- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Screening of Antileishmanial Activity of Natural Product Compounds: Determination of IC50, CC50 and SI Values
(*contributed equally to this work) Published: Vol 9, Iss 21, Nov 5, 2019 DOI: 10.21769/BioProtoc.3410 Views: 5822
Reviewed by: Alexandros AlexandratosSrujana Samhita YadavalliAdam Idoine

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sex-specific Separation of Plasmodium falciparum Gametocyte Populations
Melanie C. Ridgway [...] Alexander G. Maier
Jun 5, 2021 3889 Views

Protocol for Isolation of Cardiomyocyte from Adult Mouse and Rat
Huiliang Zhang and Peter S. Rabinovitch
May 20, 2022 5597 Views

High Content In Vitro Survival Assay of Cortical Neurons
Paolo V. Fioretti [...] Manuela Basso
Feb 5, 2026 65 Views
Abstract
Neglected tropical diseases gain the scientific interest of numerous research programs in an attempt to achieve their effective control or elimination. In this attempt, more cutting-edge public health policies and research are needed for the discovery of new, safer and effective drugs originated from natural products. Here, we describe protocols for the in vitro screening of a natural product-derived compound required for the determination of its antileishmanial potency. For this purpose, the Total Phenolic Fraction (TPF) derived from extra virgin olive oil is evaluated through the in vitro cell culture method against extracellular promastigote and intracellular amastigote Leishmania spp. forms. The aim of this article is to describe a step-by-step procedure that can be easily applied to accurately estimate the 50% inhibitory concentration (IC50), the 50% cytotoxic concentration (CC50) and the selectivity index (SI) via the resazurin reduction assay. These protocols are based on the ability of resazurin (oxidized blue form) to be irreversibly reduced by enzymes in viable cells and generate a red fluorescent resorufin product and can be easily expanded to the investigation of the antimicrobial activity in other microorganisms.
Keywords: Cytotoxic concentrationBackground
Neglected tropical diseases (NTDs) are a group of about 20 diseases, typically endemic in 149 tropical and subtropical countries that represent a significant public health impact worldwide by affecting more than 1 billion people and being responsible for over 500,000 deaths per year (Cheuka et al., 2016; Mitra and Mawson, 2017). Until nowadays, drug discovery against NTDs exhibited limited success in translating potential drug candidates into effective therapies. Indeed, the current clinically used drugs against NTDs are characterized by various disadvantages including severe adverse effects, complicated administration procedures, lengthy treatment duration and emergence of resistance (Cheuka et al., 2016). Natural products represent a valuable alternative source of novel and structurally diverse compounds that deserves attention in drug discovery against NTDs. Numerous metabolites isolated from plant, microbial and marine sources such as alkaloids, phenolic compounds, quinones, terpenes, saponins, lignans, toxoids and anthranoids have been investigated (Cheuka et al., 2016) and it has been estimated that approximately at least 25% of the presently used drugs in modern medicine are derived from plants (Lahlou, 2007).
Among NTDs, leishmaniasis is a parasitic disease caused by protozoa of the genus Leishmania spp., causing a range of manifestations and exhibiting progressively increasing frequency in non-endemic western developed countries (Burza et al., 2018). Numerous of agents of natural origin, plant derived bioactive compounds and their secondary metabolites have been tested as antileishmanial drugs, such as flavonoids, sterols, chalcones, coumarins, tannins and aurones, iridoids, quinones and quinolone alkaloids (Singh et al., 2014). The assessment of the bioactive potential of biological extracts or molecules involves numerous assays performed at animal, cell-based or molecular levels (Lage et al., 2018) while the first steps of the biological screening and evaluation of plant extracts usually involve the screening of compounds in cell-based in vitro assays (Griffiths and Sundaram, 2011).
