- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Trial-based Discrimination Procedure for Studying Drug Relapse in Rats
(*contributed equally to this work) Published: Vol 9, Iss 23, Dec 5, 2019 DOI: 10.21769/BioProtoc.3445 Views: 5008
Reviewed by: Shaarika SarasijaMarina AllerbornAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1294 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
In abstinent drug addicts, cues formerly associated with drug-taking experiences gain relapse-inducing potency (‘incubate’) over time. Animal models of incubation may help in developing treatments for relapse prevention. However, these models have primarily focused on the role of conditioned stimuli (CSs) signaling drug delivery and not on discriminative stimuli (DSs), which signal drug availability and are also known to play a major role in drug relapse. We recently showed that DS-controlled cocaine seeking in rats also incubates during abstinence and persists up to 300 days. We used a trial-based procedure to train male and female rats to discriminate between two light cues: one light cue (DS+) signaled the availability of cocaine reward and the second light cue (DS-) signaled the absence of reward. Rats learned to press a central retractable lever during trials in which the DS+ cue was presented and to suppress responding when the DS- cue was presented. Here, we provide a detailed protocol for the behavioral procedure used in our study. The trial-based design of this behavior lends itself well to time-locked in vivo recording and manipulation approaches that can be used to identify neurobiological mechanisms underlying the contributions of DSs to drug relapse.
Keywords: DrugsBackground
In abstinent drug addicts, the ability of drug-associated cues to induce relapse increases over time. This process is called ‘incubation’ (Neisewander et al., 2000; Grimm et al., 2001 and 2002; Lu et al., 2004a). Incubation has been demonstrated in both preclinical (Shalev et al., 2001; Bienkowski et al., 2004; Lu et al., 2004b; Shepard et al., 2004; Abdolahi et al., 2010) and clinical (Bedi et al., 2011; Wang et al., 2013; Li et al., 2015; Parvaz et al., 2016) studies, and is a target for development of novel treatments for the prevention of drug relapse. In preclinical models of incubation, animals are first trained to self-administer a given drug of abuse and then, following successful learning of operant responding, tested for drug seeking under extinction conditions (i.e., in the absence of drug reward) at different time points during abstinence. To date, incubation studies have focused on how discrete cues paired with drug delivery during training (i.e., conditioned stimuli, CSs) can potentiate drug seeking during abstinence. Another type of cue critical to performance of drug-taking behavior has been overlooked: the discriminative stimulus (DS). DSs are different from cues typically investigated in preclinical incubation studies in that they are neither response-contingent like CSs, nor ever-present like contextual cues. Rather, DSs precede operant responding and signal drug availability–or unavailability–thereby guiding the performance of drug-taking behavior. DS-controlled operant responding is also very difficult to extinguish (Ettenberg, 1990; McFarland and Ettenberg, 1997; Katner et al., 1999; Ghitza et al., 2003; Yun and Fields, 2003; Ciccocioppo et al., 2004; Weiss, 2010; Mihindouet al., 2013; Suto et al., 2016; Martin-Fardon et al., 2017), which makes the DS an important factor in the notorious persistence of drug addiction, and thus, a critical target for intervention. Despite the importance of DSs in stimulus control of drug taking and relapse, it was unknown whether DS-controlled drug seeking incubates during abstinence.
We recently developed a trial-based procedure (Madangopal et al., 2019) to train male and female rats to discriminatively self-administer cocaine (0.75 mg/kg/infusion) during trials in which a positive discriminative stimulus (DS+) signaled cocaine availability, while suppressing responding on the same lever during trials in which a negative discriminative stimulus (DS-) signaled cocaine unavailability during the same session. Drug infusions were not paired with CSs (Madangopal et al., 2019). Following training, we tested for the ability of DSs to control cocaine seeking at multiple time points extending to 400 days of abstinence and observed that cocaine seeking during DS+, but not DS-, presentations was maximal after 60 days of abstinence (reflecting incubation of DS-controlled cocaine-seeking) and persisted for up to 200 days despite repeated testing under extinction conditions. In contrast, following discriminated operant training for palatable food reward, DS-controlled food seeking was maximal at 1 day of abstinence, progressively decreased over time, and was no longer observed after 60 abstinence days. Thus, DS-controlled incubation was specific for cocaine and observed in the absence of CSs.
DS-controlled behaviors offer an especially promising path to treatment development because DSs are always present before and during human drug taking. The current behavioral protocol utilizes a trial-based format featuring two distinct trial types, allowing for well-controlled study of such stimuli and the potential for use in time-locked in vivo recording and manipulations in mechanistic studies of discrimination learning, discriminated drug taking, and discriminative stimulus-controlled drug relapse.
Materials and Reagents
Catheters (see Figure 1B)
- Polyester Mesh Sheet: 12” width, 12” length, 1000 microns mesh size, 58% open area (Small Parts, catalog numbers: CMY-1000-C/5PK-05)
- Medical Silicone Adhesive (Factor II Incorporated, catalog number: A-100)
- Heat shrink tubing: Fit 221, 3/64 clear, 1.169 mm (Newark Electronics, catalog number: 37F6980)
- Ortho-Jet Powder: fast curing (Patterson Dental, 459-8371 Mfg#1330-P)
- Orthodontic Resin Liquid: pink (Patterson Dental, C22-0603 Mfg#651002)
- Bolts (McMaster-Carr, catalog number: 90308A129, Female Threaded Hex Standoff Zinc-Plated Brass, 1/4" Hex Size, 5/8" Long, 6-32 Thread; see Figure 1B: Item 5)
- Cannulae: 5up 22g (Plastics One, catalog number: C313G-5up)
- Silastic Tubing (VWR, catalog number: 62999-075)
- Catheter Mold (Behavioral Pharma Inc.)
