- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Model of Chemotherapy-associated Mucositis and Oral Opportunistic Infections
Published: Vol 9, Iss 21, Nov 5, 2019 DOI: 10.21769/BioProtoc.3411 Views: 5322
Reviewed by: Kristin L. ShinglerEmmanuel Orta-ZavalzaLucy Xie

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Colibactin-associated Genotoxicity in HeLa Cells by In Cell Western (ICW) Using γ-H2AX as a Marker
Sophie Tronnet and Eric Oswald
Mar 20, 2018 8072 Views

Adhesion of Enteroaggregative E. coli Strains to HEK293 Cells
Jorge Luis Ayala-Lujan and Fernando Ruiz-Perez
Apr 20, 2018 6850 Views

Intracellular Invasion and Killing Assay to Investigate the Effects of Binge Alcohol Toxicity in Murine Alveolar Macrophages
Victor Jimenez Jr and Fernando P Monroy
Jan 20, 2019 6733 Views
Abstract
Oral mucositis is a common complication of cancer chemotherapy treatment. Because of the lack of relevant oral mucositis experimental models, it is not clear how chemotherapeutic agents injure the oral mucosa and if commensal microorganisms accelerate tissue damage. We developed an organotypic oral mucosa model that mimics cellular responses commonly associated with cytotoxic chemotherapy. The organotypic model consists of multilayer oral epithelial cells growing over a collagen type I matrix, with embedded fibroblasts. Treatment of organotypic constructs with the chemotherapeutic agent, 5-fluorouracil (5-FU), leads to major histopathologic changes resembling mucositis, such as DNA synthesis inhibition, increased apoptosis and cytoplasmic vacuolation. Candida albicans formed mucosal biofilms on these tissues and augmented the inflammatory responses to 5-FU. This model can be used in further mechanistic studies of oral mucositis and associated opportunistic infections.
Keywords: Oral mucositisBackground
Candida albicans is a fungal commensal of the upper and lower gastrointestinal tract implicated in opportunistic mucosal and disseminated infections in immunocompromised hosts (Westbrook et al., 2013). Clinical studies have shown that risk factors associated with candidemia go beyond the frequently described use of central venous catheters; they are also associated with the use of high dose chemotherapeutic agents. Under this scenario, the mucosal barrier integrity is decreased, resulting in life threatening systemic dissemination (Nucci et al., 1998; Lalla et al., 2014). We have recently started to explore the role of cytotoxic chemotherapy in Candida albicans mucosal infection. We showed that this fungus can amplify the proinflammatory epithelial response to the cytotoxic drug 5-FU in vitro, (Sobue et al., 2018) and it can promote a dysbiotic state in vivo, that causes increased oral mucosal barrier breach (Bertolini et al., 2019). 5-fluorouracil is a chemotherapeutic agent used for treatment of a variety of metastatic cancers including breast, gastrointestinal tract and head & neck. In this patient population oral thrush and/or candidemia with disseminated multi-organ infection are prevalent and most frequently attributed to C. albicans (Teoh and Pavelka, 2016).
Thus far organotypic models of oral mucositis have relied on cost-prohibitive commercially available constructs (Sonis et al., 2017) or primary epithelial cultures which vary in life span and other phenotypic characteristics, and are associated with ethical considerations for harvest (Tobita et al., 2010). Our in-house organotypic construct is based on an oral epithelial cell line which readily forms a multilayer epithelial structure and responds to 5-FU in a manner similar to oral mucosa in vivo (Sobue et al., 2018; Bertolini et al., 2019). This model will facilitate further mechanistic studies on oral mucositis and the complex relationships with fungal and bacterial organisms that overgrow on the oral mucosa during chemotherapy-driven immunosuppression. This model could lead to novel future interventions designed to prevent the initiation of oral mucositis in chemotherapy treated patients and better understand the effect of resident microbiota in aggravating oral mucosal lesions.
Materials and Reagents
- Plates/Inserts/tubes/pipettes
- 6-well Falcon Deep Well Plate (Corning, catalog number: 355467)
- Transwell 3414 (24 mm diameter inserts, 3.0 μm pore size, Corning, catalog number: 3413)
- 50 ml polypropylene tube (Fisher Scientific, catalog number: 05-539-8)
- 25 ml polystyrene pipette (Fisher Scientific, catalog number: 13-678-11)
- 10 ml polystyrene pipette (Fisher Scientific, catalog number: 13-678-11E)
- 9 inch glass pasteur pipette (Fisher Scientific, catalog number: 13-678-20C)
- Tissue embedding cassettes (Fisher Scientific, catalog number: B1000729BL)
- Tweezers (Fisher Scientific, catalog number: 12-000-132)
- 75 cm2 Cell culture flask (Corning, catalog number: 430641U)
- 100 mm Petri Dish (Fisher Scientific, catalog number: FB0875713)
- Cells
Note: Use freshly thawed vial of cells from Liquid Nitrogen. Sub-culture when cells are 70-80% confluent. Sub-culture one week after starting culture.- Fibroblast: 3T3 cell line (ATCC, catalog number: CRL-1658)
- Epithelial cells: SCC15 cell line (ATCC, catalog number: CRL-1623)
- Common reagent
- 10% normal goat serum (Jackson ImmunoResearch, catalog number: 005-000-121)
- 100%, 95%, 70% and 50% Ethanol (EtOH) (Pharmo, catalog number: 111000200)
- 30% H2O2 (Sigma-Aldrich, catalog number: H1009-100ML)
- Phosphate buffered saline (PBS, Corning, catalog number: 21-031-CV)
- Triton X-100 (Sigma-Aldrich, catalog number: T8787)
- Xylene (Fisher Scientific, catalog number: X3P-1GAL)
- 1 N Sodium Hydroxide (NaOH, Sigma-Aldrich, catalog number: S2770)
- Dimethyl sulfoxide (DMSO, Sigma-Aldrich, catalog number: 276855)