The half maximal inhibitory concentration (IC50) is a representative quantitative measure of the potency of a substance to inhibit in vitro a specific biological process by 50%, (Lahlou, 2007; Aykul and Martinez-Hackert, 2016) and is primarily defined through in vitro test models. Since Leishmania spp. have a digenetic life cycle, the antileishmanial effect of drugs is tested on both the promastigote (infective form in the intermediate host) and the amastigote form (intracellular form in the tissue of the mammalian host) (Bates and Rogers, 2004). In this regard, IC50 is determined for both promastigotes and intracellular amastigotes and is defined as the concentration of a compound that causes inhibition of growth in the 50% of Leishmania promastigotes and of intracellular amastigotes, respectively. To this end, the in vitro screening of the antileishmanial activity of Total Phenolic Fraction (TPF) is performed on L. infantum and L. major promastigotes and on Leishmania-infected J774A.1 macrophages in which amastigotes survive and multiply (Bilbao-Ramos et al., 2012). Moreover, the cytotoxicity of TPF in vitro against J774A.1 macrophages is also tested through the determination of the CC50 (cytotoxicity concentration 50%) that is the compound’s concentration that causes cytotoxicity in the 50% of the cells (Kyriazis et al., 2013). In addition, we employ the selectivity index (SI) that is defined as the ratio of the 50% cytotoxic concentration of J774A.1 macrophages to the 50% inhibitory concentration of Leishmania spp. amastigotes (CC50/IC50). A compound with SI value greater than 1 is considered to be more selective against Leishmania spp. parasites and is regarded as a promising potential agent in the treatment of leishmaniasis (Makwali et al., 2015). All the above indices are crucial in order to conclude whether or not a tested compound is appropriate as an antileishmanial agent.
Materials and Reagents
- Cell culture flasks with filter cap, 25 cm2 (Thermo Fisher Scientific, catalog number: 156367)
- Cell culture flasks, plug seal cap, 25 cm2 (Greiner Bio-One, catalog number: 690160)
- 96-well flat bottom tissue culture plates (Sarstedt, catalog number: 83.3924.005)
- Cell scrapers (Sarstedt, catalog number: 83.1830)
- Cover glasses square (VWR, catalog number: 6311570)
- Parafilm (Bemis, catalog number: HS234526B)
- Pipette tips: 0.5-10 µl, 10-200 µl, 200-1,000 µl (Greiner Bio-One, catalog numbers: 771291, 739290, 740290)
- Multichannel pipette reservoir (Brand, catalog number: BR703411)
- Pleated filter paper (Sigma-Aldrich, catalog number: WHA1201150)
- Microcentrifuge tubes, 1.5 ml (Greiner Bio-One, catalog number: 616201)
- Serological pipettes 2 ml, 5 ml, 10 ml (Sarstedt, catalog numbers: 86.1252.001, 86.1253.001, 86.1254.001)
- Syringes 5 ml (BD Emerald, catalog number: 307732)
- Sterile syringe filter 0.22 µm (Millipore, catalog number: SLGVV255F)
- Leishmania infantum promastigotes (zymodeme GH8, strain MHOM/GR/2001/GH8)
- Leishmania major promastigotes (zymodeme LV39, strain MRHO/SU/59/P)
- Immortalized macrophage cell line J774A.1 (ATCC; Rockville, USA/ ATCC No: TIB-67)
- Total Phenolic Fraction (TPF) (derived from extra virgin olive oil from agricultural cooperative in Zaros region, Crete, Greece)
- RPMI 1640 w/o L-glutamine (Biowest, catalog number: L0501)
- Schneider’s insect medium (Biosera, catalog number: LM-F0702)
- L-glutamine (Biosera, catalog number: LM-R1641)
- HEPES buffer 1 M (Biowest, catalog number: L0180)
- Penicillin-Streptomycin solution 10,000 U/ml (Biowest, catalog number: L0022)
- Dimethyl sulfoxide (DMSO) cell culture grade (PanReac Applichem, catalog number: A3672,0050)
- Ethanol absolute (Sigma-Aldrich, catalog number: 32205-M)
- Formalin (Sigma-Aldrich, catalog number: R04586-82)
- Milteforan® 20 mg/ml (Hexadecylphosphocholine, [HePC], Virbac S.A.)