- I.V. blocker: Saint-Gobain*Tygon*Microbore Tubing (cut and sealed on one end; Fisher Scientific, catalog number: 14-171-284; see Figure 1B: Item 4)
Animals
- Sprague-Dawley Rats, both sexes, 250-300 g, ~56 days old at start of experiment (Charles River)
- 100 mg/ml cocaine-HCl (cocaine) diluted in sterile normal saline (NIDA pharmacy, store at room temperature, store in a secure safe)
- Gentamicin diluted to 4.25 mg/ml (Fresenius Kabi, Multiple Dose Vial [80 mg/2 ml], catalog number:1002, store at 4 °C)
- 0.9% Sodium Chloride Injection USP (Henry Schein Medical, Sterile Saline, catalog number: 1046816, store at 4 °C)
- Brevital (Butler Schein, 500 mg MDV Injection, catalog number: 038431)–dilute to 10 mg/ml with sterile saline and store 1 ml aliquots at 4 °C
- Palatable food pellets (Test Diet, AIN-76A Rodent Tablet 45 mg, 5TUL, store at 4 °C, shelf life is 1 year)
- Ethanol diluted to 70% in deionized water (NIH Supply Center, catalog number: 201096)
- Ketofen (Ketoprofen) diluted to 2.5 mg/ml (Butler Schein, sterile solution, 100 mg, catalog number: 005487, store at 4 °C
- Froot Loops (Kellogg Company, Cereal, store at 4 °C, shelf life is 1 year)
- Plastic One Channel Swivel (Instech Laboratories Inc, Plastic Liquid Swivels, catalog number: 375/22PLS; see Figure 1A: Item 11)
- Modified needles (Thomas Scientific, BD 27G x 1/2’ Luer-LokTM Syringe Needles, catalog number: 8956A70)
- Springs (HRS Scientific, catalog number: 0219-020-13780; see Figure 1A: Item 12)
- Saint-Gobain*Tygon*Microbore Tubing (Fisher Scientific, catalog number: 14-171-284; see Figure 1A: Item 10)
Equipment
Operant Chambers
- Med Associates Modular Chamber for Rat (Med Associates Inc, catalog number: ENV-007; see Figure 1A: Item 2)
- Med Associates Sound Attenuating Chamber (Med Associates Inc, catalog number:ENV-018MD-W; see Figure 1A: Item 1)
- Stainless Steel Grid Floor (Med Associates Inc, catalog number: ENV-005; see Figure 1A: Item 13)
- Stainless Steel Waste Pan (Med Associates Inc, catalog number: ENV-007A3; see Figure 1A: Item 14)
- Single Speed Syringe Pump (Med Associates Inc, catalog number: PHM-100, 3.33 RPM; see Figure 1A: Item 8)
- Retractable Lever for Rat (Med Associates Inc, catalog number: ENV-112CM; see Figure 1A: Item 5)
- Incandescent Stimulus Light (White) for Rat Modular Chamber (Med Associates Inc, catalog number: ENV-221M; see Figure 1A: Item 4)
- Replacement Lens Cover (Red) (Med Associates Inc, catalog number: SG-221-RD; see Figure 1A: Item 3)
Software
- MED PC IV (Med Associates Inc, SOF-735)
- Microsoft Excel
Procedure
- Intravenous catheterization
In this self-administration protocol, rats learn to press a lever to receive–through a permanent catheter inserted into the jugular vein–a direct intravenous infusion of a fixed volume of cocaine dissolved in saline. This direct delivery of drug into the vein allows it to rapidly reach the brain and link the operant response to the rewarding effects felt upon drug delivery. For a schematic of the catheters we use, refer to Figure 1B.- Perform surgery to implant an intravenous catheter into the right jugular vein of each rat. For a list of parts for this surgery refer to the Equipment section, and for a detailed video of the steps of surgery please refer to Feng et al., 2015.
- We use short pieces of Tygon tubing that have been sealed off at one end (referred to as I.V . blockers; see Figure 1B: Item 4) to cap the entry port of the catheters. Zinc-coated hex bolts (referred to as bolts; see Figure 1B: Item 5) are also used to protect the threads and the entry port of the catheters. We have found that this combination works better for our catheters than other commercially available catheter blockers.
- The bolt and I.V. blocker are removed prior to behavioral training, sterilized in 70% ethanol during behavioral sessions, and reattached prior to returning rats to their home cages.
- Post-surgery care
Post-operative care includes daily monitoring of rats, and post-surgical observations are documented on each rat’s cage card for at least three days.- Measure and record body weight for each rat. Monitor weight loss and report to your institutional veterinarian if a rat loses > 10% of its body weight in a day. Typically, rats lose ~10 g the day after surgery and will regain this weight during their recovery period.
- Give rats subcutaneous injections of the veterinary nonsteroidal anti-inflammatory drug (NSAID) ketoprofen (2.5 mg/kg) once rats have shown complete recovery from anesthesia (approximately 1-2 h after surgery) and also the next day to provide analgesia and decrease inflammation. Discontinue this step after the first day unless rats show signs of distress on the following days.
- Fill a 1 ml Luer-lock syringe with gentamicin solution (4.25 mg/ml in sterile saline). Connect it to the catheter port using a piece of connecting Tygon tubing attached to a modified needle on the end of the syringe. Slowly inject 0.1 ml gentamicin solution into vein through the catheter. This step helps to minimize microbial infection following surgery and maintain catheter patency.
