- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Microbial Bioassay for Direct Contact Assessment of Soil Toxicity Based on Oxygen Consumption of Sulfur Oxidizing Bacteria
Published: Vol 10, Iss 1, Jan 5, 2020 DOI: 10.21769/BioProtoc.3470 Views: 4394
Reviewed by: Alba BlesaFrancesco Dal GrandeJan-Ulrik Dahl

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bacterial Pathogen-mediated Suppression of Host Trafficking to Lysosomes: Fluorescence Microscopy-based DQ-Red BSA Analysis
Mădălina Mocăniță [...] Vanessa M. D'Costa
Mar 5, 2024 2896 Views

Purification of Native Dentilisin Complex from Treponema denticola by Preparative Continuous Polyacrylamide Gel Electrophoresis and Functional Analysis by Gelatin Zymography
Pachiyappan Kamarajan [...] Yvonne L. Kapila
Apr 5, 2024 2091 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1659 Views
Abstract
A new direct contact assessment of soil toxicity using sulfur oxidizing bacteria (SOB) is proposed for analyzing the toxicity of soils. The proposed method is based on the ability of SOB to oxidize elemental sulfur to sulfuric acid in the presence of oxygen. Since sulfate ions are produced from sulfur by SOB oxidation activity, changes in electrical conductivity (EC) serve as a proxy to assess toxicity in water. However, in soil medium, EC values are not reliable due to the adsorption of SO42- ions by soils. Here, we suggest a new parameter which measures oxygen consumption by SOB for 6 hours to assess soil toxicity by using a lubricated glass syringe method. The proposed method is rapid, simple, cost- effective as well as sensitive and capable of assessing direct contact soil toxicity.
Keywords: Toxicity assessmentBackground
Currently, the most prevalent technologies used in detecting toxic chemicals are gas chromatography (GC), high liquid chromatography (HPLC), and atomic absorption spectroscopy (AAS) that provide accurate measurements by applying different detection principles. However, these well-established methods require both skilled personnel and expensive equipment and cannot practically measure all the toxic chemicals in soil (Brouwer, 1991; Eom et al., 2019a). In contrast, bioassays have been one of the most useful technologies for the detection of environmental toxicity. Bioassays depend on changes in the physiological responses of living organisms to toxic chemicals. Ecotoxicological tests (ET) more precisely identify the cumulative and synergistic effects of toxic contaminants even if they fail to clearly identify all toxic chemicals (Sisinno et al., 2007).
Several toxicity bioassays are based on measurements of growth inhibition, oxygen uptake, colony formation, or luminescence for screening toxicants in industrial effluents, sediments, and soils (Selivanovskaya et al., 2010). To date, few studies exist for direct contact assessment of toxicants in soil medium. To evaluate soil toxicity, both liquid phase (soil elutriates) and solid phase bioassays (direct contact tests) are commonly used (Hubálek et al., 2007). In liquid-phase bioassays, test organisms are exposed to the elutriate of toxicants previously in solid-phase after dissolution in water or organic solvents (Gälli et al., 1994; Tarradellas et al., 1996; Maxam et al., 2000). This approach provides limited information on solids-associated toxicity because, using aqueous elutriates, the elutriating process cannot accommodate the complexity of the solid-phase of soil (Ronnpagel et al., 1995; Selivanovskaya et al., 2010). Moreover, partial dissolution of toxicants in soil or synergistic effects between toxicants and an extractant can possibly underestimate or overestimate the toxicity of contaminants in soil (Ronnpagel et al., 1995; Tarradellas et al., 1996; Selivanovskaya et al., 2010).
On the other hand, direct contact toxicity tests can measure the total toxic response of diverse types of contaminants in a soil sample. A direct contact bioassay could enables the determination of actual toxicity of contaminants in a highly dynamic and complex system (soil or sediments) much better than aqueous elutriates of solids (Ronnpagel et al., 1995).
Recently, SOB bioassays have been successfully employed in water, wastewater, soil toxicity detection and assessment (Oh et al., 2011; Van Ginkel et al., 2011; Gurung and Oh, 2013; Ahmed et al., 2019, Eom et al., 2019a). Most toxicity assessment studies have been carried out in aqueous phase while few studies have investigated soil toxicity by SOB (Gurung and Oh, 2013; Ahmed et al., 2019, Eom et al., 2019b). SOB are chemoautotrophic bacteria which grow as a biofilm on the surface of elemental sulfur particles. They have the ability to oxidize sulfur (electron donor) to sulfuric acid in the presence of oxygen (electron acceptor) as shown in Eq. 1 (Oh et al., 2011; Hassan et al., 2013).