- Resazurin sodium salt (7-Hydroxy-3H-phenoxazin-3-one 10-oxide) (Sigma-Aldrich, catalog number: R7017)
- Trypan blue dye for vital staining (BDH, catalog number: 34078)
- Potassium chloride (KCl), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 746336)
- Potassium phosphate monobasic (KH2PO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795488)
- Sodium chloride 99.9% (NaCl) (Applichem, catalog number: 381659)
- Sodium phosphate dibasic (Na2HPO4), ACS reagent, ≥ 99.0% (Sigma-Aldrich, catalog number: 795410)
- Sodium Dodecyl Sulfate (SDS) (Sigma-Aldrich, catalog number: L4509)
- Fetal Bovine Serum (Biowest, catalog number: S181B) (see Recipes)
- Complete RPMI-1640 medium (see Recipes)
- Complete Schneider’s medium (see Recipes)
- Phosphate buffered saline, 1x (PBS, pH 7.2) (see Recipes)
- Resazurin solution (see Recipes)
- 0.4% (w/v) Trypan blue exclusion dye (see Recipes)
- 0.01% w/v Sodium Dodecyl Sulfate (SDS) (see Recipes)
- Lysis solution (see Recipes)
Equipment
- Agitating water bath (Labtech, catalog number: LSB-015S)
- ELISA Microplate reader (Dynatech Laboratories, catalog number: MRX)
- Gilson pipettes (Gilson, models: PIPETMAN Classic P-10, P-20, P-200, P-1000)
- Multichannel pipette (Brand, catalog number: 703710)
- Malassez counting chamber (Paul Marienfeld GmbH & Co., catalog number: 0640610)
- Microplate rotor (Centurion Scientific Ltd, catalog number: BRK 5530)
- New BrunswickTM Galaxy® 170 S CO2 Incubator (Eppendorf, catalog number: Galaxy 170 S)
- Refrigerated centrifuge (Centurion Scientific Ltd, catalog number: PrO-Research K241R)
- Optical microscope (Olympus, catalog number: BHB)
- pH meter (Thermo Fisher Scientific, catalog number: 13-644-928)
- Pipette controller (Brand, catalog number: accu-jet® pro 26300)
- Refrigerated Incubator 26 °C (Sanyo, catalog number: MIR-253)
- Sterile biosafety cabinet (Telstar, catalog number: Bio-II-A)
- Water distiller (Sartorius, catalog number: H2O-I-1-UV-T)
Software
- Microsoft® Office Excel 2010 (Microsoft)
Procedure
- In vitro cell culture method for biological evaluation of antileishmanial activity of total phenolic fraction (TPF) against Leishmania spp. promastigotes by using the resazurin reduction assay
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Note: The recovery of TPF from extra virgin olive oil was carried out using the Centrifugal Partition Extraction (CPE) technique, which is an innovative solid support free separation technique derived from Centrifugal Partition Chromatography (CPC). Briefly, liquid-liquid chromatography was performed using a laboratory scale centrifugal partition extractor FCPE300®, which was equipped with a rotor composed of 7 stacked partition disks engraved with a total of 231 partition cells, while the total volume of the column was 300 ml (Koutsoni et al., 2018). - Cultivate L. infantum and L. major promastigotes in 25 cm2 cell culture flasks containing 10 ml of complete RPMI-1640 medium (Recipe 2) at 26 °C.
Note: Long-term in vitro cultivation of Leishmania spp. leads to a progressive loss of virulence (Segovia et al., 1992). Subsequently, the maintenance of Leishmania spp. virulence is achieved by continuous passage in BALB/c mice. Tissue amastigotes are obtained after homogenization either of spleen tissue in case of L. infantum or popliteal lymph node in case of L. major. Transformation of intracellular amastigotes to infective promastigotes is achieved during culture in complete RPMI-1640 medium at 26 °C. Parasite cultures are subcultured into fresh medium (usually at day 4 depending on Leishmania strain, after every passage) until they reach the 10th subculture (Nasiri et al., 2013). The appropriate inoculum dose of Leishmania spp. promastigotes is usually 2 x 106 parasites per ml. - Allow parasites to enter stationary-growth phase at 26 °C. Then promastigotes are removed by gently pipetting using a sterile disposable transfer pipette.
Note: Leishmania spp. promastigotes usually enter the stationary-growth phase after 3 to 5 days, depending on the Leishmania strain. For example, L. major, a commonly used strain, approximately enters the stationary growth phase at Day 4 when it reaches the number of 3.5 x 107 parasites/ml (Nasiri et al., 2013). - Determine the number of Leishmania spp. promastigotes per ml by differential counting of dead and live parasites using the Trypan blue exclusion dye (Recipe 6) in a Malassez counting chamber under an optical microscope.
Note: The final dilution of promastigotes in Trypan blue dye depends on the density of the in vitro parasite culture. Usually, a 1:20 final dilution of promastigotes is suitable at the 3rd or 4rth day of the in vitro culture. Additionally, promastigotes have to be fixed with 2% (v/v) formalin before counting. - Seed 1 x 106 L. infantum promastigotes and 7.4 x 105 L. major promastigotes per well in a final volume of 100 µl of complete RPMI-1640 medium in separate 96-well flat bottom tissue culture plate for each strain, under a sterile biosafety cabinet.
