plants, and improve so plant sulfur nutrition (Gahan and Schmalenberger, 2014). One of the enzymes catalyzing such reactions is sulfatase. This enzyme, catalyzing the reaction X-O-SO3 + H2O → X-OH + HSO4-, is found in many organisms including bacteria, fungi, and humans, but is not present in plants (Gunal et al., 2019). Sulfatase is induced by sulfate limitation and is the basis of plant growth promoting effects of some bacteria (Kertesz and Mirleau, 2004). Sulfatase activity in soil reflects the activity of microbial communities and can be used for estimation of soil health after various treatments (Tejada et al., 2006; Zaborowska et al., 2018) alongside activities of, e.g., β-glucosidase, cellobiohydrolase, chitinase, leucine aminopeptidase phosphatase, and tyrosine aminopeptidase (Maharjan et al., 2017).
However, the sulfatase can be used also as a tool to study plant-microbe interactions. The role of the plant microbiome in improving plant performance and fitness has been increasingly recognized and great progress in the understanding of the assembly of plant microbiome has been achieved (Bulgarelli et al., 2013; Bai et al., 2015). Clearly, plants shape their microbiome composition, even though the mechanisms are largely unknown. However, most microbiome studies are based on DNA sequencing and therefore taxonomic description of the microbiome composition (Jacoby et al., 2017). We have used sulfatase activity to identify mechanisms by which plants shape their microbiome (Koprivova et al., 2019). Through analyzing the effects of Arabidopsis accessions on sulfatase in soil and using the activity for genome-wide association mapping we revealed an important role of the phytoalexin camalexin in the interactions between plant roots and rhizosphere bacteria (Koprivova et al., 2019). The sulfatase assay, which was adapted from (Margesin et al., 2014), proved to be an excellent tool to assess the microbiome activity and the effect of plant genotype on such activity. Therefore, here we present a protocol not only for the core enzymatic activity but also for a full assessment of the effects of Arabidopsis genotypes (accessions or mutants) on microbiome function.
Materials and Reagents
- Tape
- 0.5 ml Eppendorf tubes
- Toothpicks
- 1.5 ml cuvettes
- Plastic trays, Plant Pots Direct, Heavyweight full seed tray (no holes), catalog number: 2012137PT (Figure 1)
- Plastic inserts (Plant Pots Direct, Seed Tray Inserts 40, catalog number: 2012111PT) (Figure 1)
- Plastic Petri dishes (Sarstedt, catalog number: 821.473)
- Pipette tips (Sarstedt)
- 2 ml plastic tubes (Sarstedt, catalog number: 72.695.500)
- Plastic rack for 2 ml tubes, Eppendorf
- Plastic cuvettes (Brand, catalog number: 759115)
- Seeds of Arabidopsis thaliana (can be obtained, e.g., from NASC Arabidopsis Stock Centre, http://arabidopsis.info/BasicForm)
- Sand, Quarzwerke (Frechen, Germany)
- Soil (e.g., CAS11 soil, Bulgarelli et al., 2012)
- Murashige Skoog (MS) medium (Duchefa Biochemie, catalog number: MO222.0025), stored at RT
- Sucrose (Sigma, catalog number: S7903-1KG), stored at RT
- Potassium-4-nitrophenyl sulfate (Sigma, catalog number: N3877-1G), stored at -20 °C
- P-nitrophenol (Merck, catalog number: 48549), stored at -20 °C
- Calcium chloride dihydrate (Sigma, catalog number C3881-1KG), stored at RT
- Sodium hydroxide (NaOH) (Sigma, catalog number: 71687-500G), stored at RT
- Sodium acetate trihydrate (Sigma, catalog number: S8625-500G), stored at RT
- Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) (Merck, catalog number: C1396), stored at RT
- Potassium nitrate (KNO3) (Merck catalog number: P8394), stored at RT
- Potassium dihydrogenphosphate (KH2PO4) (Merck, catalog number: 1.04873), stored at RT
- Ferric EDTA (Fe-EDTA) (Merck, catalogue number: E6760), stored at RT
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Merck, catalog number: M2670), stored at RT
- Liquid nitrogen
- Glacial acetic acid (Merck, catalog number: A6283), stored at RT
- Sodium hypochlorite solution (NaClO), 12%, Cl (Roth, catalog number: 9062.4)
- Hydrochloric acid (HCl) 37% (Merck KGaA, catalog number: 1.00317.1000), stored at RT
- Agarose (Sigma, catalog number: A9539-500G), stored at RT
- Toluene (Sigma, catalog number: 34866-100 ml), stored under fume hood at RT
- Modified Long Ashton solution (see Recipes)
- 0.5 M Acetate buffer (see Recipes)
- 0.005 M p-nitrophenyl solution (see Recipes)
- 0.5 M calcium chloride solution (see Recipes)
- 0.5 M sodium hydroxide solution (see Recipes)
- Standard p-nitrophenol solution 10 mM (100 ml) (see Recipes)
- Half strength MS medium with sucrose (1 L) (see Recipes)
Figure 1. Photos of (A) plastic trays and (B) inserts used for plant growth
Equipment
- Glass beaker 250 ml
- Forceps
- Pipettes (Eppendorf)
- Balances (Sartorius)
- Glass beaker
- Sanyo growth chamber, 10 h light/14 h darkness, 22 °C
- Rotating shaker, LTF (Labortechnik, Intelli-Mixer, RM-2L)
- Vortex (LMS, model: VTX-3000L)
- Incubator for 37 °C (Thermo Scientific, Heratherm Incubator)
- Centrifuge (Eppendorf, 5424)
- Spectrophotometer (Eppendorf)
- Fume hood
- Computer
- Desiccator
Procedure
- Arabidopsis thaliana seed sterilization
- Place small portion of seeds (ca. 10 µl) into 0.5 ml Eppendorf tubes.