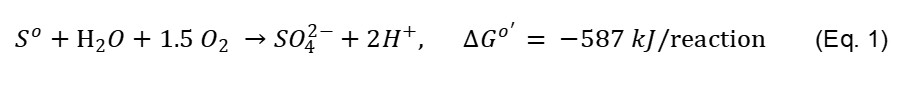
During oxidation, SOB consumes molecular oxygen and produce sulfate (SO42-) and two protons (H+). Production of H+ acidifies the medium, thereby reducing pH and increasing EC (Oh et al., 2011). Upon introduction of a toxic contaminant into the SOB reactors in aqueous media, SOB growth and oxidation becomes inhibited resulting in lower increase of EC (Hassan et al., 2013). However, the heterogeneous nature of soils and their ability to adsorb sulfate ions prevents EC in soils from increasing, making the use of EC an unreliable indicator of soil toxicity assessment using SOB. Our proposed method introduces the direct contact assessment of soil toxicity using oxygen consumption by SOB during a defined period of incubation as a new parameter.
Materials and Reagents
- 0.5 and 2.0 mm testing sieve (Chunggye Sanggong sa, Seoul, Korea)
- Sulfur granules (MIDAS-SG, Miwon Commercial Co. Ltd, Korea)
- 10 ml glass syringe (Truth, Top Syringe Mfg Co (P) Ltd, India)
- 12 L plastic container (Influent and effluent tanks)
- 1.3 L fabricated acrylic container (0.13 m x 0.13 m x 0.15 m)
- 6 DRAM CLR round glass vials 20-400TD (KD brand, catalog number: 324020-2385)
- Stainless steel wire test tube rack (model: TRS-2350, catalog number: ROI-11-590)
- 20 MMX.100 PTFE Teflon rubber stoppers (857518197L-610050-20, USA)
- Open top screwcaps (Daihan Scientific, WH.W240506 cap, screw, 8-425)
- Plastic weighing dish (Lab Korea, B17-132-034-1815-002)
- CuSO4·5H2O (Sigma-Aldrich, catalog number: 209198-100G)
- (NH4)6Mo7O24·4H2O (Daejung, catalog number: 1073-4475)
- FeSO4·7H2O (Sigma-Aldrich, catalog number: 7782-63-0)
- NH4Cl (Junsei, catalog number: 18075-0350)
- KCl (Daejung, catalog number: 6566-4400)
- NaH2PO4 (Daejung, catalog number: 7615-4400)
- Na2HPO4 (Daejung, catalog number: 7613-4400)
- NaHCO3 (Daejung, catalog number: 7566-4400)
- Yeast extract (Bacto, catalog number: 212750)
- CaCl2·2H2O (Sigma-Aldrich, catalog number: C3881-500G)
- MnCl2·4H2O (Daejung, catalog number: 5526-4400)
- ZnSO4·7H2O (Daejung, catalog number: 8607-4400)
- K2Cr2O7 (Daejung, catalog number: 6572-4400)
- CoCl2·6H2O
- Dish washing detergent
- Synthetic medium (see Recipes)
Equipment
- Adjustable volume micropipettes, 0-200 μl and 100-1,000 μl (Biorad Industries, USA)
- Refrigerated low temperature BOD incubator (JSI Industries, Korea JSBI-150C)
- Shaking water bath (Lab Companion BS-31 (55 L), model: SKU: V018. AAH44311K)
- Digital precise water bath (Daihan Scientific, Korea WB-11)
- Electrical conductivity meter (Lutron, model: YK-2005CD)
- Digital electronic scale (Scharwz, model: SCH1812S)
- Air pump (PhilGreen, model: BT-6500)
- 20-30 cm flexible air sparger for the sulfur master culture reactor (SMCR)
- Peristatic pump (Techno, Lab system, model: PP-150D; POOLIM. CO, Korea)
- Cisa BA 200N electromagnetic digital sieve shaker (Cisa Cedaceria Industrial S.L, Spain)
- MasterFlex peristaltic tubing
Software
- Sigmaplot (Systat software Inc, https://systatsoftware.com/products/sigmaplot/)
- Toxicalc (Tidepool Scientific Software, https://tidepool-scientific.com/ToxCalc/ToxCalc.html)
Procedure
- Sulfur master culture reactor (SMCR)
- The SMCR is maintained to provide consistent and repeatable SOB cultures attached to sulfur particles for the soil toxicity tests.