- 0.05 % Trypsin-EDTA (Gibco, catalog number: 25300-054)
- 20x Citrate buffer (Thermo Fisher Scientific, catalog number: 005000, see Recipes)
- 16% Paraformaldehyde (Electron Microscopy Science, catalog number: 15710, see Recipes)
- Fibroblast Culture Media: DMEM-10% FBS-1% Pen/Strep
- Dulbecco's Modified Eagle Medium (DMEM) liquid (high glucose) without Pyruvate (Gibco, catalog number: 11965-084)
- Fetal bovine serum (FBS, Gibco, catalog number: 16000044)
- Penicillin/streptomycin (Pen/Strep, Gibco, catalog number: 15140-122)
- Epithelial Cell Culture Media
KSFM with Bovine Pituitary Extract (BPE) (1 tube/each bottle), 0.2 ng/ml human epithelial growth factor (hEGF) (Invitrogen, catalog number: 17005-042), 0.4 mM CaCl2 (Sigma-Aldrich, catalog number: C-3306) and Pen/Strep (1:100). - Matrix and organotypic cell culture media components
- Type I Collagen (Organogenesis, catalog number: 200/50)
- Matrigel Basement Membrane Matrix (BD Biosciences, catalog number: 354234)
- Newborn Calf Serum (HyClone, catalog number: SH 3011802)
- DMEM (-) (Gibco, catalog number: 11965-084)
- Ham's F12 (Gibco, catalog number: 11765-054)
- 10x EMEM (BioWhittaker, catalog number: 12-684F)
- 7.5% Sodium-Bicarbonate (Na-Bicarbonate, BioWhittaker, catalog number: 17-613E)
- FBS (Gibco, catalog number: 16000044)
- L-Glutamine (Cellgro, catalog number: 25-005CI)
- Gentamicin, 50 mg/ml (Cellgro, catalog number: MT30-0005-CR)
- ITES - Insulin, Transferrin, Ethanolamine and Selenium (BioWhittaker, catalog number: 17839Z)
- Hydrocortisone (Sigma-Aldrich, catalog number: H0888, see Recipes)
- O-phosphorylethanolamine (Sigma-Aldrich, catalog number: P0503, see Recipes)
- Adenine (Sigma-Aldrich, catalog number: A9795, see Recipes)
- Progesterone (Sigma-Aldrich, catalog number: P8783, see Recipes)
- Triiodothyronine (Sigma-Aldrich, catalog number: T5516, see Recipes)
- 5-Fluorouracil, 5-FU (Sigma-Aldrich, catalog number: F6627, see Recipes)
- 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside, BrdU (Sigma-Aldrich, catalog number: B4252, see Recipes)
- Microorganisms used and microbiological media
- Candida albicans strain SN425 (reference strain, derived from SC5314, provided by Dr. Clarissa J. Nobile, University of California, Merced, CA)
- YPD medium and Agar (see Recipes)
- Yeast Extract (Sigma-Aldrich, catalog number: Y1625)
- Peptone (BD, catalog number: 211677)
- Dextrose (J. T. Baker, catalog number: 1616-05)
- Agar (BD, catalog number: 214530)
- Immunofluorescence stain
- Coplin Staining Jar (Thermo Scientific, catalog number: 19-4)
- Rat anti BrdU monoclonal antibody (Abcam, catalog number: ab6326)
- Goat anti rat Alexa Fluor 555 conjugated secondary antibody (Thermo Fisher scientific, catalog number: A21434)
- Goat anti rabbit anti active Caspase-3 antibody (Abcam, catalog number: Ab32042)
- Alexa Flour 568 conjugated secondary antibody (Thermo Fisher scientific, catalog number: A11011)
- Rabbit FITC conjugated anti Candida albicans polyclonal antibody (Meridian Life Science, catalog number: B65411F)
- Hoechst 33258 (10 mg/ml, Thermo Fisher scientific, catalog number: H3569)
- Fluoro-Gel (Electron Microscopy Sciences, catalog number: 17895-11)
Equipment
- Fluorescence microscope (Zeiss Axio Imager M1)
- Objective lens (EC Plan-Neofluar 20x/0.5)
- Tissue culture incubator (Thermo Forma, catalog number: 3110)
- Biological safety cabinet (Thermo Forma, catalog number: 1284)
- Autoclave
- Orbital Shaker (New Brunswick Scientific, catalog number: C1A88IC-C1)
- Centrifuge machine (Thermo Scientific, catalog number: CL2)
- Hemocytometer (Hausser Scientific, Fisher Scientific, catalog number: 0267110)
Software
- AxioVision LE64 (Zeiss)
Procedure
- Organotypic constructs (OTC)
The organotypic oral mucosal model has been described previously (Dongari-Bagtzoglou and Kashleva, 2006a; Dongari-Bagtzoglou and Kashleva, 2006b; Sobue et al., 2016). The protocol was recently modified based on work by Nakagawa and colleagues (Nakagawa et al., 2015). The organotypic model consists of human immortalized oral keratinocytes (squamous cell carcinoma) SCC15 and type I collagen matrix-embedded mouse fibroblasts, 3T3. Mucosal constructs take approximately 2 weeks to form, resulting in a non-keratinizing, multilayer squamous epithelium which is then exposed to the chemotherapy agent 5-FU (Figure 1).- A week ahead: Start growing 3T3 cells
- Thaw a frozen vial in 9 ml of DMEM with 10% FBS 1% Pen/Strep in a 50 ml tube.
- Centrifuge the tube 3 min with 670 x g in a centrifuge machine.
- Aspirate supernatant from the tube.
- Add 10 ml of DMEM with 10% FBS 1% Pen/Strep into 50 ml tube and resuspend gently.
- Seed 10 ml cell suspension on 75 cm2 cell culture flask and incubate the flask in the incubator with 37 °C and 5% CO2.
- Change the culture medium every 2 to 3 days.
- Grow until they reach 80% confluent in DMEM with 10% FBS 1% Pen/Strep.
- Day 0: Thaw Matrigel overnight (one vial for 2.5 OTC plates) at 4 °C.
- Day 1: Matrix preparation
15-30 min prior to matrix preparation:- Place FBS, L-Glutamine, 10x EMEM and 7.5% Na-bicarbonate, Matrigel and type I collagen on ice.
- Place transwell inserts into each well in 6-well Falcon Deep Well Plate.
- Add 10x EMEM, FBS, L-Glutamine, Na-bicarbonate and type I collagen in this order in a 50 ml tube on ice (Table 1).
- Mix gently using a 25 ml pipette in a 50 ml tube on ice.
Note: When making acellular and cellular layers, mix components gently on ice to avoid bubbles. - Pour 1 ml per insert using a 10 ml pipette.
- Leave inside the tissue culture hood while preparing fibroblasts and cellular layer.
Table 1. Acellular layer media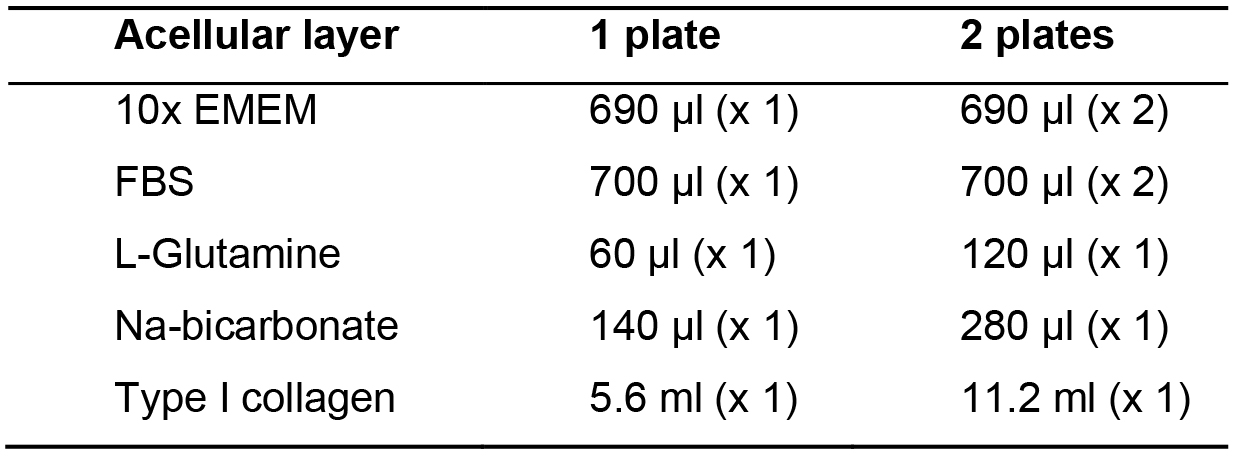
- Aspirate condition medium from cell culture flask.
