- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Stimulation of Peritoneal Macrophages with Apoptotic Jurkat Cells to Produce IL-10
Published: Vol 9, Iss 24, Dec 20, 2019 DOI: 10.21769/BioProtoc.3467 Views: 6116
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2232 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views
Abstract
Clearance of apoptotic cells by macrophages is critical to ensuring cellular homeostasis and suppression of autoimmunity. Macrophage recognition of apoptotic cells triggers an anti-inflammatory response, which is mediated by the release of IL-10, TGF-β etc. with concurrent inhibition of pro-inflammatory cytokines (such as TNFα, IL-12, IL-1β). To characterize cytokine profile produced by macrophages during phagocytosis of apoptotic cells, we developed an effective, more physiologic system using isolated murine peritoneal macrophages and T-lymphocyte cell line Jurkat as a source of apoptotic cells. Apoptosis of Jurkat cells is induced with staurosporine, a protein kinase C (PKC) inhibitor and detected by Annexin V/propidium iodide staining. This in vitro assay demonstrates that murine peritoneal macrophages produce large amounts of IL-10 following exposure to apoptotic Jurkat cells.
Keywords: ApoptosisBackground
Production of IL-10, a major immunoregulatory cytokine, by phagocytes during clearance of apoptotic cells is critical to ensuring cellular homeostasis and suppression of autoimmunity (Chung et al., 2007). Little is known about the regulatory mechanisms in this fundamental process. To elucidate the molecular mechanisms involved in the regulation of IL-10 gene expression in macrophages upon interaction with apoptotic cells, we developed this protocol to explore key regulators of IL-10 production induced by apoptotic cells.
Materials and Reagents
- 24-well plate (Corning, catalog number: 3563847)
- T-75 flask (Corning, catalog number: 431464)
- Falcon® 50 ml High Clarity PP Centrifuge Tube (Corning, catalog number: 352070)
- Posi-Click 1.7 ml microcentrifuge tube (Denville, catalog number: C2170)
- 10-ml syringe (BD, catalog number: 309695)
- 25 G and 18 G needles (BD)
- C57BL/6 mice (6-8 weeks old)
- Cell line: Jurkat, Clone E6-1 (ATCC, catalog number: TIB-152)
- Fetal Bovine Serum (Thermo Fisher, catalog number: 16000)
- RPMI 1640 Medium, GlutaMAXTM Supplement, and HEPES (Thermo Fisher, catalog number: 72400)
- Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher, catalog number: 15140)
- GlutaMAXTM Supplement (Thermo Fisher, catalog number: 35050)
- Staurosporine (Sigma-Aldrich, catalog number: 19-123)
- DPBS, no calcium, no magnesium (Thermo Fisher, catalog number: 14190)
- FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen, catalog number: 556547)
- Mouse IL-10 ELISA Set (BD, catalog number: 565252)
- BD BBL Dehydrated Culture Media (Fisher Scientific, catalog number: 211716)
- Dimethyl sulfoxide (Sigma-Aldrich, catalog number: D2560)
- 70% Ethanol
- Complete media for Jurkat T cells (see Recipe 1)
- 3% (w/v) Brewer thioglycollate medium (see Recipe 2)
- 1x Binding buffer(see Recipe 3)
- RPMI-1640-10 (see Recipe 4)
- 0.5 mg/ml Staurosporine (1,000x stock) (see Recipe 5)
Equipment
- Tissue culture hood (biosafety cabinet, Forma Scientific, model: 1284)
- 37 °C, 5% CO2 humidified incubator (Thermo Forma, model: 3110 Series II)
- Centrifuge (Thermo Scientific, ST8 Benchtop Centrifuge)
- Optical microscopy (Olympus, CK30)
- Flow cytometer (BD, FACSCalibur)
- Sterile Surgical Scissors (Miltex, MH5-302)
- Hemacytometer (Hausser Scientific, catalog number: 02-671-5)
- Autoclave
Software
- FlowJo software (7.6.1)
- GraphPad Prism
Procedure
- Subculturing Jurkat cell Line
- Transfer growing cells from T-75 flask to a 50 ml centrifuge tube followed by centrifugation at 300 x g for 5 min at room temperature.