- Place open tubes into tube rack inside of desiccator (Figure 2).
- Add 125 ml of sodium hypochlorite solution into 250 ml glass beaker and place near the seeds.
- Add 2.5 ml of concentrated HCl into the liquid, which forms chlorine gas, and quickly close the desiccator lid.
- Sterilize seeds for 3 h.
Figure 2. Photos of (A) open or (B) closed desiccator for seed sterilization
- Initial plates preparation
- Autoclave half-strength MS medium with 0.8% agarose and 0.5% sucrose and pour it into round Petri dishes, let it set.
- Carefully place sterile Arabidopsis seeds onto agar, using sterile toothpicks, approx. 1 seed per cm2.
- Seal the plates with tape.
- Place the plates into fridge for 2-3 days for stratification.
- Place plates into a plant growth cabinet for 9 days (10 h light/14 h dark; 22 °C, 100 µE m-2 s-1).
- Preparation of trays
- Mix soil with sterile (autoclaved) sand 1:9 (V/V).
- Place plastic insert into the tray.
- Fill inserts with soil-sand mix.
- Water slightly.
- Using forceps carefully transfer one seedling per insert, covering roots lightly.
- Water a bit more, to keep soil moist.
- Cover tray with the lid.
- Place trays into plant growth cabinet (10 h light/14 h dark; 22 °C, 100 µE m-2 s-1) for 2 weeks, uncover it after 3 days, and water daily with modified Long Ashton solution.
Figure 3. Photos of (A) Petri dish with seedlings before transfer and (B) trays with Arabidopsis ecotypes ready for material collection
- Collection of samples for sulfatase activity
- Carefully remove plants from soil.
- Into a 2 ml microcentrifuge tube collect about 1 g of the soil-sand mix, which was closest to the roots of the plant, i.e., rhizosphere, record fresh weight.
- Collect at least 2 samples per plant.
- Freeze in liquid nitrogen.
- Sulfatase activity measurement
- Defrost the samples in a rack.
- Add 400 µl of 0.5 M acetate buffer.
- Vortex each sample for at least 5 s.
- Under fume hood add 25 µl of toluene.
- Close tubes and vortex for at least 5 s.
- Place tubes into rotating rack for 6 min at 100 rpm.
- Add 100 µl of p-nitrophenyl sulfate solution under fume hood.
- Vortex each sample for 10 s.
- Place tubes for additional vigorous shaking for 5 min in an Eppendorf shaker at 1,000 rpm.
- Place rack with the tubes into 37 °C incubator for 1 h, mixing the whole rack every 10 min by reversing several times.
- To stop the reaction under fume hood add 100 µl of 0.5 M CaCl2 solution and 400 µl of 0.5 M sodium hydroxide solution.
- Vortex each sample for 10 s.
- Centrifuge tubes at RT at maximum speed for 20 min.
- Under fume hood carefully transfer the supernatant into plastic 1.5 ml cuvettes.
- Measure absorption at 400 nm. Use water as blank.
- Prepare standards by diluting 0, 20, 40, 80, 120, 160, and 200 µl of 10 mM p-nitrophenol standard to 1 ml H2O and measure in the same way.
- Calculate the p-nitrophenol content of the samples from a calibration curve (standards: 0, 200, 400, 800, 1,200, 1,600, and 2,000 nmol ml-1 p-nitrophenol).