- Wash cells with 10 ml PBS and aspirate PBS. Repeat one more time.
- Add 1 ml 0.05% Trypsin/EDTA to cell culture flask. Tap the flask gently and keep in the incubator for up to 2 min.
- Once cells detach from the bottom of flask, add 9 ml of DMEM with 10 % FBS 1% Pen/Strep and suspend cells with a 10 ml pipette.
- Collect cell suspension and place to a new 50 ml tube.
- Centrifuge the tube 3 min with 670 x g in a centrifuge machine.
- Aspirate supernatant and resuspend the cell pellet with 10 ml of DMEM with 10 % FBS 1% Pen/Strep.
- Count cell number with a hemocytometer and prepare cell suspension (6 x 105/ml, 2 ml for each plate).
- Add 10x EMEM, FBS, L-Glutamine, Na-bicarbonate, type I collagen, Matrigel and fibroblasts in this order into a new 50 ml tube on ice (Table 2).
- Mix gently using a 25 ml pipette in the 50 ml tube on ice.
- Pour 3 ml per insert using a 10 ml pipette.
- Incubate for 30-45 min in the tissue culture incubator (37 °C, 5% CO2).
- Add DMEM-10% FBS-1% Pen/Strep: 10 ml into the bottom of the wells, 2 ml into the insert.
Table 2. Cellular layer media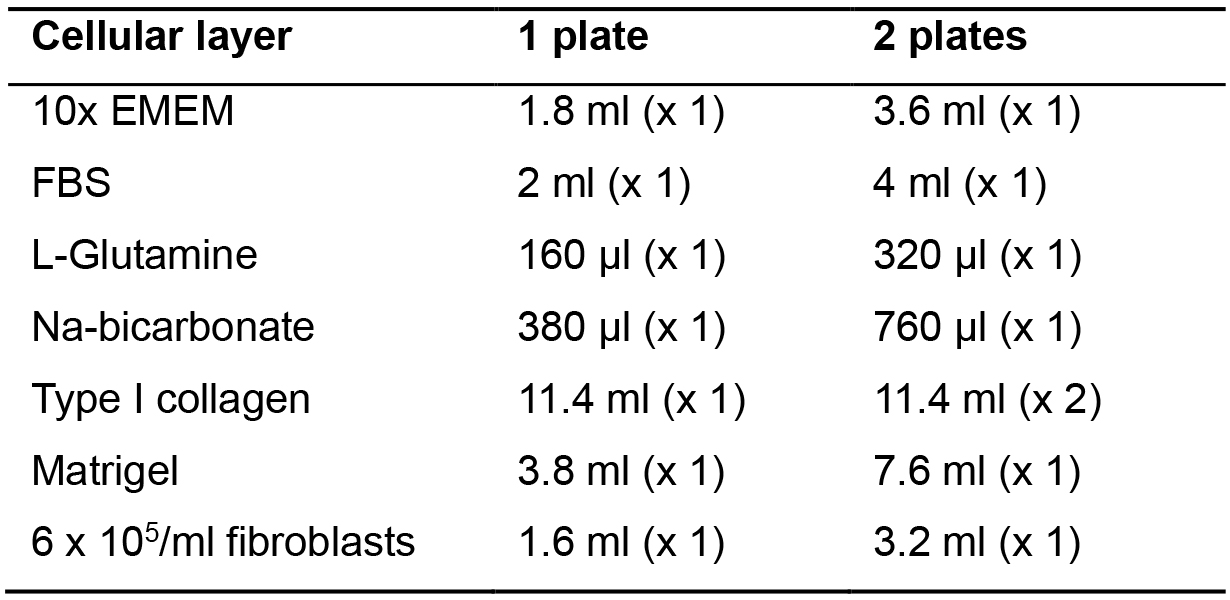
- Thaw a frozen vial in 9 ml of KSFM with BPE, 0.2 ng/ml hEGF, 0.4 mM and 1% Pen/Strep in a 50 ml tube.
- Centrifuge the tube for 3 min with 670 x g in a centrifuge machine at room temperature.
- Aspirate supernatant from the tube.
- Add 10 ml of KSFM with BPE, 0.2 ng/ml hEGF, 0.4 mM and 1% Pen/Strep into the 50 ml tube and resuspend gently.
- Seed 10 ml cell suspension on 75 cm2 cell culture flask and incubate the flask in the incubator with 37 °C and 5% CO2.
- Change the culture medium every 2 to 3 days.
- Grow until they reach 80% confluent in KSFM with BPE, 0.2 ng/ml hEGF, 0.4 mM and 1% Pen/Strep.
- Day 2: Dislodge matrix and add 2 ml of DMEM-10% FBS-1% Pen/Strep into the insert.
- Use a sterile glass Pasteur pipette to go around (2-3 times) the matrix along the inner wall of the insert. Feel the friction at the tip on the transwell membrane, but try not to pierce it.
Note: The matrix will contract over the next few days. Additional 2 ml needs to submerge the OTC. - No need to change medium. Wait at least 4 days before seeding epithelial cells.
- Use a sterile glass Pasteur pipette to go around (2-3 times) the matrix along the inner wall of the insert. Feel the friction at the tip on the transwell membrane, but try not to pierce it.
- Day 5 or later (up to Day 7): Seeding epithelial cells
- Make DMEM (-)/F12 (Table 3) to pre-saturate the OTC matrix.
- Remove old medium from the OTC plates/inserts.
Note: Gentle aspiration is recommended to reduce the risk of distracting OTC. - Add DMEM (-)/F12. Add 10 ml to the bottom well and 2 ml into the insert.
- Incubate for 1 h to equilibrate the matrix in the tissue culture incubator (37 °C, 5% CO2).
- Aspirate condition medium from the SCC15 cell culture flask.
- Wash cells with 10 ml PBS and aspirate PBS. Repeat one more time.
- Add 1 ml 0.05% Trypsin/EDTA to cell culture flask. Tap the flask gently and keep in the incubator (37 °C, 5% CO2) up to 2 min.
- Once cells detach from the bottom of flask, add 9 ml of DMEM with 10% FBS 1% Pen/Strep and suspend cells with a 10 ml pipette.
- Collect cell suspension and place to a new 50 ml tube. Count cell number with hemocytometer.
- Centrifuge the tube 3 min with 670 x g in a centrifuge machine at room temperature.
- Aspirate supernatant and resuspend cell pellet with KSFM with BPE, 0.2 ng/ml hEGF, 0.4 mM and 1% Pen/Strep. The volume of medium needs to be adjusted based on the counting cell number in Step A5i.
Note: Prepare 1 x 107/ml epithelial cell suspension (300 µl per plate). 5 x 105 epithelial cells are seeded per insert/well. One ~80% confluent 75 cm2 flask yields about 2-3 x 106 SCC15 cells. - Remove DMEM (-)/F12 from the OTC plates/inserts.