- Remove and discard the supernatant (old growth medium from above the cell pellet) and resuspend the cell pellet with fresh complete growth medium.
- Add a proper volume of Jurkat cell suspension to a new T-75 flask containing 15 ml of complete growth medium with approximately 105 viable cells/flask and subculture every 3-4 days.
- Place the culture flask in a 37 °C, 5% CO2 humidified incubator.
- Inducing apoptosis of Jurkat cells
- Harvest exponentially growing Jurkat cells (initial concentration of 2 x 105 cells/ml, and final concentration of 6-8 x 105 cells /ml, usually two days after seeding) by centrifugation at 300 x g for 5 min (same procedure for Jurkat cells subculturing/harvesting/washing).
- Resuspend cells in fresh medium to a final concentration of 1 x 106 cells/ml and seed into a new T-75 flask. Another flask will be used as the negative control for non-induced cells (note that there would be two groups, one for apoptosis, and the other for non-apoptosis control group).
- Add staurosporine into Jurkat cell culture with a final concentration of 0.5 μg/ml, and incubate in a 37 °C, 5% CO2 humidified incubator for 6-8 h. Add the same volume of DMSO into control Jurkat T cell culture.
- Harvest cells for staining at different times (i.e., 3, 6, 12 h) after the addition of staurosporin/DMSO by transferring 2 ml of cell culture medium into a fresh Falcon tube and centrifugation. Keep culturing the rest of cells in T-75 flask in a 37 °C, 5% CO2 humidified incubator.
- Detection apoptosis of Jurkat cells with FITC Annexin V Apoptosis Detection Kit
- Wash harvested staurosporine-treated Jurkat cells twice with cold DPBS to stop the reaction.
- Resuspend cells in 1x Binding buffer at a concentration of 5 x 106 cells/ml.
- Transfer 100 μl of the solution (~5 x 105 cells) to a new 1.7 ml microcentrifuge tube.
- Add 2 μl of FITC Annexin V and 2 μl of PI, gently vortex the cells and incubate for 15 min at room temperature or 30 min on ice, protecting from light under either condition.
- Add 400 μl of 1x Binding buffer to each tube, wash twice (centrifugation at 400 x g for 5 min) and analyze by flow cytometer within 1 h (Figure 1).
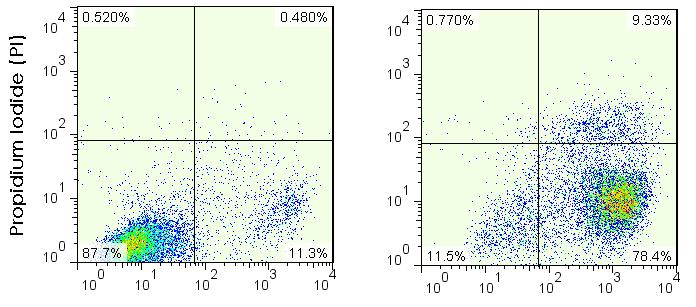
Figure 1. Apoptosis of Jurkat cells induced by staurosporine. After 6 h-induction, the percentage of early- and late-apoptotic cells was quantified by flow cytometry analysis with Annexin V and propidium iodide (PI) staining. The numbers indicate the percentage of subpopulations.
- Intraperitoneal injection of thioglycollate
- Fill a 10-ml syringe with 3% Brewer thioglycollate medium with a 25 G needle.
- Inject 2 ml of solution per mouse into the peritoneal cavity, allowing inflammatory response to proceed for 4 days.
- Harvest and culture thioglycollate-elicited peritoneal macrophages
- Euthanize mouse with CO2 and clean abdomen with 70% ethanol.
- Make a small incision along the midline with sterile scissors and retract the abdominal skin manually to expose the transparent peritoneal wall.
- Fill a 10-ml syringe with 9 ml of DPBS or RPMI-1640-10 (Recipe 4), then inject harvest medium with a 25-G needle into the peritoneal cavity.