- Using recorded FW data and incubation time of 1 h calculate sulfatase activity in nmol g FW-1 h-1.
Data analysis
The assay determines the end concentration of the sulfatase product, p-nitrophenol, using a calibration curve (Figure 1) and the activity can be calculated from the weight of soil and time of the assay. All calculations can be easily performed in standard office software, e.g., Excel (Table 1). Five biological replicates with two technical replicates each should be used for each plant genotype.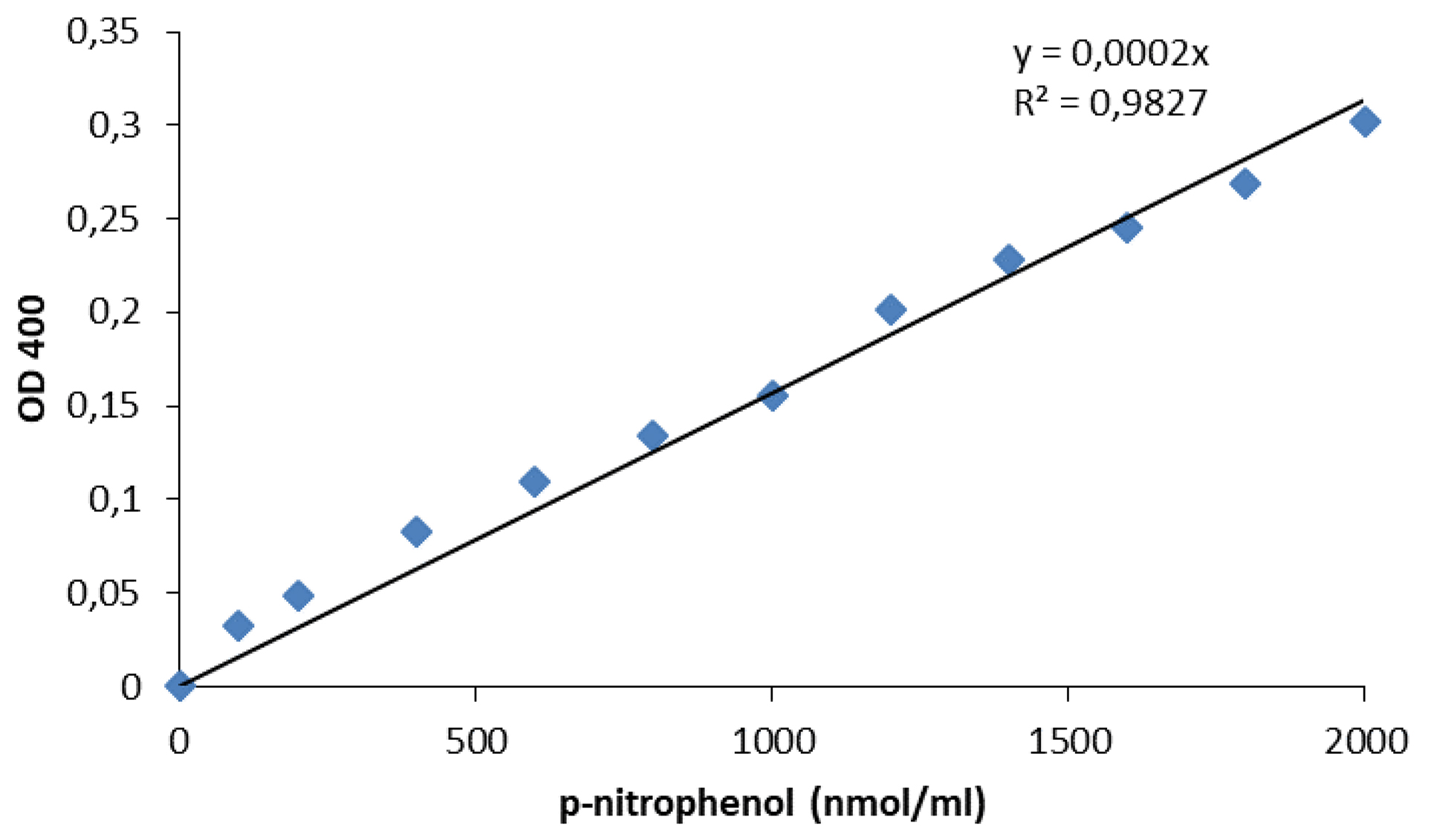
Figure 4. Calibration curve for measurement of sulfatase activity. Standard p-nitrophenol solution was diluted by water to contain 0-2,000 nmol per ml and OD400 was measured.