- Seed epithelial cells by adding 50 μl (5 x 105) of cell suspension per well onto the center of the surface of contracted matrix.
- Incubate for 2 h in the tissue culture incubator without medium.
- Make EP2 media. Mix all cell culture media components seen in Table 4 and store at 4 °C.
- Add EP2 media to the plate–10 ml into the bottom well and 2 ml into the insert. And incubate the plate in the tissue culture incubator (37 °C, 5% CO2).
Table 3. DMEM/F12 Media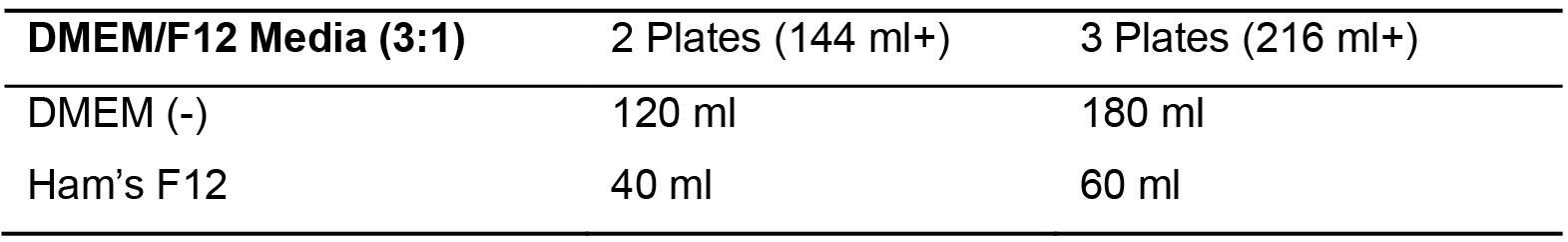
Table 4. EP2 Media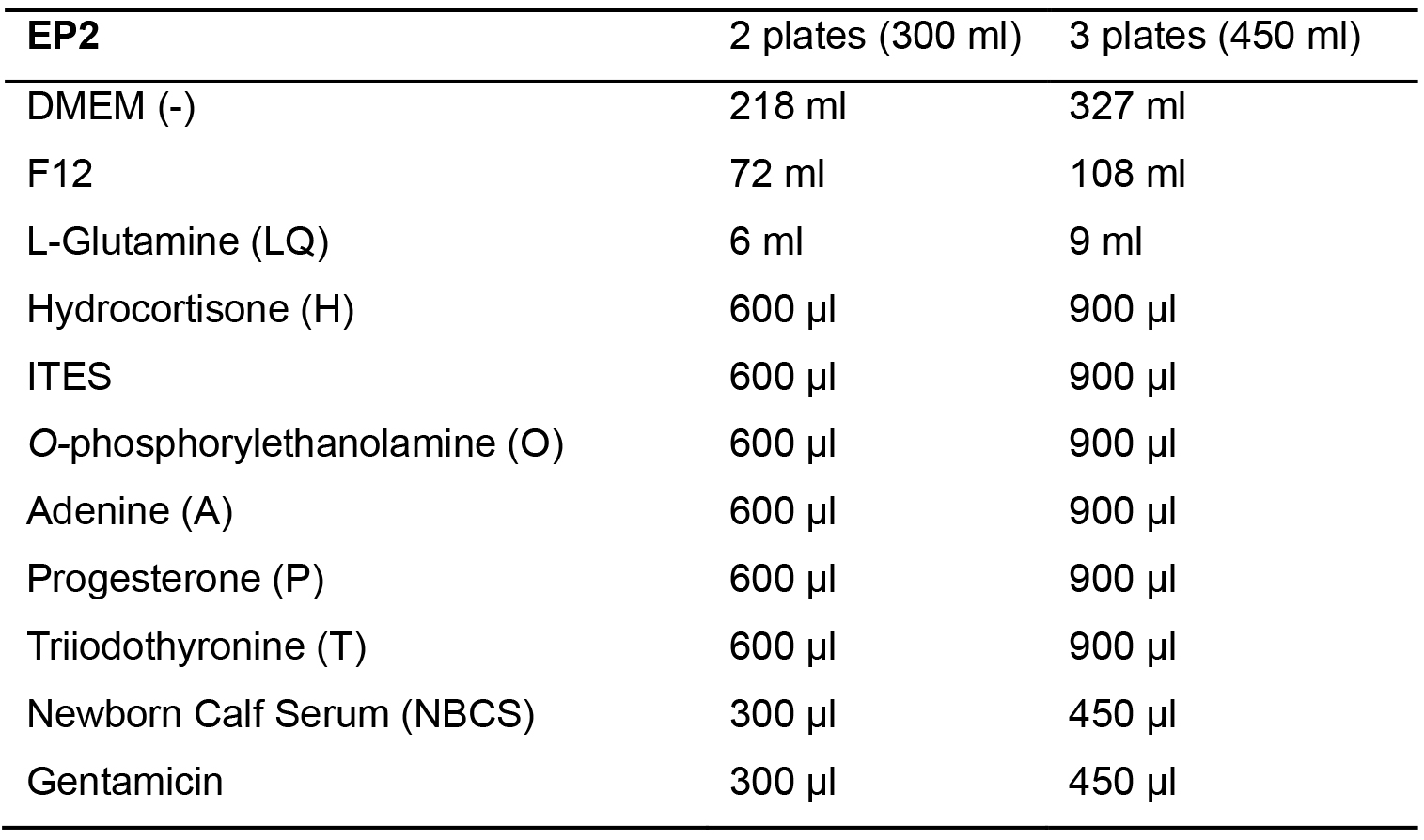
- Day 7 (or 2 days after seeding epithelial cells): Medium change with EP2 media.
- Remove old medium from the OTC plates/inserts.
- Add EP2 media: add 10 ml to the bottom well and 2 ml into the insert.
- Incubate the plate in the tissue culture incubator (37 °C, 5% CO2).
- Day 9 (or 4 days after seeding epithelial cells, Air Lifting): Replace EP2 media with EP3 media.
- Make EP3 media. Mix all cell culture media components seen in Table 5 and store at 4 °C.
- Remove old medium from both inserts and the bottom well.
- Add 7.5 ml of EP3 media into the bottom wells only.
- Incubate the plate in the tissue culture incubator (37 °C, 5% CO2).