- Behavior room setup
The behavior room, sound attenuating chambers, and behavioral testing boxes should all be sanitized prior to starting any behavioral run. Additionally, test all equipment (pumps, lights, levers) and behavioral monitoring programs, assign rats to individual behavior boxes, and set up individual drug syringes for each rat on the day before the first session. In our original study, we used a cocaine dose of 0.75 mg/kg/infusion and an infusion volume of 0.1 ml/infusion during all phases of behavioral training. Please refer to Figure 1A for a detailed schematic showing the setup of the behavioral chambers and the components described during this protocol.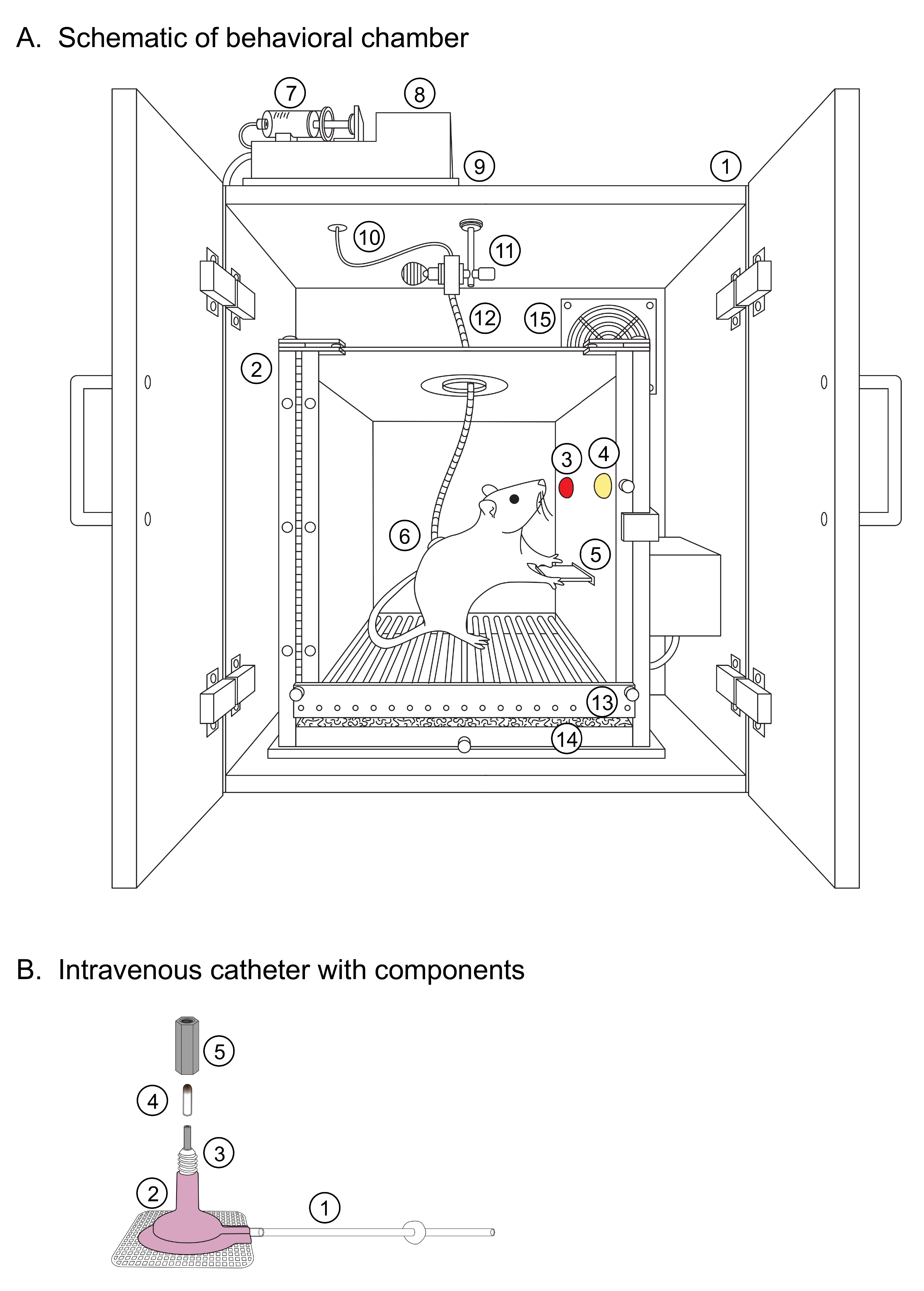
Figure 1. Behavioral Apparatus. A. Schematic of behavioral chamber. (1) Sound-attenuating chamber, (2) Operant chamber, (3,4) DS+/- light cues, counterbalanced, (5) Central retractable lever, (6) Catheter back-mount, (7) (20 ml) drug syringe, (8) Fixed-speed infusion pump, (9) Sound-attenuating foam pad, (10) Drug infusion line, (11) Liquid swivel, (12) Protective spring, (13) Stainless steel grid floor, (14) Stainless steel waste pan with bedding, (15) Ventilation fan. B. Intravenous catheter with components. (1) Intravenous tubing, (2) Back-mount, (3) Threaded connection port, (4) Intravenous blocker, (5) Zinc-coated hex bolt.- Drug syringes: Weigh each rat the day before the first training session and assign correct dosages based on individual weights. Refer to Cocaine dose calculations in Notes for an example of cocaine dose calculation. Label each syringe with the rat number, the number of the behavior chamber in which they are trained, and the dose of cocaine they need based on their weight. Each rat’s drug dose should also be checked against their weight periodically to account for changes of dose based on weight increases during the course of training.
- Counterbalancing cues: As discussed earlier, we used two different light cues as the discriminative stimuli in our task. We used different color caps (red vs. white) and different spatial locations (left vs. right side of the central lever) to help rats discriminate between these two stimuli during training. Although we did not observe any differences in training based on which light cue was used as the DS+ or DS-, it is advisable to counterbalance the two colors across your behavior boxes. For example, if the two light colors are white and red, set up your boxes so that the DS+ light is red in half of the boxes and white in the other half.
- Lever tightness: When using Med Associates levers, downward deflection of the lever (i.e., a lever press) leads to the temporary closure of a switch that is in turn read as an operant response by the computer. The tension on these levers can be adjusted (by tightening a spring) to precisely control how much force is necessary to trigger the switch. As rats of different weights (e.g., male vs. female rats of similar ages) might have varying amounts of strength, we tune the lever ‘tightness’ based on the size of the rats but keep it consistent between boxes of rats with similar weights. To optimize lever tightness, we recommend that you observe the rats on the first few days of training and confirm that they are able to depress the lever (such that it reads as a response on the computer) when they lean onto it with both front paws. If a rat is unable to depress the lever (because it is too tight) and receive reinforcement, it will not learn that responses on the lever lead to reward, making it unlikely that it will learn the task.