Table 1. Example of calculation of sulfatase activity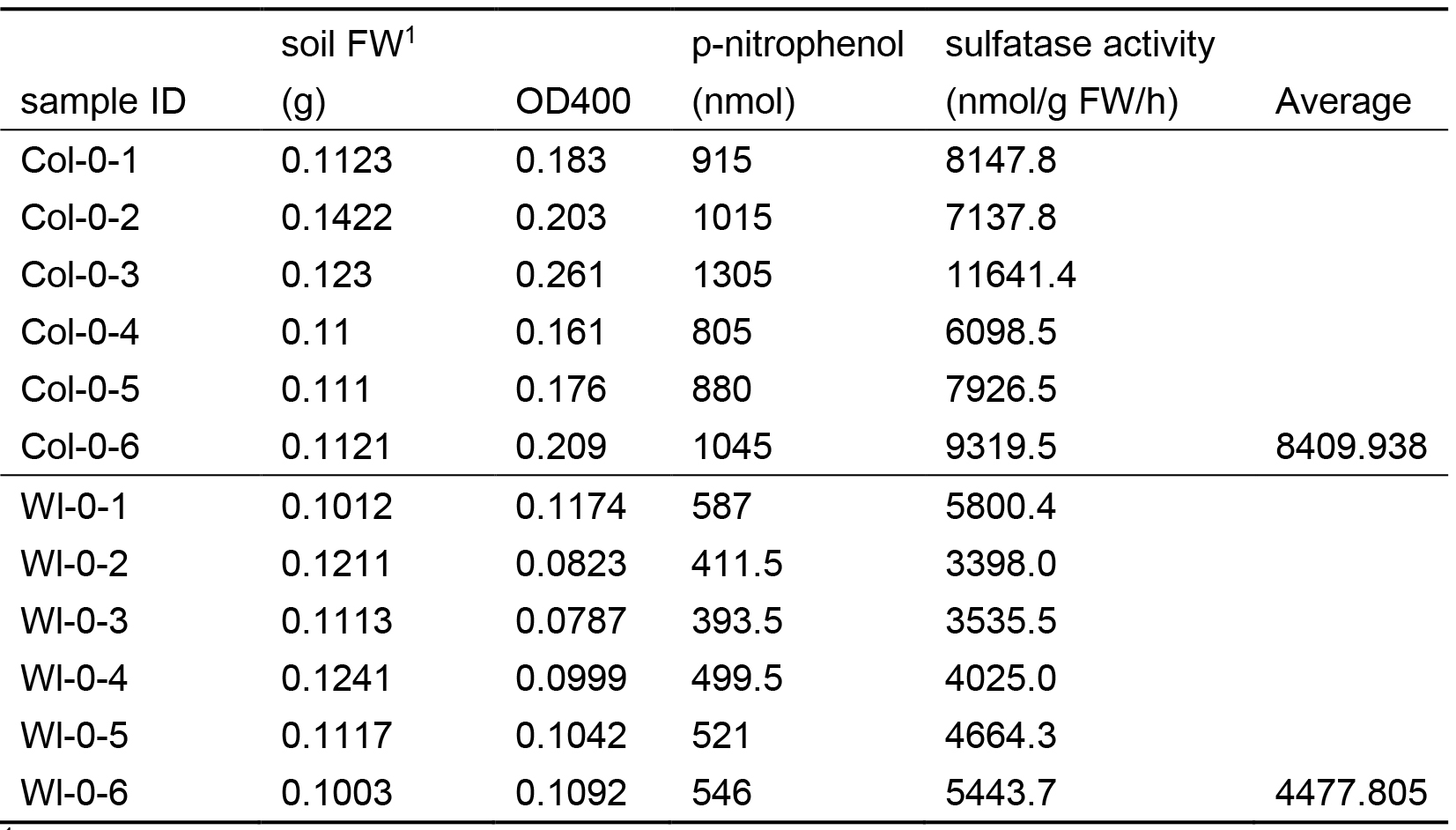
1The soil FW is adjusted from the FW of soil/sand sample and the ratio of soil/sand mix, here 10% soil.
Notes
The assay can easily be adapted for different plant species, different sizes of pots, or different soils.