- Prepare a 1.3 L SMCR made of acrylic with a working capacity of 0.6 L in an incubator maintained at 38 °C.
- Place 500 ml of 0.5-2 mm sulfur particles in the SMCR (Figure 1A) filled with 600 ml of synthetic medium (composition is given in Recipes) and maintain at a temperature of 38 °C.
- Introduce air into the SMCR using a flexible air diffuser and a air pump at a flow rate of 2-3 L/min and operate the reactor in fed-batch mode in the incubator. Influent and the air pump should also be placed in the incubator to maintain the temperature of 38 ± 1 °C.
- Use 10 ml of aerobic return activated sludge from a wastewater treatment plant as the initial inoculum.
- Feed the SMCR using synthetic medium (38 °C) at a cycle of 5 min (20 ml/min) and 2 h 55 min of batch reaction mode.
- Alternatively, the SMCR can be operated manually using a 1 L beaker by feeding 100 ml and wasting 100 ml of the medium twice daily.
- Activity of SOB in the SMCR can be determined by monitoring changes in EC. An increase in EC indicates SOB growth in the SMCR. Before using the SMCR for toxicity tests, it should be operated continuously for more than 5 days in fed-batch mode.
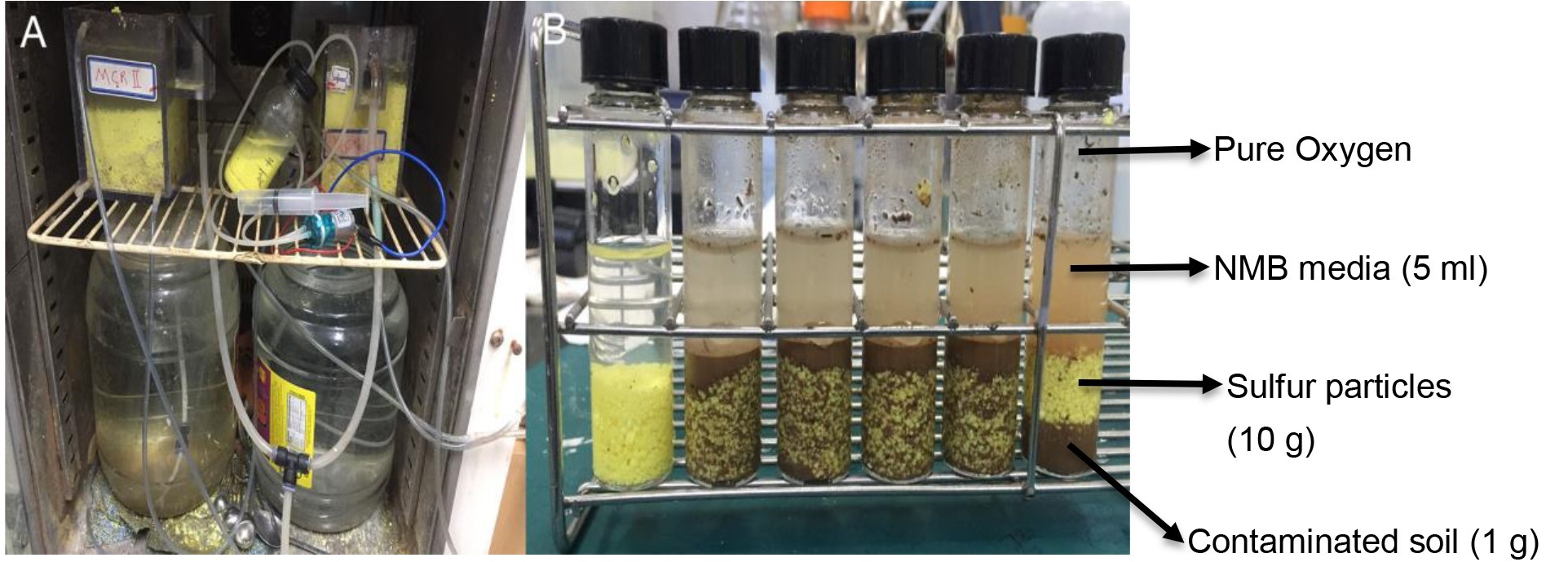
Figure 1. Sulfur master culture reactor (A) and toxicity test vials (B)
- Soil collection
- Remove all the gravel, concrete, and plant debris present at the sampling site.