Note: Different concentrations of stationary phase L. infantum and L. major promastigotes (e.g., 104, 2.5 x 104, 5 x 104, 7.5 x 104, 105, 2.5 x 105, 5 x 105, 7.5 x 105, 106 in a final volume of 200 µl/well) are usually screened before the experiment’s set-up in order to determine the optimal cell density (Corral et al., 2013). - Add various increasing concentrations of TPF (5-400 µg/ml) in triplicates and fill in the wells with complete RPMI-1640 medium until the final volume of 200 µl per well.
Note: Concentrations of plant extracts may vary depending on the natural product. - Add one reference drug (i.e., one drug currently subscribed for the chemotherapy of leishmaniasis) in triplicate at the appropriate 50% inhibitory concentration (IC50). Hexadecylphosphocholine (HePC) is used at the concentrations of 3.3 µg/ml and 6.4 µg/ml for L. infantum and L. major promastigotes respectively, as previously described (Koutsoni et al., 2018).
- Triplicates of different kinds of negative controls are also included. More specifically, Leishmania spp. promastigotes cultured only in the presence of complete RPMI-1640 medium and Leishmania spp. promastigotes cultured in equivalent volumes of TPF’s solvents are included in triplicates (Figure 1).
Note: A quadruplicate of parasite-free complete RPMI-1640 medium is also included in order to serve as blank.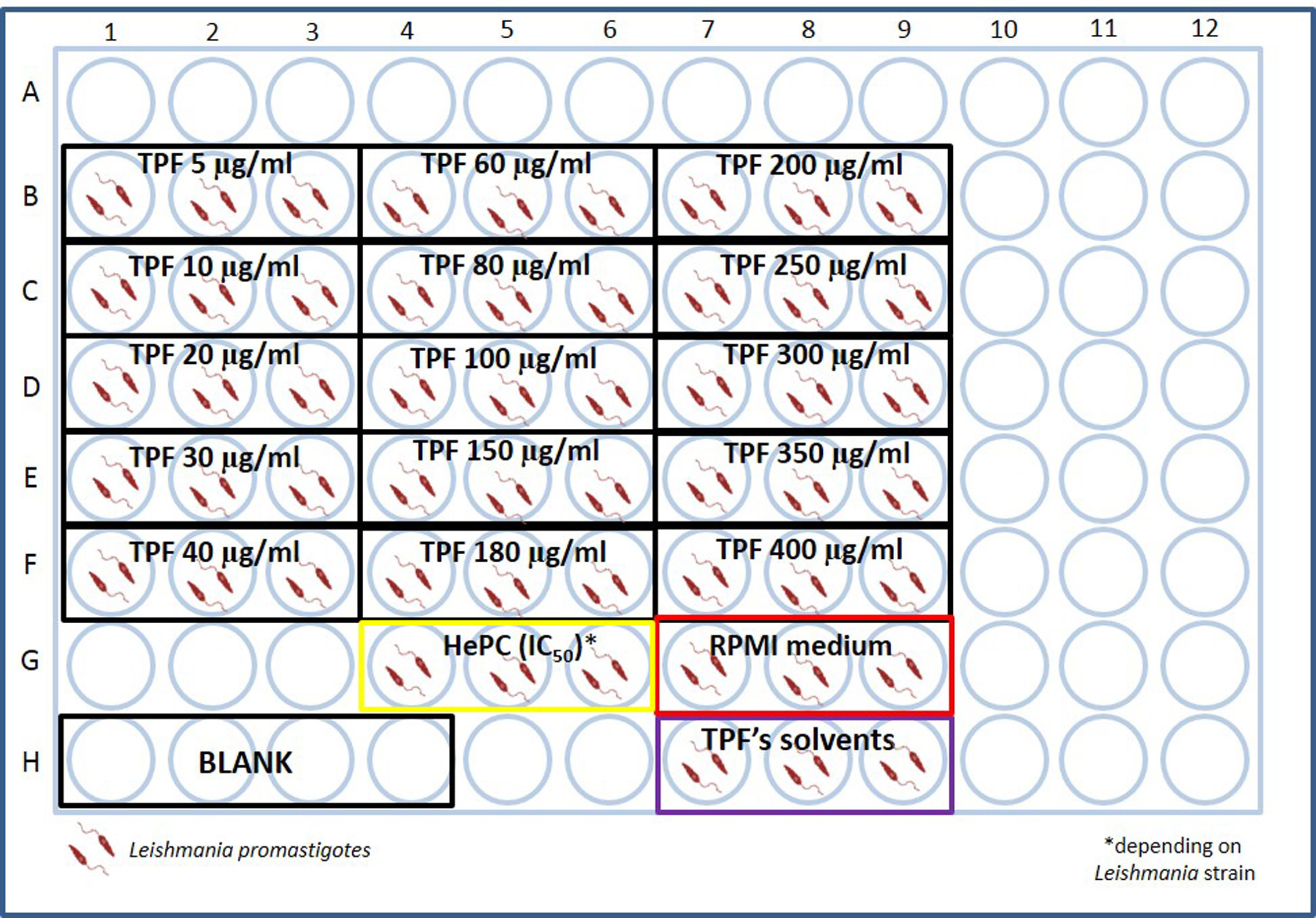
Figure 1. Example test plate designed to estimate IC50 of TPF extract for Leishmania spp. promastigotes - Seal the plates with parafilm and incubate them in a non-inverted position, in a 26 °C incubator for 60 h.