- Cocaine self-administration training stage 1: Continuous access (2 x 3 h sessions per day, 3 consecutive days, see Figure 2A)
The goal of this stage is to train rats to press the central retractable lever to receive an infusion of cocaine. In this stage, the lever is permanently available throughout the session, and the light on the left of the lever is always on. This is the light that will eventually turn on during DS+ trials in the trial-based task. Responses on the lever result in a single 3.5 s infusion of cocaine (0.75 mg/kg/inf) through the back-mounted intravenous catheter. Additional responses during this 3.5 s period are not reinforced. By the end of this stage, rats should stably administer 25-30 infusions of cocaine in each 3 h session.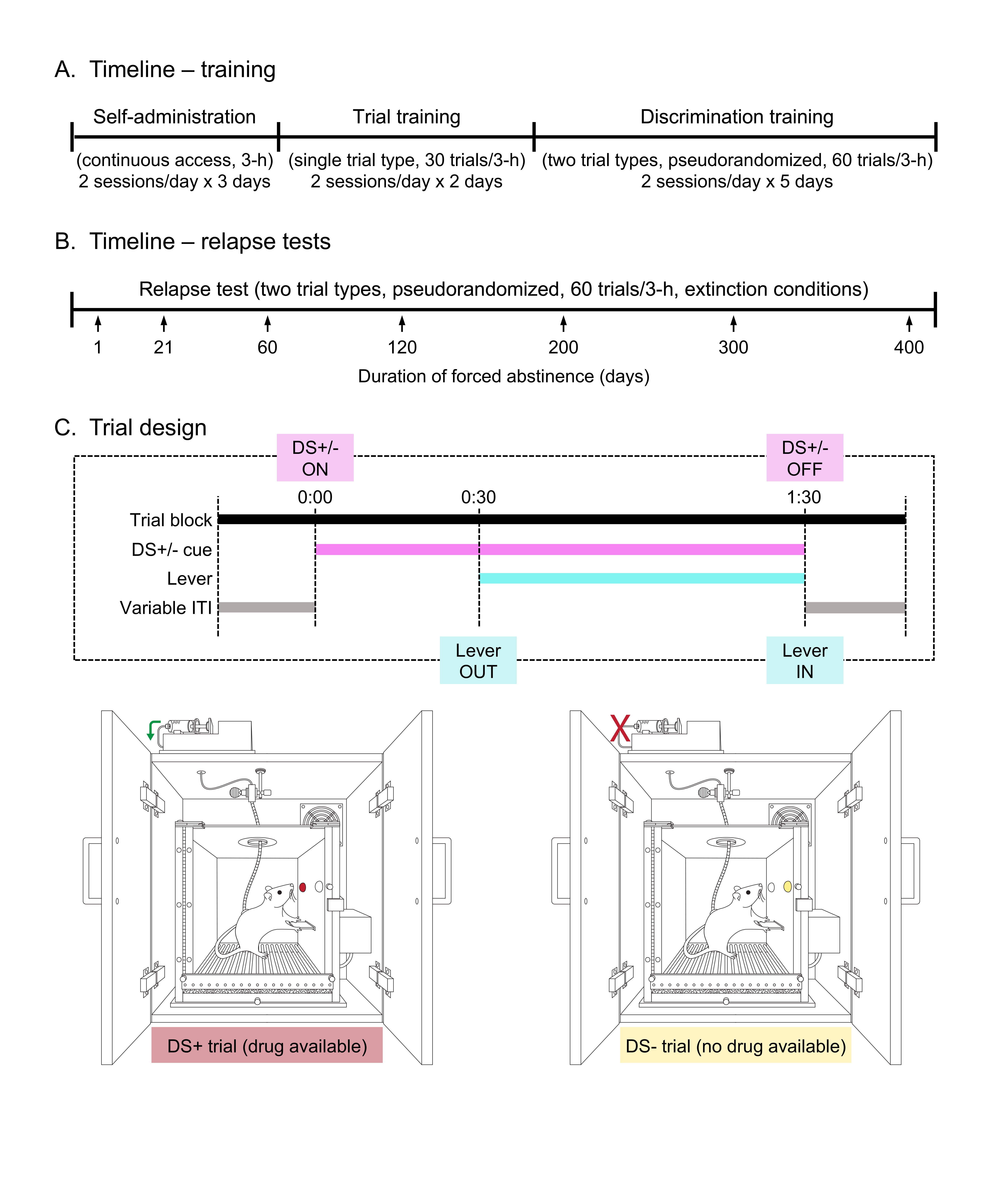
Figure 2. Behavioral procedure. A. Timeline-training. Timeline for self-administration training, consisting of three stages: self-administration (also referred to as continuous access), trial training, and discrimination training. B. Timeline-relapse tests. Relapse tests are conducted immediately following training, after increasing periods of forced abstinence. C. Trial design. Timing of events during a single trial block and distinction between the two trial types.- Bring rats out of the vivarium and to the room in which behavioral procedures will be conducted.
- Measure and record body weight for each rat at the start of the day. Always check to ensure that rats have not lost weight (> 10% decrease in body weight) compared to the previous day. Typically, rats will gain ~2-5 g each day during cocaine self-administration training.
- Disconnect the bolt and I.V. blocker from the catheter port. Place them in 70% ethanol.
- Administer 0.1 ml gentamicin intravenously (as described in Procedure B) to each rat and connect its back-mounted catheter to the drug line and swivel-tethered protective spring sleeve in its assigned operant box. Immediately prior to attaching a rat to its drug line, manually activate the pump motor to run the line through with drug and confirm that it is dripping consistently out of the end of the line. This allows you to test the infusion pumps and to ensure that there are no interruptions and/or air bubbles in the fluid line.