- Collect the soil from a depth of 0-30 cm using a shovel or stainless steel spoon.
- Completely fill the soil samples in labeled containers or bags to remove any headspace and immediately transfer to the laboratory.
- Pass soil samples through a 2-mm sieve. Store the sieved sample in tightly sealed plastic containers until used for toxicity tests.
- Soil toxicity tests
- Place 1 g soil samples in 25 ml flat-bottomed glass vials equipped with plastic caps and Teflon-lined rubber stoppers.
- Add 5 ml NMB medium to the glass vials placed in a water bath set at 38 °C to maintain media temperature of 38 °C (Figure 2A).
- When the medium temperature reach to 38 °C, carefully transfer 10 g SOB attached sulfur particles into the glass vials kept in the water bath set at 38 °C (Figure 2A).
- A headspace volume of 15.0 ml is allotted for oxygen (Figure 1B). Purge the headspace with pure oxygen for 6 s.
- Cap the vials immediately with plastic caps and Teflon-lined rubber stoppers. Immediately transfer these prepared vials into a shaking water bath set at 38 °C and agitate at 90 rpm for 10 min.
- After that, briefly remove the vials from the shaking water bath. Equilibrate the pressures by inserting a 26 G needle into the Teflon rubber stoppers for 5 s and immediately re-incubate in the shaking water bath.
- Run two control samples: one control sample with unpolluted soil; the other, without soil (i.e., NMB media and sulfur particles contain SOB).
- Run the controls and test samples in triplicate. Strictly maintain the temperature (38 °C) throughout the process of sample preparation as SOB activity is temperature dependent.
- After 6 h incubation and agitation in the shaking water bath, measure oxygen consumption using the lubricated glass syringe method. Briefly, oxygen volume consumed by SOB can be measured by movement of the glass plunger into the syringe’s barrel. Before measuring the oxygen volume consumed by SOB in each vial, the plunger and the barrel of the glass syringe is lubricated using an aqueous solution comprised of two drops of dish liquid detergent in 100 ml of distilled