- Add resazurin solution (Recipe 5) at a final concentration of 20 µg/ml in each well and mix thoroughly by pipetting.
- Seal the plates with parafilm and further incubate them in a non-inverted position, in a 26 °C incubator until a fluorescent red color is observed in the negative control group (Figure 2).
Note: Resazurin is a non-toxic, cell-permeable compound that converts from a non-fluorescent blue dye to the highly fluorescent red dye resorufin in response to changes of the reducing environment within the cytosol of the cell (Ahmed et al., 1994; Nociari et al., 1998). Noticeably, the incubation period depends on various parameters such as the parasite strain, varying from 3 to 72 h (Mikus and Steverding, 2000; Bilbao-Ramos et al., 2012; Corral et al., 2013; Kyriazis et al., 2013).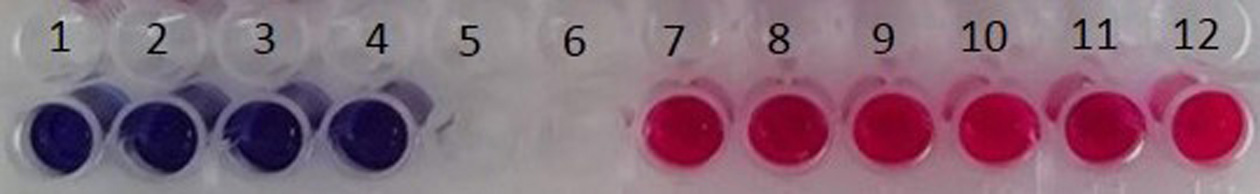
Figure 2. Representative non-fluorescent blue wells (numbered 1-4) and fluorescent red wells (numbered 7-12) - Read the plates by using an absorbance microplate reader with excitation at 570 nm and reference filter at 630 nm.
Note: The excitation and emission spectra of resorufin are fairly broad, excitation filters between 530 and 570 nm can be used (Czekanska, 2011).
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
- In vitro resazurin reduction assay to assess the concentration of TPF that results in 50% cytotoxicity (CC50) in cultured macrophages
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Note: The recovery of TPF from extra virgin olive oil was carried out using the Centrifugal Partition Extraction (CPE) technique, which is an innovative solid support free separation technique derived from Centrifugal Partition Chromatography (CPC), as mentioned in Procedure section, Step A1. - Cultivate J774A.1 macrophages in 25 cm2 cell culture flasks with filter cap containing 10 ml of complete RPMI-1640 medium at 37 °C under 5% CO2 humidified air.
Note: The J774A.1 macrophages used in experiments are long-term maintained in liquid nitrogen. More specifically, the J774A.1 macrophage cell line is freezed in complete RPMI-1640 medium supplemented with 10% DMSO and stored in liquid nitrogen in appropriate cryovials. For thawing procedure, cryovials are immediately placed in a 37 °C water bath and upon thawing, are transferred into a centrifugation tube containing pre-warmed complete growth medium. Cell suspension is centrifuged at 300 x g for 5 min. Gently, cells are resuspended in complete RPMI-1640 medium and transferred into 25 cm2 cell culture flasks with filter cap at 37 °C under 5% humidified air. - Allow macrophages to reach a high density population of about 70% confluence.