- Massage abdomen for ~30 s and recover peritoneal fluid without puncturing any abdominal organs using a 10 ml syringe with 18 G needle (~8 ml fluid could be recovered from per mouse) (Video 1).Video 1. Harvest peritoneal macrophages (Steps E2-E4)
- Remove the needle from the syringe and dispense peritoneal fluid into a 15 ml Centrifuge tube on ice (Cells should be kept cool throughout the procedure).
- Centrifuge the peritoneal cells in a refrigerated centrifuge for 5 min at 400 x g. Discard supernatant and resuspend the cell pellet in 5 ml of RPMI-1640-10 medium by gently pipetting up and down.
- Count cells using hemacytometer (with 1:10 dilution by mixing 100 μl of cell suspension with 900 μl of trypan blue).
- Adjust cell concentration with RPMI-1640-10 and seed into 24-well plate at 5 x 105 cells/well (500 μl of medium per well).
- Cells are allowed to adhere to the plate by culturing for 2-3 h in 37 °C, 5% CO2 humidified incubator. Non-adherent cells are removed by gently washing three times with 1 ml of warmed PBS (Remaining cells should be greater than 90% macrophages).
- Stimulate peritoneal macrophages with apoptotic Jurkat cells
- After overnight culture, media for peritoneal macrophages are changed with 500 μl of RPMI-1640 without FBS.
- Add 100 μl of induced and evaluated apoptotic Jurkat cells (suspended in RPMI-6140 without FBS, with a concentration of 25 x 106 cells/ml) into macrophage cultures at a 5:1 ratio.
- Harvest cell culture supernatants at 8 h, 16 h after apoptotic cell challenge.
- Centrifuge harvested samples in a refrigerated centrifuge for 10 min at 500 x g, and then carefully transfer cleared supernatant (~600 μl) to a new tube without disturbing cell pellet.
- Detection of IL-10 production by ELISA (Figure 2)
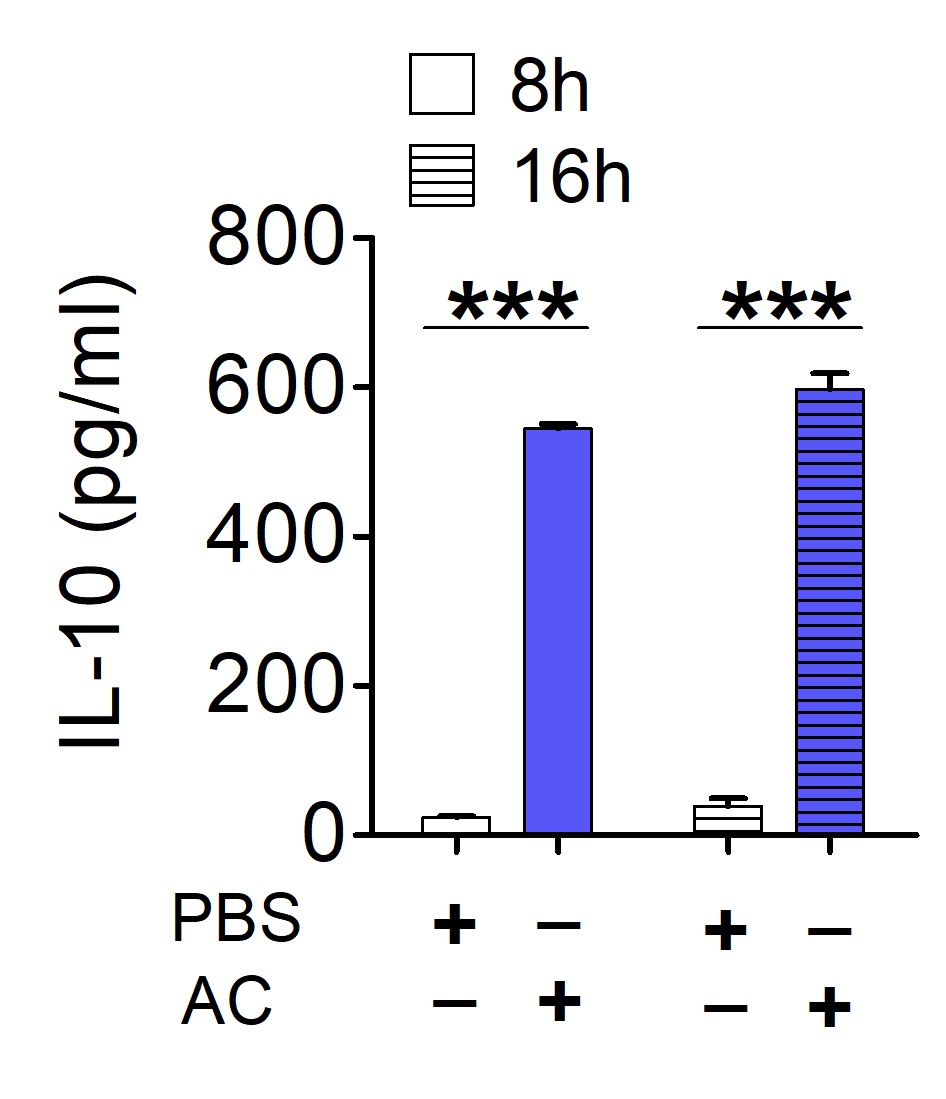
Figure 2. Macrophages produce IL-10 in response to apoptotic cells. 5 x 105 thioglycollate-elicited peritoneal macrophages were stimulated with apoptotic Jurkat cells (AC) (5:1 ratio of AC to macrophages) for indicated times and analyzed for IL-10 production.
Data analysis
- Analysis was performed using FlowJo software (7.6.1). Average three independent replicates to obtain a single read to each sample.