- Close the doors of both the behavioral chamber and the sound-attenuating chamber. Once all rats are in their respective boxes, set up the MedPC program and start the behavioral session.
- The sequence of steps described above in Steps D1-D5 is hereafter referred to in the protocol as ‘loading rats’.
- Perform checks on behavior of rats to ensure that they are not becoming tangled in their tethers and/or detached from their drug lines. Also, observe that the timing of infusions is regular and uniformly spaced during the session. Rats typically take 8-12 infusions per hour at the 0.75 mg/kg/inf. dose recommended for this procedure. It is recommended that you check on the rats once every 30-45 min. Perform checks more frequently if rats are rapidly increasing their levels of cocaine self-administration, as it may become more likely that they will become tangled/detached as a result of increased activity.
- At this stage of training, not all rats will readily pick up self-administration (i.e., press the lever to complete the operant requirement to receive cocaine reward). Priming may be necessary to facilitate learning for any rats that have not performed a lever press within the first 45 min of the first training session. Follow the Cocaine priming procedure in Notes. If any rats show signs of loss of catheter patency, conduct appropriate tests to confirm patency (see Notes 6-7).
- Wait 30 min after the end of the first session of each day before starting the second session. Do not remove rats from boxes between the first and second session of the day.
- At the end of the afternoon session, disconnect each rat from its spring and infusion line, flush with 0.1 ml gentamicin and reattach the bolt and I.V. blocker to its catheter. Place each rat back in its homecage and return to the vivarium. This sequence of steps is hereafter referred to in the protocol as ‘unloading rats’.
- Log the body weight of each rat, the number of lever responses made per session, and the number of infusions received during each 3-h session. Plot these measures as the mean of all rats in the group each day.
- Repeat Steps D1-D11 for 3 days. If rats do not achieve stable levels of drug intake, this stage of training can be extended for an additional 2 days (4 more sessions).
- Give rats a day off from behavior training prior to starting the next stage of training. We typically train rats that have not reached stable responding on two additional remedial sessions during this day off.
- Cocaine self-administration training stage 2: Introduction to trial format (2 x 3 h sessions per day, 30 DS+ only trials in the morning, 30 DS- only trials in the afternoon, 2 consecutive days, see Figures 2A and 2C)
The goal of this stage is to train rats to continue pressing the central retractable lever when the task format is switched from continuous access to a trial-based design. In this stage, rats get 30 DS+ trials in the morning session and 30 DS- trials in the afternoon session. Each trial begins by the illumination of the respective cue light for 30 s, followed by the extension of the lever. After 60 s of lever access, the trial ends as the cue light turns off and the lever is retracted. The end of each trial is followed by a variable inter-trial interval (ITI-270 s average), after which a new trial begins. During DS+ trials, the light on the left side of the lever is illuminated and responses on the lever result in a single 3.5 s infusion of cocaine (0.75 mk/kg/inf). Additional responses during this 3.5 s period are not reinforced. During DS- trials, the light on the right side of the lever is illuminated and responses on the lever do not result in cocaine infusions. Thus, during this phase, rats learn the trial-based format and also experience both trial types, but in isolation. By the end of this phase, rats should still stably administer 25-30 infusions of cocaine in the DS+ only session but should reduce responding during the DS- only session.- Load rats and run them on the appropriate MedPC program by following Steps D1-D5.
- Perform checks on behavior of rats as described earlier (Step D7).
- A sudden change in rates of responding can be an indicator that a rat is not receiving its expected level of drug reward per infusion–this could happen because of pump failure, catheter failure, lever malfunction, depletion of the syringe, or disconnection of the rat’s drug infusion line during the session. If this occurs, first disconnect the rat from its line and then perform necessary checks to determine the source of the problem. Only reconnect the rat once you have corrected the situation. For more details on loss of patency, refer to Checking for Catheter Patency in Notes.
- At this stage of training, prime any rat who appears to be responding significantly less in the morning sessions because of the switch to the trial format and/or those who are still not responding consistently. Do not prime the rats during DS- only sessions. Follow the Cocaine priming procedure in Notes. If any rat shows signs of loss of catheter patency, conduct appropriate tests to confirm patency (see Notes 6-7).
- Wait 30 min after the end of the first session of each day before starting the second session. Do not remove rats from boxes between the first and second session of the day.
- At the end of the afternoon session, disconnect each rat from its spring and infusion line, flush with 0.1 ml gentamicin and reattach the bolt and I.V. blocker to its catheter. Place each animal back in its homecage and return to the vivarium.
- Record the body weight of each rat, as well as the number of trials of each type in which an animal makes at least one response (“trials”). Also, record the number of lever responses made during each trial type per session (“lever presses”) and the number of infusions received across all DS+ trials in a 3 h session (“infusions”). Plot these measures as the mean of all rats in the group each day.
- Repeat Steps E1-E7 for 2 days. If rats do not achieve stable levels of drug intake, this stage of training can be extended for an additional 2 days (4 more sessions).
- Give rats a day off from behavior training prior to starting the next stage of training. We typically train rats that haven’t reached stable responding on two additional remedial sessions during this day off.
- Cocaine self-administration training stage 3: Discrimination (2 x 3 h sessions per day with pseudorandomized presentation of 30 DS+ and 30 DS- trials in every session, 5-10 days, see Figures 2A and 2C)
The goal of this stage is to train rats to discriminate between DS+ and DS- trials and only respond on the central retractable lever during DS+ trials. In this stage, rats get 30 DS+ and 30 DS- trials in each 3 h session. Trial timing and reward rules for each trial type are the same as in the previous phase, but the inter-trial intervals are reduced to a 90 s average to accommodate for the additional trials in each 3 h session, and DS+ and DS- trials are presented in a pseudorandomized manner so that rats are required to pay attention to the DS in order to determine whether cocaine is available. Thus, during this stage, rats learn to respond discriminatively (i.e., to respond only during DS+ trials) and stably administer 25-30 infusions of cocaine in each 3 h session. We average each rat’s discrimination ratio (DR, total number of lever presses during all DS+ trials/total number of lever presses during all DS- trials) over the last 3 sessions and consider a rat to be trained when its DR ≥ 3.- Load rats and run them on the appropriate MedPC program by following Steps D1-D5.