Note: J774A.1 macrophages cultured in complete RPMI-1640 medium at 37 °C under 5% CO2 humidified air, usually reach 70% confluence within 3 to 4 days. - Then, remove the majority of culture medium and leave about 2 ml medium in the flask and detach macrophage monolayer by scrapping cells gently and slowly with a cell scraper at 45° angle (see Video 1) (References 23 and 29)Video 1. Scraping
- Determine the number of macrophages per ml by differential counting of dead and live cells using the Trypan blue exclusion dye in a Malassez counting chamber under an optical microscope.
Note: The final dilution of macrophages in Trypan blue dye depends on the density of the culture. Usually, a 1:20 final dilution of macrophages is suitable at the 3rd or 4rth day of the in vitro culture. - Seed 4 x 104 J774A.1 macrophages per well in a final volume of 100 µl of complete RPMI-1640 medium in a 96-well flat bottom tissue culture plate under a sterile biosafety cabinet.
- Incubate the plate for 18 h at 37 °C under 5% CO2 humidified air, in a non-inverted position, in order to achieve cell adhesion.
- Add various increasing concentrations of TPF (5-400 µg/ml) in triplicates and fill in the wells with complete RPMI-1640 medium until the final volume of 200 µl per well.
Note: Concentrations of plant extracts may vary depending on the natural product. - Add one reference drug in triplicate at the appropriate 50% inhibitory concentration (IC50). Hexadecylphosphocholine (HePC) is used at the concentrations of 60.2 µg/ml, as previously described (Koutsoni et al., 2018).
- Triplicates of different kinds of negative controls are also included. More specifically, J774A.1 macrophages cultured only in the presence of complete RPMI-1640 medium at a final volume of 200 µl and J774A.1 macrophages cultured in equivalent volumes of TPF’s solvents are included.
Note: A quadruplicate of macrophages-free complete RPMI-1640 medium is also included in order to serve as blank. - Incubate the plate in a non-inverted position, at 37 °C under 5% CO2 humidified air for 72 h.
- Add resazurin solution at a final concentration of 20 µg/ml in each well and mix thoroughly by pipetting.
- Incubate the plate in a non-inverted position, at 37 °C under 5% CO2 humidified air until a fluorescent red color is observed in negative control group.
Note: The incubation period of J774A.1 macrophages cultured only in the presence of complete RPMI-1640 medium, until the development of red color is usually 24 h. - Read the plate by using an absorbance microplate reader with excitation at 570 nm and reference filter at 630 nm.
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
- In vitro cell culture method for biological evaluation of antileishmanial activity of TPF extract against intracellular Leishmania spp. amastigotes by using the resazurin reduction assay
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Note: The recovery of TPF from extra virgin olive oil was carried out by Centrifugal Partition Extraction (CPE) technique, which is an innovative solid support free separation technique derived from Centrifugal Partition Chromatography (CPC), as mentioned in Procedure section, Step A1. - Cultivate J774A.1 macrophages in 25 cm2 cell culture flasks with filter cap containing 10 ml of complete RPMI-1640 medium at 37 °C under 5% CO2 humidified air.
- Allow macrophages to reach a high density population of about 70% confluence.
Note: J774A.1 macrophages cultured in complete RPMI-1640 medium at 37 °C under 5% CO2 humidified air, usually reach 70% confluence within 3 to 4 days. - Then, remove the majority of culture medium with a sterile disposable transfer pipette and leave about 2 ml medium in the flask and detach macrophage monolayer by scrapping cells gently with a cell scraper at 45° angle (see Video 1) (References 23 and 29).
- Determine the number of macrophages per ml by differential counting of dead and live cells using the Trypan blue exclusion dye in a Malassez counting chamber under an optical microscope.
Note: See the note of Step B5 in Procedure section. - Seed 5 x 104 J774A.1 macrophages per well in a final volume of 100 µl of complete RPMI-1640 medium in a 96-well flat bottom tissue culture plate under a sterile biosafety cabinet.
- Incubate the plate in a non-inverted position, for 18 h at 37 °C under 5% CO2 humidified air in order to achieve cell adhesion.
- Seed 7.5 x 105 Leishmania spp. early stationary phase promastigotes in each well (i.e., ratio of 15:1 parasites:macrophage) in a total final volume of 200 µl of complete RPMI-1640 medium.
Note: Control triplicates of promastigote-free macrophages are also included in order to serve as infection negative control. - Incubate the plate in a non-inverted position, for 48 h at 37 °C under 5% CO2 humidified air.