water. After lubricating the syringe, the plunger is set at the 10 ml mark and the needle is then inserted parallel to the ground through the Teflon rubber stopper into the test vial. The plunger is allowed to move into the barrel of the syringe and equilibrate between the atmospheric pressures. The value on the syringe corresponds to the amount of oxygen consumed by SOB, i.e., a decrease in oxygen in the head space of the test vial (Figure 2B).
Note: The amount of oxygen consumption in the water control should exceed 5 ml after 6 h incubation to reliably confirm toxicity in soil.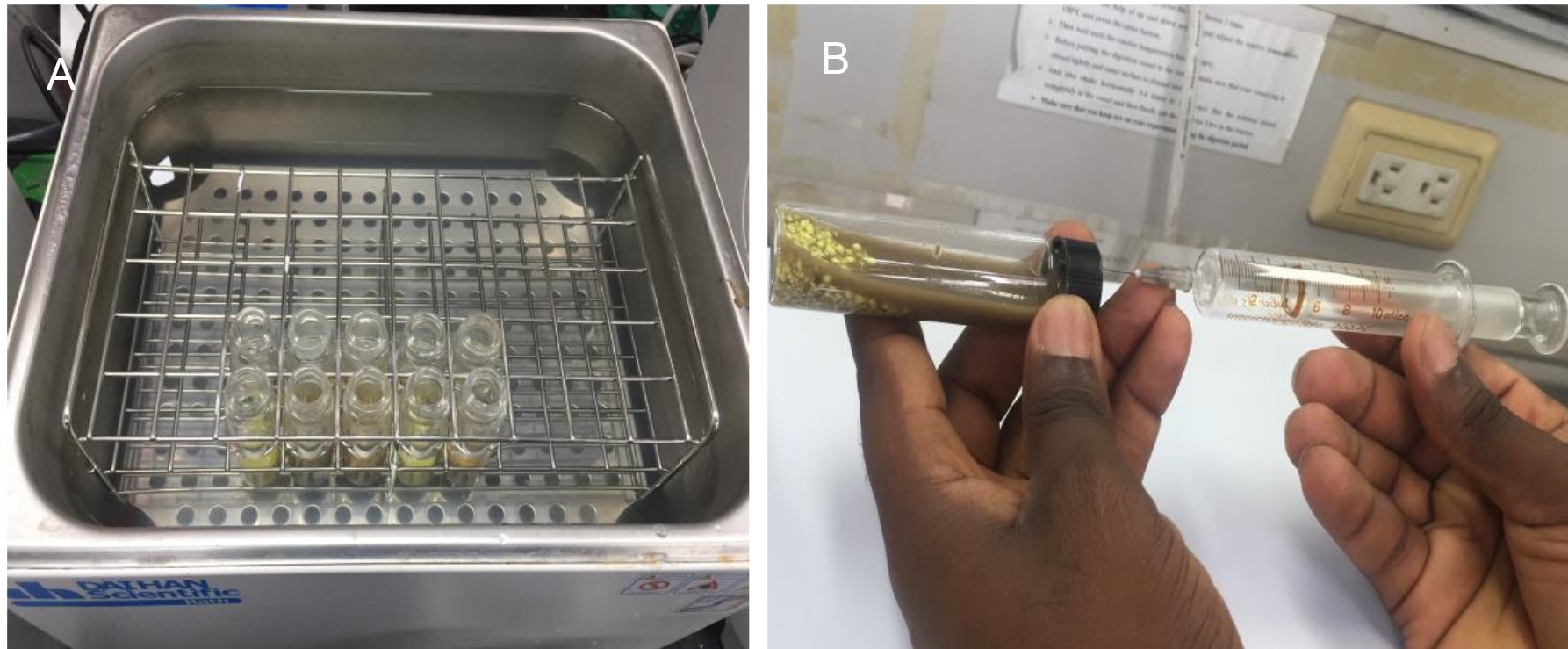
Figure 2. Test vials containing contaminated soil, sulfur particles, and medium kept in a water bath (A) and oxygen consumption measurement by a 10 ml glass syringe (B)
Data analysis
A decrease in the oxygen consumed in the headspace of each test vial is determined by the glass syringe method. The inhibitory effect of the tested toxic chemicals on SOB activity in soil is determined by Eq. 2.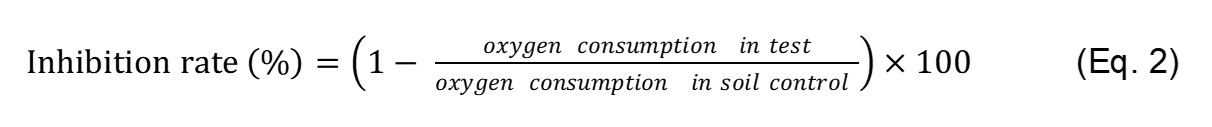
The results from the polluted soils are given in Figure 3 which clearly shows less oxygen consumption than from unpolluted soil (control). Determined inhibitions (%) for A and B samples are 89.4% ± 4.2 and 99.4% ± 0.97, respectively.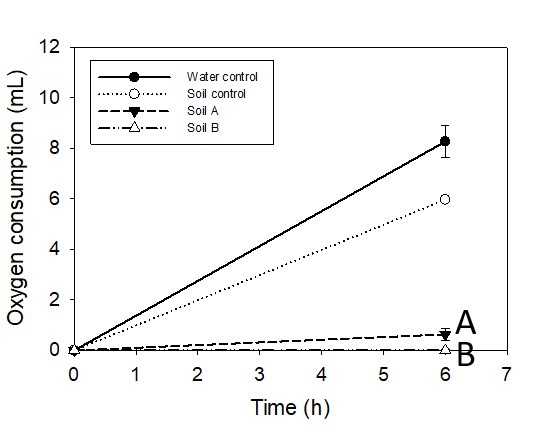
Figure 3. Oxygen consumption for the polluted soil samples
Recipes
- Synthetic medium
- Nutrient mineral buffer solution
3.1 g/L NH4Cl
1.3 g/L KCl
4.22 g/L NaH2PO4
2.75 g/L Na2HPO4 - Trace mineral solution
7.34 mg/L CaCl2·2H2O
5 mg/L FeSO4·7H2O
2.5 mg/L MnCl2·4H2O
2.5 mg/L ZnSO4·7H2O
0.5 mg/L (NH4)6Mo7O24·4H2O
0.5 mg/L CoCl2·6H2O
0.2 mg/L CuSO4·5H2O
The following components are added after 100x dilution of the nutrient mineral buffer solution (Recipe 1a) with distilled water (pH 6.8-7.0):
200 mg/L NaHCO3
5 ml/L trace mineral solution (Recipe 1b)
50 mg/L yeast extract - Nutrient mineral buffer solution
Acknowledgments
This study is supported by Korea Ministry of Environment as “The SEM project : 2018002450001”
Competing interests
The authors declared no conflicts of interest.