- At this stage of training, prime rats who demonstrate low overall levels of responding (i.e., those who do not appear to be responding consistently enough to achieve at least 10 infusions per session-see Expected behavioral response timing in Notes for more details on “consistent” responding). Do not prime during DS- trials. Cease all priming efforts starting at the third-to-last training session before the Day 1 relapse test. Follow the Cocaine priming procedure in Notes. If any rats show signs of loss of catheter patency, conduct appropriate tests to confirm patency (see Notes 6-7).
- Perform checks on behavior of rats as described earlier (Step D7).
- Continue to ensure that any apparent sudden behavioral changes are not the result of failures of any of the mechanisms involved in drug delivery (for more details on this, see Step E3).
- Wait 30 min after the end of the first session of each day before starting the second session. Do not remove rats from boxes between the first and second session of the day.
- See Step D10 for details on unloading rats after the second session of the day and Step E7 for details on recording data.
- Repeat Steps F1-F6 until rats show stable discriminated responding for cocaine. Typically, rats achieve stable discrimination over 10-16 sessions (5-8 days).
- Give rats a day off from behavior after 3-5 consecutive training days and ensure that they have at least 3 consecutive days of training prior to their first relapse test (day 1 relapse test).
- Abstinence (1- 400 days, see Figure 2B)
In this phase, rats are kept in their home cages in the vivarium. They do not receive any additional training, and hence, do not have access to cocaine.- Check on rats twice a week and ensure that they still have their bolts and I.V. blockers on their catheters.
- Handle rats to keep them socialized during long durations of abstinence. This can help to minimize handling-related stress during testing.
- Relapse to cocaine seeking test (1 x 3 h session on each test day with pseudorandomized presentation of 30 DS+ and 30 DS- trials, see Figures 2B and 2C)
In this phase, we test rats for DS-controlled drug-seeking (i.e., responding on the lever that they previously associated with cocaine access, but while no drug is available during the session). The session format and trial spacing are the same as during discrimination training.- Clean behavior boxes and set up the room in the same way as at the start of behavioral training (see Procedure C) but with the exceptions noted below.
- Turn off the syringe pumps and disconnect all syringes from their infusion lines.
- Ensure that all lines formerly used for drug delivery are cleared of any traces of drug by flushing first with D.I. water, then 100% ethanol, and finally with air through the line to clear traces of ethanol.
- Load rats into individual operant boxes after weighing and mock intravenous gentamicin administration (follow the steps described in Post-Surgery Care, but do not infuse any gentamicin through the catheter). Perform only mock intravenous gentamicin administration during loading on relapse test days, as loss of catheter patency over time may cause flushing to become painful and thereby stress rats before the test.
- Set up the MedPC program and start the behavioral session.
- At the end of the session, disconnect each rat from its spring and infusion line, perform mock flush with 0.1 ml gentamicin and reattach the bolt and I.V. blocker to its catheter. Place each rat back in its homecage and return to the vivarium.
- Record the body weight of each rat, as well as the number of trials of each type in which an animal makes at least one response (“trials”). Also record the number of lever responses made during each trial type per session (“lever presses”). Plot these measures as the mean of all rats in the group each day.
- Clean behavior boxes and set up the room in the same way as at the start of behavioral training (see Procedure C) but with the exceptions noted below.
Data analysis
Please refer to the original article (Madangopal et al., 2019) for behavioral measures used for different phases of the study, recommended sample sizes, and statistical tests employed for analysis. Details of inclusion/exclusion criteria can also be found in the statistical analysis section of the same paper.
Notes
- Light cycle conditions
Ensure that proper light cycle conditions are maintained in the room in which all training and testing occur. In our experiments, rats were housed on a reverse dark cycle (i.e., white room lights off at 9:00 A.M. and back on at 9:00 P.M.). All behavioral procedures (training and relapse tests) were conducted in MedPC sound-attenuated behavioral chambers during their dark cycle. Rats were housed in the vivarium (in their homecages) between behavioral sessions and during abstinence. - Daily maintenance of behavioral chambers
Daily maintenance of boxes includes refilling drug syringes at the end of each day and changing bedding in boxes every other day. The goal of refilling syringes is to ensure that each syringe contains enough drug for the sessions that will take place the following training day. To achieve this, we have found that it is best to refill any syringe that contains less than 13 ml of drug following the end of the second session of the day. In order to decrease the chance that any rat will run out of drug during the second session of a given day, it is also advisable to check syringes between the first and second session of the day to ensure that there is at least 10 ml of drug in each syringe. These standards are only general guidelines based on our observation of the average rate of cocaine self-administration by rats in our experiments (typically ~30 infusions per 3 h session when using 0.75 mg/kg/inf. cocaine). It is recommended that appropriate adjustments be made based on observed self-administration patterns of each animal. - Cocaine dose calculations