- Then, remove the non-internalized promastigotes by washing thrice with RPMI-1640 medium pre-warmed at 37 °C with the use of a multichannel pipette at 45° angle.
- Add various increasing concentrations of TPF (5-400 µg/ml) in triplicates and fill in the wells with complete RPMI-1640 medium until the final volume of 200 µl per well.
Note: Concentrations of plant extracts may vary depending on the natural product. - Add one reference drug in triplicate at the appropriate 50% inhibitory concentration (IC50). Hexadecylphosphocholine (HePC) is used at the concentrations of 0.6 µg/ml and 3.2 µg/ml for L. infantum and L. major respectively, as previously described (Koutsoni et al., 2018).
- Triplicates of different kinds of negative controls are also included. More specifically, Leishmania-infected J774A.1 macrophages cultured only in the presence of complete RPMI-1640 medium without drug influence and Leishmania-infected J774A.1 macrophages cultured in equivalent volumes of TPF’s solvents are included (Figure 3).
Note: A quadruplicate of parasite- and cell-free complete RPMI-1640 medium is also included in order to serve as blank.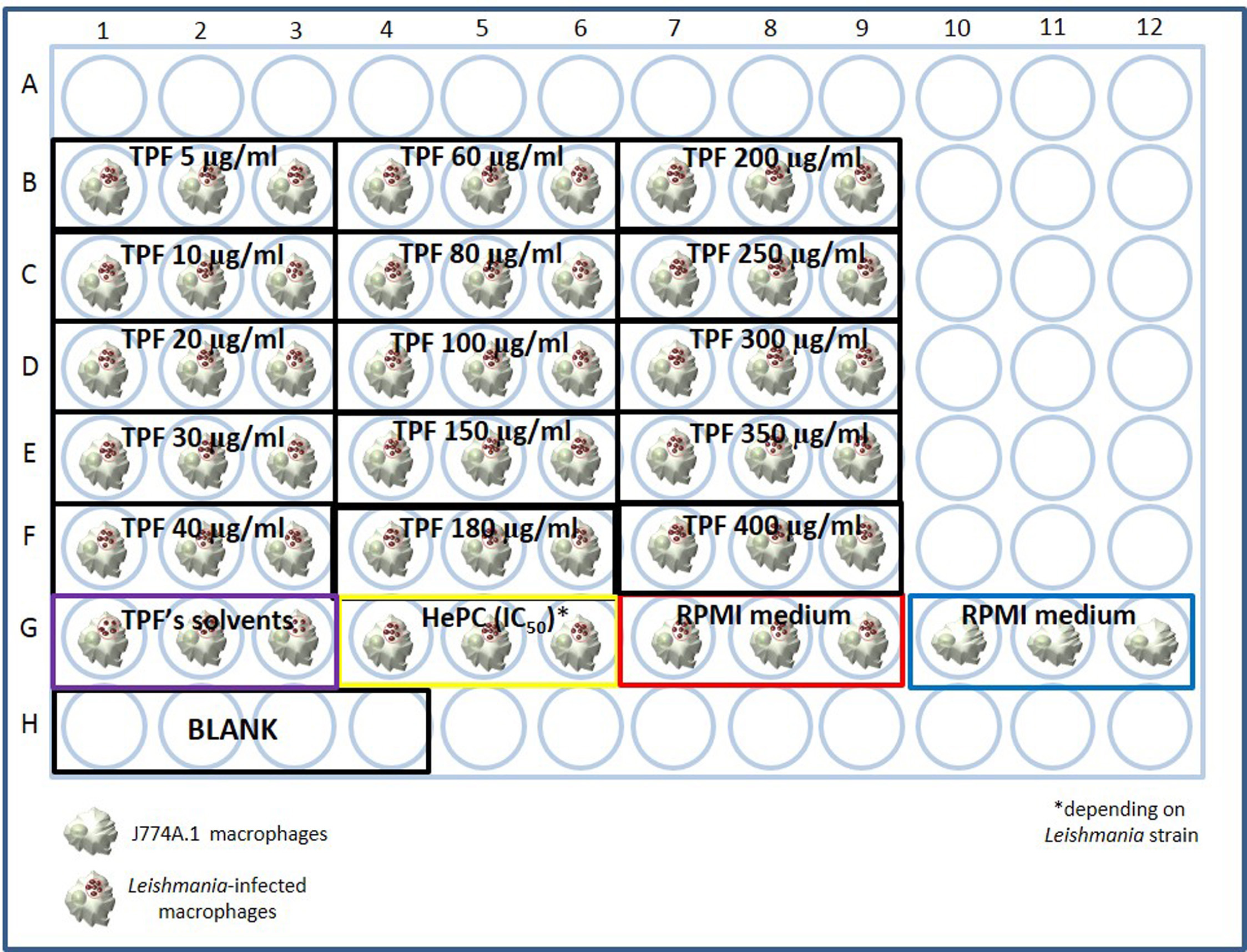
Figure 3. Example test plate designed to estimate IC50 of TPF extract for Leishmania spp. amastigotes - Incubate the plate in a non-inverted position, for 48 h at 37 °C under 5% CO2 humidified air.