Table 5. EP3 Media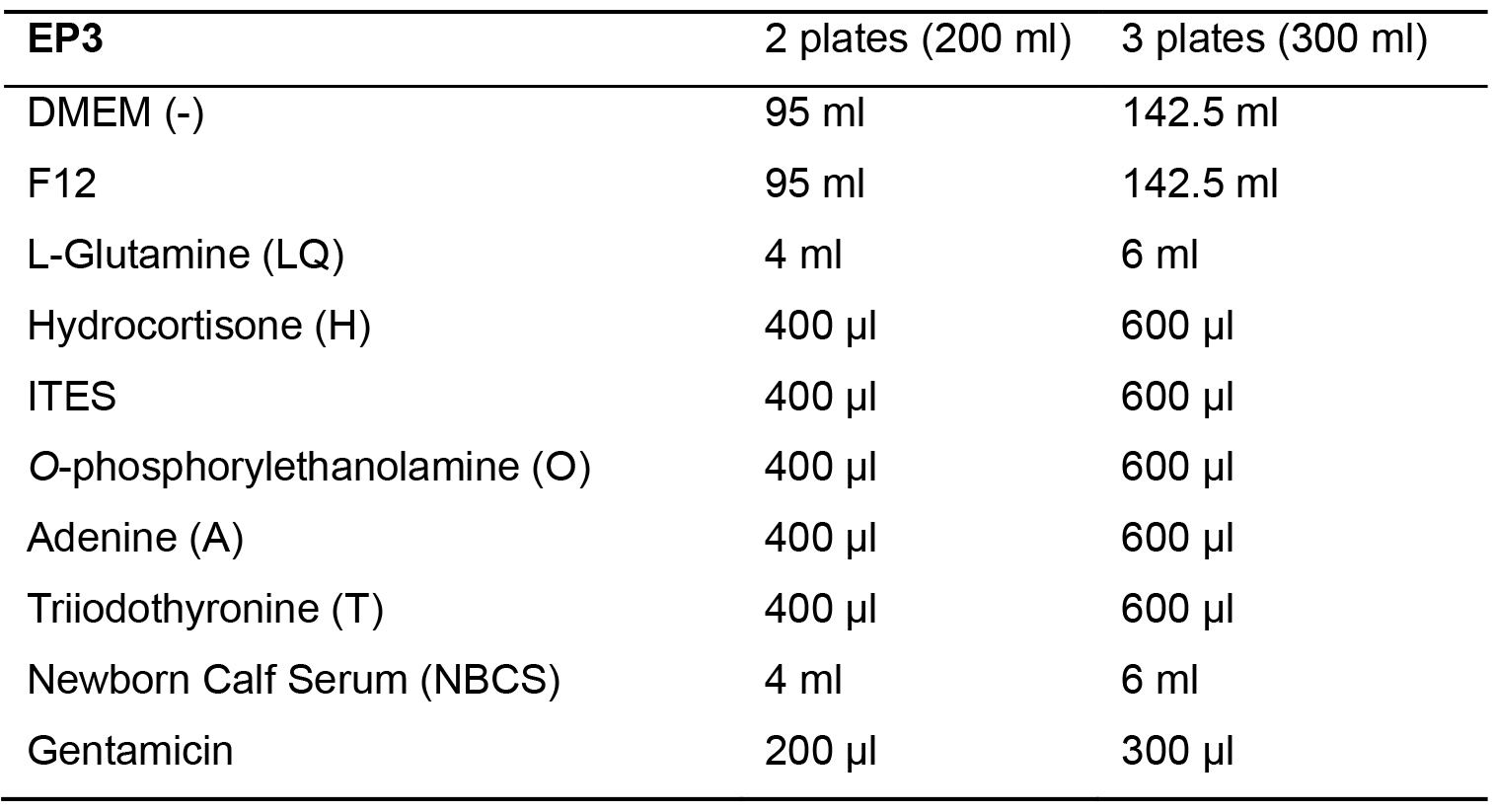
- Day 11 (or 6 days after seeding epithelial cells): Medium change with EP3 media, prepared on Day 9.
- Remove old medium from both inserts and the bottom well.
Note: After air lifting, further significant contraction will be seen. Contraction can be better assessed during medium change. - Add 7.5 ml of EP3 media into the bottom wells only.
- Incubate the plate in the tissue culture incubator (37 °C, 5% CO2).
- Remove old medium from both inserts and the bottom well.
- Day 13 (or 8 days after seeding epithelial cells): Medium change with EP3 media, prepared on Day 9.
- Remove old medium from both inserts and the bottom well.
- Add 7.5 ml of EP3 media into the bottom wells only.
- Incubate the plate in the tissue culture incubator (37 °C, 5% CO2).
- Day 14 (or 9 days after seeding epithelial cells): Three-dimensional mucosal organotypic culture is now ready to use for 5-FU treatment followed by microbial inoculation.
- A week ahead: Start growing 3T3 cells
- 5-FU treatment of organotypic construct and confirmation of the drug effect on cell proliferation
- Prepare EP3 media with 10 μM 5-FU (2 ml for each well).
- Transfer inserts to a new 6 well dish with sterile tweezers.
- Add 2 ml EP3 media with 5-FU to basolateral side only to expose the submucosal compartment to the drug.
- Add 1 ml of fresh cell culture media without 5-FU to the apical mucosal surface.
- Add 100x concentrated BrdU (final concentration: 10 μM) on both apical mucosal surface (100 μl) and basolateral (200 μl) sides.
Note: BrdU labeling is needed to confirm the anti-proliferative effect of 5-FU. Incorporation should be allowed for a minimum of 16 h since most of epithelial cells are slowly proliferating after the multilayer construct is formed. - Incubate mucosal tissues in a transwell plate for 16 h at 37 °C with 5% CO2.
- Candida albicans infection of 5-FU treated organotypic construct
- Candida albicans (C. albicans) was inoculated flasks from colonies growing on YPD Agar plates into 10 ml YPD broth and incubate aerobically with gentle agitation, at room temperature, on a shaker.
- Remove all cell culture media from both sides of transwells (apical and basolateral) after 16 h incubation with 5-FU.
- Add EP3 media to both sides of transwells and incubate for 1 h at 37 °C with 5% CO2. Repeat twice more in total 3 h to wash out 5-FU from mucosal tissues.
Note: Removing 5-FU prior to microbial inoculation is necessary because it is toxic to most organisms. - Count cell number of C. albicans with a hemocytometer and prepare cell suspension (106 fungi cells in 20 µl EP3 media for each well).
- Remove all cell culture media from both sides of the transwell plate.
- Add the inoculum of C. albicans on mucosal surface side and leave for 45 min at room temperature.
Note: Place the inoculum gently on the center of mucosal surface; to avoid the excessive spread of the liquid media to the sides use a small volume (up to 20 μl) and ensure microorganisms are inoculated on the surface. - Add 1.5 ml of EP3 media to basolateral side only and incubate for 2 h at 37 °C with 5% CO2.
- Add 0.5 ml of EP3 media to mucosal surface side and incubate the plate for 16 h at 37 °C with 5% CO2.