We recommend titrating the cocaine concentration for each rat to achieve a final cocaine dose of 0.75 mg/kg/inf. throughout training. For example, if a rat weighs 300 g, the final concentration required is 2.25 mg/ml. See below for the equation: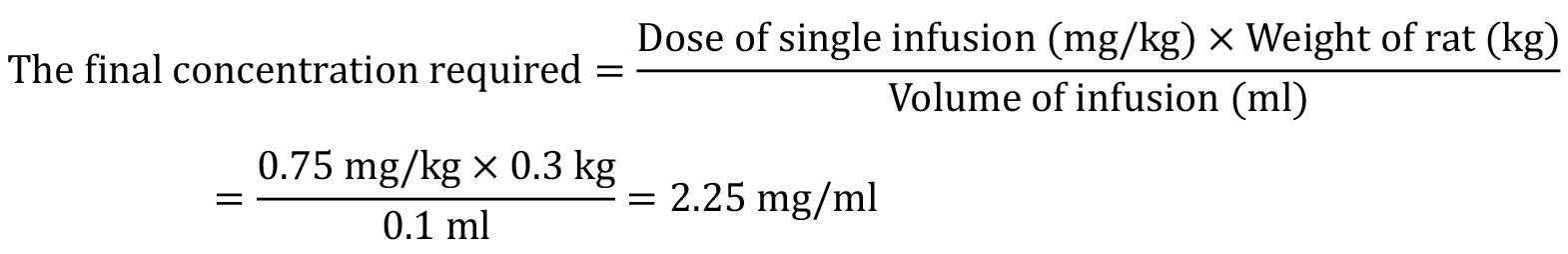
To make a 20 ml syringe of cocaine for this rat at this concentration using a stock solution of 100 mg/ml, you will need: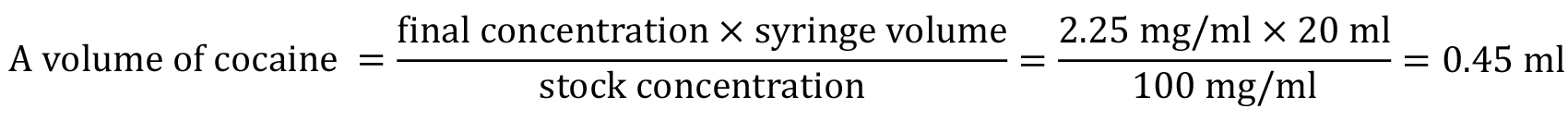
Therefore, you need to add 0.45 ml stock cocaine to 19.55 ml of sterile saline to make this drug syringe. - Food restriction procedure
Food restriction to facilitate training involves the removal of all but approximately two standard diet chow pellets (15-20 g) from the home cage. Rats are given unrestricted access to water in their homecages during this time. The purpose of this restriction is to increase (1) the likelihood that food-based priming will result in interaction with the lever and (2) rats’ general activity levels that may result in lever pressing. It is important to note that rats should be maintained at > 85% of their ad libitum feeding weight throughout the study and restricted feeding should not be used on consecutive days. - Overnight housing procedure
Any rat who appears to be anxious as a result of exposure to the novel operant box environment (i.e., during the beginning of stage 1 of training) may be housed in the box overnight to attenuate this anxiety. Following the end of the second session of the day, disconnect the rat from the drug line and intravenously administer 0.1 ml of gentamicin at a concentration of 4.25 mg/ml as usual. Return the rat to the box without tethering the rat to the drug line. Provide the rat with access to sufficient food and water unless food restricting rat (if food restricting, follow Procedure 4 above). Additionally, ensure that any openings in the box through which the drug line traveled are covered so that the rat cannot escape the box during overnight housing. When housing rats in their behavior chambers, also make sure that the testing room is properly equipped to maintain their light cycle in the same way as the vivarium and leave one door of the sound attenuating chamber open overnight to allow the rat to be exposed to the light cycle change in the room. - Cocaine priming procedure
The goal of priming rats during training is to facilitate the formation of the association between the operant response (i.e., the lever press) and the receipt of reward (i.e., the infusion). For a given rat, the priming procedure should always proceed in the following order (a., b., and c. below). Once a rat has reached the point of manual priming, continue priming only via this form of priming (i.e., do not revert to forms a and b).- The first instance of priming should be a simple “lever jiggle” in which the lever is briefly brought into and out of the operant box to draw the rat’s attention to the lever.
- The second instance of priming involves the use of crushed Froot Loops placed onto the lever in order to prompt the rat to interact with the lever. Prior to using this approach, please ensure that rats have had access to a small quantity of Froot Loops in their home cages to counteract potential effects of innate neophobia. While we routinely use Froot Loops in our lab for this process, these can be replaced with other dry palatable foods that can be crushed into a coarse powder. Use of food restriction can also facilitate food priming-based training in this phase.
- Manual priming by the experimenter is used as a final attempt to facilitate training. In manual priming, the experimenter physically guides the rat to perform the lever press motion while also ensuring that this interaction involves minimal stress for the animal. Once this form of priming has been used for a rat, avoid reverting to previous forms of priming and continue only with manual priming for said animal.
- Checking for catheter patency
The following tests of catheter patency are performed only when the experimenter suspects loss of patency. Loss of catheter patency may result in a sudden change in behavioral responding that is not attributable to the natural progression of the training timeline (e.g.,the switch to trial format) or another acute experience by the animal of which the experimenter is aware (e.g., stress from handling). Loss of patency may also be suspected when such a behavioral change is accompanied by subcutaneous swelling at the neck, clear fluid leakage at the back catheter port, and/or a noticeable change in the resistance to flushing of the catheter (either significantly increased or decreased resistance). Before performing a brevital-based test, first check for patency either by infusing a larger volume of saline (see a. below), attempting to draw blood via the catheter (see b. below), or by observing a rat’s behavioral response to cocaine infusion during training (see c. below). Proceed to brevital-based testing if none of these checks reveal patency.- Slowly inject 2 ml of saline through the catheter port using a flusher. If the catheter is cracked at the base, this will result in clear fluid leakage around the backport. If the catheter has slipped out of the vein this will result in the formation of a liquid bubble around the site of catheter insertion into the vein (at the animal’s neck).
- First slowly inject 2 ml of saline through the catheter port and then slowly pull back to draw a small amount of blood (less than 0.01 ml) from the rat. If blood can be drawn via the catheter, it is very likely that the catheter is patent. When catheters crack or slip out of the vein, air or clear fluid can be seen in the line of the flusher attached to the catheter while attempting to draw blood into the line.
- Checks for patency can also be performed by observing a rat’s behavior following a cocaine infusion in the operant box. A rat with a patent catheter will typically display apparent stereotyped behaviors (e.g., rapid grooming, repetitive circular head movements, and rapid sniffing) within seconds following a cocaine infusion at the 0.75 mg/kg dosage.