Recipes
- Modified Long Ashton solution
1.5 mM Ca(NO3)2·4H2O
1 mM KNO3
0.75 mM KH2PO4
0.1 mM Fe-EDTA
0.75 mM MgCl2·6H2O
pH 5.7 - 0.5 M acetate buffer, pH 5.8 (1 L)
64 g sodium acetate trihydrate
1.7 ml glacial acetic acid
Stored at 4 °C - 0.005 M p-nitrophenyl sulfate solution (100 ml)
0.1287 g of p-nitrophenyl dissolved in 100 ml of 0.5 M acetate buffer
Stored at 4 °C - 0.5 M calcium chloride solution (1 L)
73.5 g CaCl2·2H2O dissolved in 1 L H2O
Stored at RT - 0.5 M sodium hydroxide solution (1 L)
20 g NaOH dissolved in 1 L H2O
Stored at RT - Standard p-nitrophenol solution 10 mM (100 ml)
139 mg p-nitrophenol dissolved in 100 ml H2O
Stored at 4 °C - Half strength MS medium with sucrose (1 L)
2.2 g MS medium
5 g sucrose
8 g agarose
pH 5.7 with 1 M KOH
Acknowledgments
The sulfatase assay was adapted from Margesin et al., 2014. SK’s research is funded by the Deutsche Forschungsgemeinschaft (DFG) under Germany’s Excellence Strategy, EXC-Nummer 2048/1, project 390686111. AS was funded by FP7-Peoople (293429) and the Irish Research Council New Foundation “S-cycle”.
Competing interests
The authors declare no competing interests.
References
- Bai, Y., Muller, D. B., Srinivas, G., Garrido-Oter, R., Potthoff, E., Rott, M., Dombrowski, N., Munch, P. C., Spaepen, S., Remus-Emsermann, M., Huttel, B., McHardy, A. C., Vorholt, J. A. and Schulze-Lefert, P. (2015). Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528(7582): 364-369.
- Bulgarelli, D., Rott, M., Schlaeppi, K., Ver Loren van Themaat, E., Ahmadinejad, N., Assenza, F., Rauf, P., Huettel, B., Reinhardt, R., Schmelzer, E., Peplies, J., Gloeckner, F. O., Amann, R., Eickhorst, T. andSchulze-Lefert, P. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488: 91-95.
- Bulgarelli, D., Schlaeppi, K., Spaepen, S., Ver Loren van Themaat, E. and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64: 807-838.
- Gahan, J. and Schmalenberger, A. (2014). The role of bacteria and mycorrhiza in plant sulfur supply. Front Plant Sci 5: 723.
- Gunal, S., Hardman, R., Kopriva, S.and Mueller, J. W. (2019). Sulfation pathways from red to green. J Biol Chem 294: 12293-12312.
- Jacoby, R., Peukert, M., Succurro, A., Koprivova, A. and Kopriva, S. (2017). The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci 8: 1617.
- Kertesz, M. A. and Mirleau, P. (2004). The role of soil microbes in plant sulphur nutrition. J Exp Bot 55(404): 1939-1945.
- Koprivova, A., Schuck, S., Jacoby, R. P., Klinkhammer, I., Welter, B., Leson, L., Martyn, A., Nauen, J., Grabenhorst, N., Mandelkow, J. F., Zuccaro, A., Zeier, J. and Kopriva, S. (2019). Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proc Natl Acad Sci U S A 116(31): 15735-15744.
- Margesin, R., Minerbi, S. and Schinner, F. (2014). Long-term monitoring of soil microbiological activities in two forest sites in South tyrol in the italian alps. Microbes Environ 29: 277-285.
- Maharjan, M., Sanaullah, M., Razavi, B. S. and Kuzyakov, Y. (2017). Effect of land use and management practices on microbial biomass and enzyme activities in subtropical top-and sub-soils. Appl Soil Ecol 113: 22-28.
- Stringlis, I. A., Yu, K., Feussner, K., de Jonge, R., Van Bentum, S., Van Verk, M. C., Berendsen, R. L., Bakker, P., Feussner, I. and Pieterse, C. M. J. (2018). MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci U S A 115: E5213-E5222.
- Tejada, M., Hernandez, M. T. and Garcia, C. (2006). Application of two organic amendments on soil restoration: effects on the soil biological properties. J Environ Qual 35: 1010-1017.
- Zaborowska, M., Kucharski, J. and Wyszkowska, J. (2018). Biochemical and microbiological activity of soil contaminated with o-cresol and biostimulated with Perna canaliculus mussel meal. Environ Monit Assess 190: 602.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Koprivova, A., Schmalenberger, A. and Kopriva, S. (2020). Sulfatase Assay to Determine Influence of Plants on Microbial Activity in Soil. Bio-protocol 10(2): e3490. DOI: 10.21769/BioProtoc.3490.
Category
Microbiology > Microbial metabolism > Nutrient transport
Plant Science
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