- To evaluate apoptosis of Jurkat cells, use unstained negative control to determine the absolute boundaries for both Annexin V-FITC and propidium iodide (PI) positive cells. Be sure to compare data generated with control cells (DMSO treated cells) to that of the unstained negative control.
- For ELISA, subtract the value of blank sample to avoid background reads.
- Mean values and standard deviation (S.D.) were used as descriptive statistics. Comparisons between two groups were performed using an unpaired two-tailed Student’s t-test (P < 0.05 was considered statistically significant).
Recipes
- Complete media for Jurkat cells
RPMI 1640 Medium, GlutaMAXTM Supplement, and HEPES (450 ml)
50 ml (10%) Fetal Bovine Serum
5 ml (100x) Penicillin-Streptomycin
5 ml (100x) GlutaMAXTM Supplement - 3% (w/v) Brewer thioglycollate medium
- Suspend 30 g of BD BBL Dehydrated Culture Media (Fisher scientific) in 1,000 ml of distilled water and autoclave to sterilize
- After cooling, aliquot to 50 ml Centrifuge Tubes, and could be stored in the dark at room temperature for 3 months
- 1x Binding buffer
10x Annexin V Binding Buffer (component no. 51-66121E): 0.1 M HEPES/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2
For a 1x working solution, dilute 1 part of the 10x Annexin V Binding Buffer to 9 parts of distilled water
Note: 1x Binding buffer should be freshly prepared. - RPMI-1640-10
To prepare RPMI-1640-10, additionally supplement RPMI-1640 medium with 10% (v/v) fetal bovine serum - 0.5 mg/ml Staurosporine (1,000x stock)
- Resolve 200 μg of Staurosporine (Sigma-Aldrich) in 400 μl Dimethyl sulfoxide (Sigma-Aldrich)
- Aliquot and store at -20 °C and thaw an aliquot before each use
Acknowledgments
This work was supported by an NIH grant (1R21AI110815) to X.M. This protocol was adapted from Chung EY et al. (2007).
Competing interests
The authors declare no conflicts of interest.
References
- Chung, E. Y., Liu, J., Homma, Y., Zhang, Y., Brendolan, A., Saggese, M., Han, J., Silverstein, R., Selleri, L. and Ma, X. (2007). Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27(6): 952-964.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Song, M. and Ma, X. (2019). Isolation and Stimulation of Peritoneal Macrophages with Apoptotic Jurkat Cells to Produce IL-10. Bio-protocol 9(24): e3467. DOI: 10.21769/BioProtoc.3467.
Category
Immunology > Immune cell isolation > Macrophage
Immunology > Immune cell isolation > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link