- Brevital testing procedure
Brevital (methohexital sodium) is an ultra-short-acting barbiturate. In general, a total loss of muscle tone within 3 s after a methohexital sodium injection (10 mg/ml, 2 mg/rat, injected intravenously), indicates catheter patency. Before intravenous injection of brevital, prepare a syringe with 6 ml of 0.9% saline with which to flush the animal immediately following observation of loss of muscle tone. Flush the animal with saline even if loss of muscle tone is not observed. In the case that the response is not observed, recatheterization surgery is necessary if the animal is to be included in the experiment.
Acknowledgments
All work was conducted at the National Institute on Drug Abuse (NIDA) and was supported by the NIDA Intramural Research Program funds to the lab of Dr. Bruce T. Hope. The procedures described here are also detailed in the original study published in eLife (Madangopal et al., 2019). We thank Dr. Bruce T. Hope for thoughtful comments during the writing of this manuscript and M. Raley (NIDA IRP Visual Media) for assistance with illustrations.
Competing interests
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the text of the paper.
Ethics
The procedures described here are in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals (8th edition; http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf) and are approved by the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the Intramural Research Program of the National Institute on Drug Abuse. Animal experiment approval ID: 17-BNRB-203.
References
- Abdolahi, A., Acosta, G., Breslin, F. J., Hemby, S. E. and Lynch, W. J. (2010). Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31(4): 733-741.
- Bedi, G., Preston, K. L., Epstein, D. H., Heishman, S. J., Marrone, G. F., Shaham, Y. and de Wit, H. (2011). Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69(7): 708-711.
- Bienkowski, P., Rogowski, A., Korkosz, A., Mierzejewski, P., Radwanska, K., Kaczmarek, L., Bogucka-Bonikowska, A. and Kostowski, W. (2004). Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol 14(5): 355-360.
- Ciccocioppo, R., Martin-Fardon, R. and Weiss, F. (2004). Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci 7: 495-496.
- Ettenberg, A. (1990). Haloperidol prevents the reinstatement of amphetamine-rewarded runway responding in rats. Pharmacol Biochem Behav 36(3): 635-638.
- Feng, J., Fitz, Y., Li, Y., Fernandez, M., Cortes Puch, I., Wang, D., Pazniokas, S., Bucher, B., Cui, X. and Solomon, S. B. (2015). Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. J vis exp (95).
- Ghitza, U. E., Fabbricatore, A. T., Prokopenko, V., Pawlak, A. P. and West, M. O. (2003). Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci 23(19): 7239-7245.
- Grimm, J. W., Hope, B. T., Wise, R. A. and Shaham, Y. (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412(6843): 141-142.
- Grimm, J. W., Shaham, Y. and Hope, B. T. (2002). Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13(5-6): 379-388.
- Katner, S. N., Magalong, J. G. and Weiss, F. (1999). Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20(5): 471-479.
- Li, X., Caprioli, D. and Marchant, N. J. (2015). Recent updates on incubation of drug craving: a mini-review. Addict Biol 20(5): 872-876.
- Lu, L., Grimm, J. W., Dempsey, J. and Shaham, Y. (2004a). Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 176(1): 101-108.
- Lu, L., Grimm, J. W., Hope, B. T. and Shaham, Y. (2004b). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 Suppl 1: 214-226.
- Madangopal, R., Tunstall, B. J., Komer, L. E., Weber, S. J., Hoots, J. K., Lennon, V. A., Bossert, J. M., Epstein, D. H., Shaham, Y. and Hope, B. T. (2019). Discriminative stimuli are sufficient for incubation of cocaine craving. eLife 8: e44427.
- Martin-Fardon, R. and Weiss, F. (2017). Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol 22(4): 923-932.
- McFarland, K. and Ettenberg, A. (1997). Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl) 131(1): 86-92.
- Mihindou, C., Guillem, K., Navailles, S., Vouillac, C. and Ahmed, S. H. (2013). Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol Psychiatry 73(3): 271-279.
- Neisewander, J. L., Baker, D. A., Fuchs, R. A., Tran-Nguyen, L. T., Palmer, A. and Marshall, J. F. (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20(2): 798-805.
- Parvaz, M. A., Moeller, S. J. and Goldstein, R. Z. (2016). Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73(11): 1127-1134.
- Shalev, U., Morales, M., Hope, B., Yap, J. and Shaham, Y. (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 156(1): 98-107.
- Shepard, J. D., Bossert, J. M., Liu, S. Y. and Shaham, Y. (2004). The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55(11): 1082-1089.
- Suto, N., Laque, A., De Ness, G. L., Wagner, G. E., Watry, D., Kerr, T., Koya, E., Mayford, M. R., Hope, B. T. and Weiss, F. (2016). Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. eLife 5: e21920.
- Wang, G., Shi, J., Chen, N., Xu, L., Li, J., Li, P., Sun, Y. and Lu, L. (2013). Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One 8(7): e68791.
- Weiss, F. (2010). Advances in animal models of relapse for addiction research. Advances in the Neuroscience of Addiction. C. M. Kuhn and G. F. Koob. Boca Raton (FL), CRC Press/Taylor & Francis.
- Yun, I. A. and Fields, H. L. (2003). Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience 121(3): 747-757.
Article Information
Copyright
Lennon et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Lennon, V. A., Brenner, M. B., Weber, S. J., Komer, L. E. and Madangopal, R. (2019). Trial-based Discrimination Procedure for Studying Drug Relapse in Rats. Bio-protocol 9(23): e3445. DOI: 10.21769/BioProtoc.3445.
- Madangopal, R., Tunstall, B. J., Komer, L. E., Weber, S. J., Hoots, J. K., Lennon, V. A., Bossert, J. M., Epstein, D. H., Shaham, Y. and Hope, B. T. (2019). Discriminative stimuli are sufficient for incubation of cocaine craving. eLife 8: e44427.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