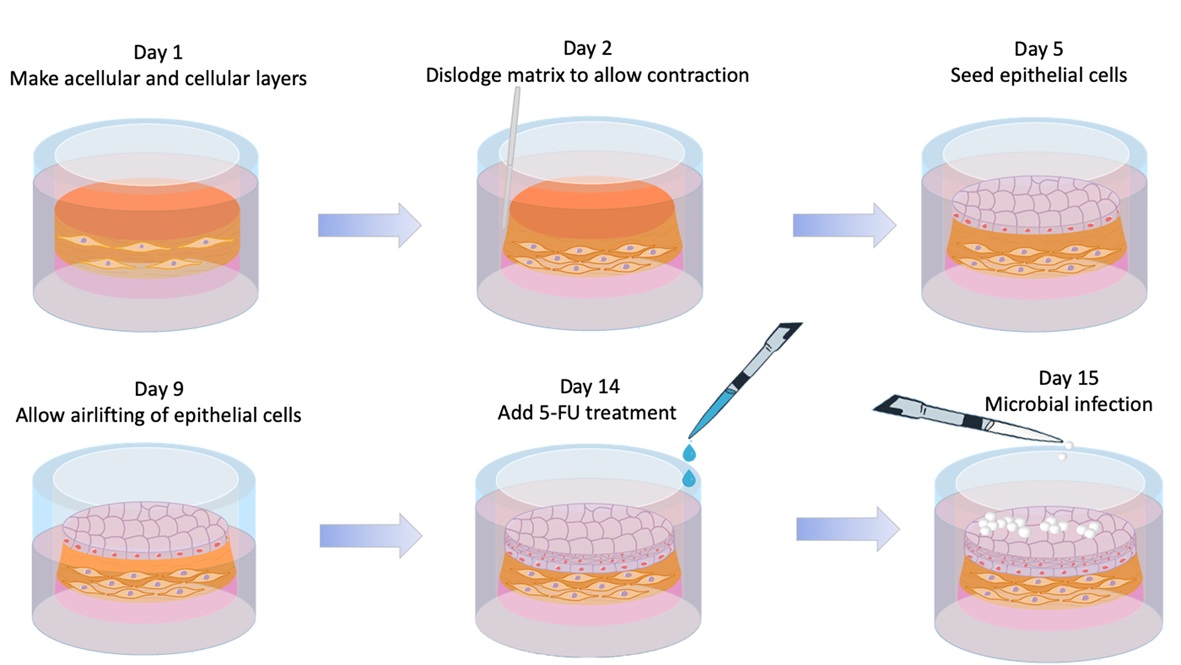
Figure 1. Organotypic Oral Mucosa model
- Preparation for analysis
- Submerge mucosal tissues in a new 6 well cell culture plate with 4% paraformaldehyde for fixation. Keep culture plates for 2 h at 4 °C.
Note: Do not over-fix. It may mask certain antigens. Two hours are sufficient for fixation with 4% paraformaldehyde. - Wash mucosal tissues with 4 ml of PBS twice for 3 min each at room temperature.
- Remove mucosal tissues from cell culture inserts and place the tissue into tissue embedding and processing cassettes.
- Process ordinal dehydration and paraffin embedding steps and cut 5 μm thick section for histological and Immunofluorescence analysis.
- Submerge mucosal tissues in a new 6 well cell culture plate with 4% paraformaldehyde for fixation. Keep culture plates for 2 h at 4 °C.
- Immunofluorescence analysis
- Deparaffinize by immersion in xylene, rinse in xylene and rehydrate in a series of ethanol washes.
- Antigen retrieval with 70 ml of Citrate buffer, pH 6.0 in Coplin jar for 10 min at 94 °C.
Note: To maximize antigen-antibody binding, antigen retrieval step is necessary for unmasking epitope. - Leave the slides at room temperature for 20 min.
- Wash with 70 ml of PBS for 3 min and repeat twice more, total 3 times in Coplin jar at room temperature.
- Immerse the slides in 70 ml of 0.05% H2O2 in PBS for 30 min in Coplin jar at room temperature.
- Wash with 70 ml of PBS for 3 min and repeat twice more, total 3 times in Coplin jar at room temperature.
- Incubate the slides in 300 µl of 10% normal goat serum, 0.2% triton X-100 in PBS (blocking buffer) for 30 min at room temperature.
- Incubate the slides with 300 µl of anti BrdU antibody (1:40 in blocking buffer) or anti active Caspase-3 antibody (1:100 in blocking buffer) overnight at 4 °C. To stain C. albicans, incubate with 300 µl of anti C. albicans antibody (1:20 in PBS) for 2 h in the dark at room temperature.
- Wash with 70 ml of PBS for 3 min and repeat twice more, total 3 times in Coplin jar at room temperature.
- For BrdU and active Caspase-3 stains, incubate with 300 µl of secondary antibody (A21434 for BrdU and A11011, 1:100 in PBS) for 1 h in the dark at room temperature.
- Wash with 70 ml of PBS for 3 min and repeat twice more, total 3 times in Coplin jar at room temperature.
- Counter staining with 300 µl of Hoescht 33258 (1:5000 in PBS) for 30 min in the dark at room temperature.
- Rinse with 70 ml of PBS and distilled water 3 min each and add a few drops of mounting solution, Fluoro-Gel, with coverslip.
- Sections can be observed under fluorescence microscope with 20x objective lens and further analysis can be conducted with AxioVision software.
Data analysis
- Histological response to 5-FU treatment can also be observed in hematoxylin and eosin (H&E) stained organotypic constructs under microscope. Although no significant structural change of multiple epithelium layers is noted, there is extensive widening of epithelial intercellular spaces and cytoplasmic vacuolation in 5-FU treated tissues compared to control (Figure 2A), consistent with histopathologic characteristics of oral mucositis in humans (von Bültzingslöwen et al., 2001).
- To reveal the inhibitory effect of 5-FU on cell proliferation, organotypic constructs are labeled with BrdU during 5-FU treatment. In Figure 2B immunofluorescence stain of BrdU incorporation revealed that the untreated tissues contained multiple BrdU-positive cells (cells in S phase) in all epithelial layers, consistent with the squamous carcinoma phenotype of SCC15 cells. The BrdU positive cells were almost completely absent with 5-FU treatment, indicating lack of DNA synthesis (Figure 2B).
- We also examined whether the treatment of 5-FU affected cell apoptosis in mucosal constructs. Immunofluorescence stain of active Caspase-3 revealed an increase in the number of apoptotic cells in the 5-FU-treated tissues, whereas minimal epithelial cell apoptosis was observed in untreated tissues (Figure 2C). Collectively these results showed that treatment of organotypic constructs for 16 h with 5-FU reproduced the major histopathologic characteristics of oral mucositis.