References
- Ahmed, N., Ok, Y. S., Jeon, B. H., Kim, J. R., Chae, K. J. and Oh, S. E. (2019). Assessment of benzene, toluene, ethyl-benzene, and xylene (BTEX) toxicity in soil using sulfur-oxidizing bacterial (SOB) bioassay. Chemosphere 220: 651-657.
- Brouwer, H. (1991). Testing for chemical toxicity using bacteria: An undergraduate laboratory experiment. J Chem Educ 68(8): 695.
- Eom, H., Hwang, J. H., Hassan, S. H. A., Joo, J. H., Hur, J. H., Chon, K., Jeon, B. H., Song, Y. C., Chae, K. J. and Oh, S. E. (2019a). Rapid detection of heavy metal-induced toxicity in water using a fed-batch sulfur-oxidizing bacteria (SOB) bioreactor. J microbiol methods 161, 35-42.
- Eom, H., Ashun, E.,Toor, U. A. and Oh, S. E. (2019b). A solid-phase direct contact bioassay using sulfur-oxidizing bacteria (SOB) to evaluate toxicity of soil contaminated with heavy metals. Sensors and Actuators B: Chemical (in press).
- Gälli, R., Munz, C. D. and Scholtz, R. (1994). Evaluation and application of aquatic toxicity tests: use of the Microtox test for the prediction of toxicity based upon concentrations of contaminants in soil. Hydrobiologia 273(3): 179-189.
- Gurung, A. and Oh, S. E. (2013). Use of sulfur-oxidizing bacteria for assessment of chromium-contaminated soil. Environ Earth Sci 70(1): 139-143.
- Hassan, S. H., Van Ginkel, S. W. and Oh, S. E. (2013). Effect of organics and alkalinity on the sulfur oxidizing bacteria (SOB) biosensor. Chemosphere 90(3): 965-970.
- Hubálek, T., Vosáhlová, S., Matějů, V., Kováčová, N. and Novotný, Č. (2007). Ecotoxicity monitoring of hydrocarbon-contaminated soil during bioremediation: a case study. Arch Environ Contam Toxicol 52(1): 1-7.
- Maxam, G., Rila, J. P., Dott, W. and Eisentraeger, A. (2000). Use of bioassays for assessment of water-extractable ecotoxic potential of soils. Ecotoxicol Environ Saf 45(3): 240-246.
- Oh, S. E., Hassan, S. H. A. and Van Ginkel, S. W. (2011). A novel biosensor for detecting toxicity in water using sulfur-oxidizing bacteria. Sensor Actuat B-Chem 154(1): 17-21.
- Ronnpagel, K., Liss, W. and Ahlf, W. (1995). Microbial bioassays to assess the toxicity of solid-associated contaminants. Ecotoxicol Environ Saf 31(2): 99-103.
- Selivanovskaya, S., Galitskaya, P., Schnell, S. and Hung, Y. T. (2010). A comparison of microbial contact bioassay with conventional elutriate assays for evaluation of wastes hazard. Int J Environ Waste Manage 6(1-2), 183-196.
- Sisinno, C. L. S., Rizzo, A. C. L., Bulus, M. R. M., Rocha, D. A., Soriano, A. U., Vital, R. L. and Moreira, J. C. (2007). Application of ecotoxicological tests in a preliminary evaluation of soils treated on bioreactor. J Braz Soc Ecotoxicol 2(2): 157-161.
- Tarradellas, J., Bitton, G. and Rossel, D. (1996). Soil ecotoxicology. CRC press.
- Van Ginkel, S. W., Hassan, S. H., Ok, Y. S., Yang, J. E., Kim, Y.-S. and Oh, S. E. (2011). Detecting oxidized contaminants in water using sulfur-oxidizing bacteria. Environ Sci Technol 45(8): 3739-3745.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ashun, E., Toor, U. A., Eom, H. and Oh, S. (2020). A Microbial Bioassay for Direct Contact Assessment of Soil Toxicity Based on Oxygen Consumption of Sulfur Oxidizing Bacteria. Bio-protocol 10(1): e3470. DOI: 10.21769/BioProtoc.3470.
Category
Microbiology > Microbial genetics > Gene mapping and cloning
Microbiology > Microbial biofilm > Biofilm culture
Microbiology > Microbe-host interactions > Bacterium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