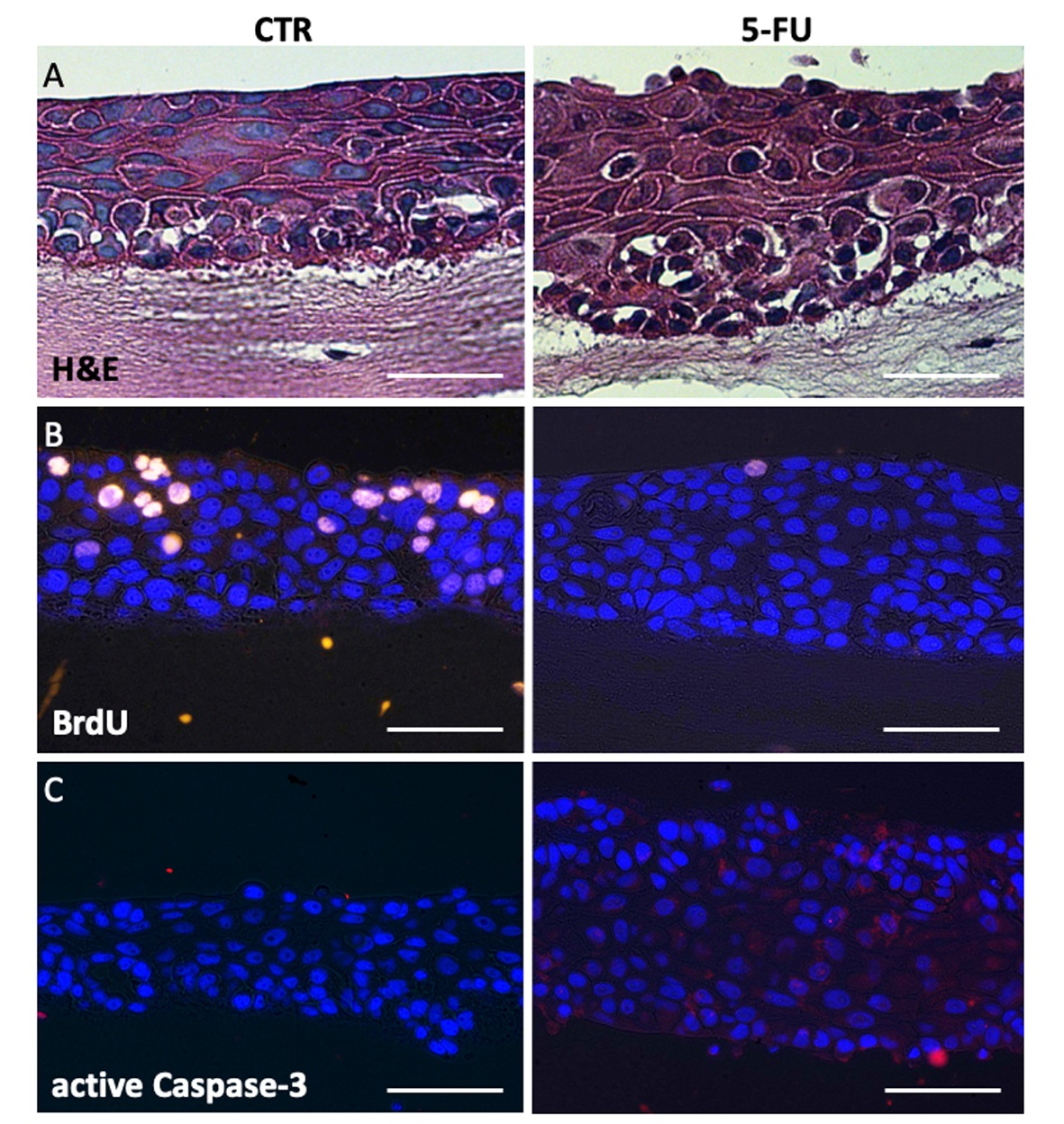
Figure 2. 5-FU treatment on organotypic oral mucosa model. A. H&E stain revealed 5-FU treatment resulted in a widening of epithelial intercellular spaces and cytoplasmic vacuolation. B. Immunofluorescence staining for BrdU incorporation revealed 5-FU treatment decreased DNA synthesis. C. Immunofluorescence staining for active Caspase 3 revealed 5-FU treatment increased apoptosis (Red spots, right panel). Bars = 50 µm. - We examined whether 5-FU-treated mucosal constructs promote fungal growth. Mucosal constructs were first exposed 5-FU for 16 h followed by infection with C.albicans for 16 h. H&E stain revealed C.albicans invasion on mucosal constructs regardless of 5-FU treatment (Figure 3A). 5-FU treatment increased C.albicans invasion in the submucosal area (Figures 3A and 3B), consistent with the increase in intercellular spaces (Figure 2A) and E-cadherin dissolution observed with 5-FU treatment (Sobue et al., 2018).
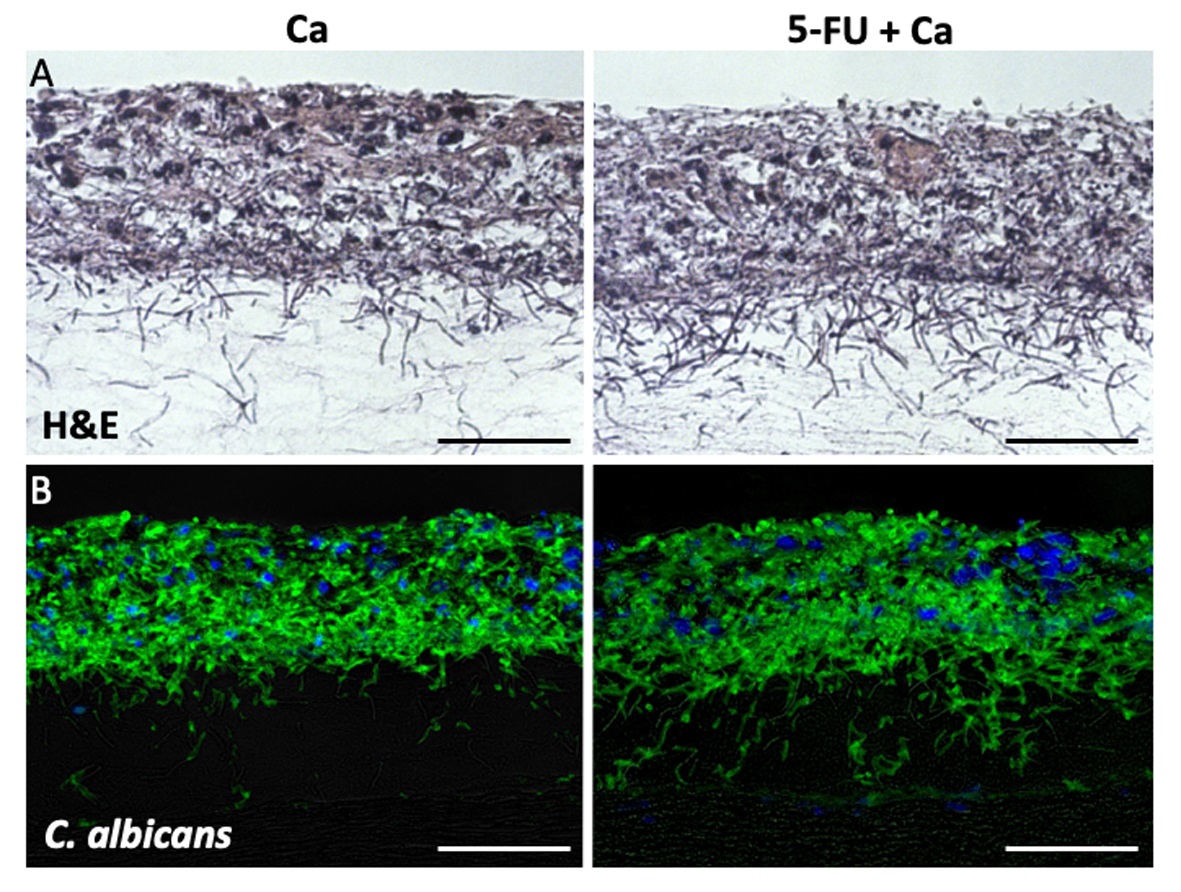
Figure 3. Candida albicans infection on 5-FU treated organotypic oral mucosa model. Candida albicans biofilms growing on untreated (Ca) or 5-FU treated (5-FU + Ca) mucosal constructs for 16 h. A: H&E stain. B: Immunofluorescence staining for Candida albicans. Bars = 50 µm.
Recipes
- Hydrocortisone (MW = 362.46 g/mol)
- Dissolve 0.0269 g hydrocortisone in 2.5 ml EtOH
- Add into 97.5 ml DMEM (0.74 mM)
- Filter-sterilize, dispense into aliquots (2 ml) and store at -20 °C
- O-phosphorylethanolamine
- Dissolve 0.705 g O-phosphorylethanolamine in 100 ml DMEM (50 mM)
- Filter-sterilize, dispense into aliquots (2 ml) and store at -20 °C
- Adenine
- Dissolve 1.55 g adenine in 100 ml warm (37 °C) ddH2O (0.09 M)
- Filter-sterilize, dispense into aliquots (2 ml) and store at -20 °C
- Progesterone
- Dissolve 1 mg progesterone in 1 ml EtOH
- Add 14.7 ml ddH2O
- Dilute 1ml of that in 100 ml DMEM (2.0 μM)
- Filter-sterilize, dispense into aliquots (2 ml) and store at -20 °C
- Triiodothyronine
- Dissolve 1 mg triiodothyronine in 1 ml 1 N NaOH
- Add 19 ml of DMEM (-)
- Dilute 4 μl of that in 31 ml plain DMEM (1 nM)
- Filter-sterilize, dispense into aliquots (2 ml) and store at -20 °C
- YPD medium
- Dissolve 5 g of yeast extract, 10 g of peptone in 450 ml of distilled water
- Autoclave the mixture, YP medium for sterilization (121 °C, 15 lb/in² for 20 min)
- Mix 1 part of 20% Dextrose with 9 part of YP medium to make YPD medium
- YPD Agar
- Dissolve 10 g of yeast extract, 20 g of peptone, 20 g of agar in 900 ml of distilled water
- Autoclave the mixture for sterilization (121 °C, 15 lb/in² for 20 min)
- Leave YPD Agar to cool down to 55 degree
- Add 100 ml of 20% Dextrose to the mixture to make YPD Agar
- Poor 10 ml each to 100 mm petri dish
- 5-FU (10 μM)
- Stock solution (50 mg/ml): Resolve 50 mg of 5-FU powder in 1 ml DMSO. Keep it at 4 °C for 3 months
- Working solution (10 μM): Make fresh immediately before use. Dilute 10 μl of stock solution with 3830 μl of cell culture media to make 1 mM 5-FU. Dilute 200 μl of 1 mM 5-FU with 20 ml of cell culture media to make 10 μM 5-FU supplemented cell culture media
- BrdU (1 mM)
- Stock solution (10 mg/ml): Resolve 10 mg of BrdU powder in 1 ml distilled water. Keep it at -20 °C
- Working solution (1 mM): Make fresh immediately before use. Dilute 3.1 μl of stock solution with 100 μl of EP3 media
- Citrate buffer
- Dilute 5 ml of 20x concentrated Citrate buffer with 95 ml of distilled water
- Preheat the buffer to 95 °C
- 4 % PFA
- Dilute 10 ml of 16% PFA with 30 ml of PBS
- Use 4 % PFA in one week and keep it at 4 °C
Acknowledgments
This protocol was developed based on previously published studies by the authors (Dongari-Bagtzoglou and Kashleva, 2006a; Dongari-Bagtzoglou and Kashleva, 2006b; Sobue et al., 2018). Funding was supplied by Public Health Service grants DE013986 and DE023632 from the National Institute of Dental and Craniofacial Research, NIH.
Competing interests
No competing interests.
References
- Bertolini, M., Ranjan, A., Thompson, A., Diaz, P. I., Sobue, T., Maas, K. and Dongari-Bagtzoglou, A. (2019). Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog 15(4): e1007717.
- Dongari-Bagtzoglou, A. and Kashleva, H. (2006a). Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat Protoc 1(4): 2012-2018.
- Dongari-Bagtzoglou, A. and Kashleva, H. (2006b). Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog 40(6): 271-278.
- Lalla, R. V., Bowen, J., Barasch, A., Elting, L., Epstein, J., Keefe, D. M., McGuire, D. B., Migliorati, C., Nicolatou-Galitis, O., Peterson, D. E., Raber-Durlacher, J. E., Sonis, S. T., Elad, S., Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in, C. and International Society of Oral, O. (2014). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10): 1453-1461.
- Nakagawa, H., Whelan, K., Lynch, J. P. (2015). Mechanisms of Barrett's oesophagus: intestinal differentiation, stem cells, and tissue models. Best Pract Res Clin Gastroenterol 29(1): 3-16.
- Nucci, M., Silveira, M. I., Spector, N., Silveira, F., Velasco, E., Akiti, T., Barreiros, G., Derossi, A., Colombo, A. L. and Pulcheri, W. (1998). Risk factors for death among cancer patients with fungemia. Clin Infect Dis 27(1): 107-111.
- Sobue, T., Bertolini, M., Thompson, A., Peterson, D. E., Diaz, P. I. and Dongari-Bagtzoglou, A. (2018). Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol Oral Microbiol 33(3): 212-223.
- Sobue, T., Diaz, P., Xu, H., Bertolini, M. and Dongari-Bagtzoglou, A. (2016). Experimental Models of C. albicans-Streptococcal Co-infection. Methods Mol Biol 1356: 137-152.
- Sonis, S., Andreotta, P. W. and Lyng, G. (2017). On the pathogenesis of mTOR inhibitor-associated stomatitis (mIAS)-studies using an organotypic model of the oral mucosa. Oral Dis 23(3): 347-352.
- Teoh, F. and Pavelka, N. (2016). How chemotherapy increases the risk of systemic candidiasis in cancer patients: current paradigm and future directions. Pathogens 5(1).
- Tobita, T., Izumi, K. and Feinberg, S. E. (2010). Development of an in vitro model for radiation-induced effects on oral keratinocytes. Int J Oral Maxillofac Surg 39(4): 364-370.
- von Bültzingslöwen, I., Jontell, M., Hurst, P., Nannmark, U. and Kardos, T. (2001). 5-Fluorouracil induces autophagic degeneration in rat oral keratinocytes. Oral Oncol 37(6): 537-544.
- Westbrook, S. D., Kirkpatrick, W. R., Wiederhold, N. P., Freytes, C. O., Toro, J. J., Patterson, T. F. and Redding, S. W. (2013). Microbiology and epidemiology of oral yeast colonization in hemopoietic progenitor cell transplant recipients. Oral Surg Oral Med Oral Pathol Oral Radiol 115(3): 354-358.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sobue, T., Bertolini, M., Thompson, A. and Dongari-Bagtzoglou, A. (2019). Model of Chemotherapy-associated Mucositis and Oral Opportunistic Infections. Bio-protocol 9(21): e3411. DOI: 10.21769/BioProtoc.3411.
Category
Microbiology > Microbe-host interactions > In vitro model > Cell line
Microbiology > Microbial cell biology > Cell viability
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