- Remove culture supernatants with a multichannel pipette and disrupt macrophages’ membranes by adding 50 µl of lysis solution (Recipe 8) (Bilbao-Ramos et al., 2012).
- Incubate the plate in a non-inverted position, for 20 min at room temperature.
- Centrifuge the plate at 300 x g for 5 min in order to remove the cell lysis solution.
- Remove supernatant and replace by 200 µl/well of complete Schneider’s insect medium (Recipe 3).
Note: Schneider’s insect medium is preferred as it is a more enriched in amino acids medium compared to RPMI-1640 medium and it is considered as a more effective medium in primary isolation from Leishmania spp. lesions (Grekov et al., 2011). - Seal the plate with parafilm and further incubate in a non-inverted position, in a 26 °C incubator for 72 h in order to allow transformation of viable amastigotes into promastigotes and proliferation.
- Add resazurin solution at a final concentration of 60 µg/ml in each well and mix thoroughly by pipetting.
- Seal the plate with parafilm and further incubate in a non-inverted position, at 26 °C until a fluorescent red color is observed in the negative control group (approximately for 24 h).
- Read the plate by using an absorbance microplate reader with excitation at 570 nm and reference filter at 630 nm.
- Prepare a known concentration of the plant extract using the appropriate solvent. TPF is dissolved in 62.5% pure ethanol, 31.25% sterile distilled water and 6.25% DMSO.
Data analysis
Resazurin assays offer a simple and rapid measurement for cell viability. Living cells are metabolically active and are able to reduce via mitochondrial reductase, the non-fluorescent dye resazurin to the strongly red-fluorescent dye resorufin (Rezende et al., 2019). The fluorescence output is proportional to the number of viable cells over a wide concentration range (O'Brien et al., 2000).
- Data analysis for the estimation of 50% inhibitory concentration (IC50) for Leishmania spp. promastigotes
The correlation between promastigote density and the Optical Density (OD) acquired values is assessed by linear regression analysis with estimation of the coefficient of determination (R2) (Schneider et al., 2010).- Calculate the average of the OD values acquired from the quadruplicate of parasite-free complete RPMI-1640 medium that serves as blank.
- Subtract this average value from every other OD value.
- Calculate the average OD values from every triplicate.
- The average OD value acquired from the triplicate of Leishmania spp. promastigotes cultured only in the presence of complete RPMI-1640 medium which serves as negative control, represents the 100% of parasite survival and growth and therefore a 0% of inhibition.
- Calculate the % of growth and correspondingly the % of inhibition for the various increasing concentrations of TPF, based on the average that refers to 100% of growth.
- Select all the values and insert a scatter plot that depicts the TPF concentrations in the x-axis and the % of inhibition in the y-axis.
Note: The values on the y-axis are depicted as real numbers between 0 and 1. - Set the x-axis to logarithmic scale and final graph must be approximately linear over some range.
Note: The linear regression method of calculating the IC50 value builts on the basis of the fact that all the data points for which the calculated percent of inhibition is more than 0% and less than 100%. This method assumes a linear relationship in the entire dose-response curve, which is rarely the case as it typically has a sigmoidal shape. To employ this method for proper IC50 calculation, it usually has to be combined with the logarithmic transformation of one or both axes to ensure the proper conversion of the dose-response curve into the linear approximation (Nevozhay, 2014). - Select “add trendline” and pick “linear” trendline options.
Note: A trendline is most reliable when its R-squared value is at or near 1. - Using the linear (y = ax + b) equation on this graph for y = 0.5 value, the x point equals to the IC50 value (Figure 4). The IC50 values are expressed in µg/ml.

Figure 4. IC50 calculation in Excel
- Data analysis for the estimation of 50% cytotoxic concentration (CC50) for J774A.1 macrophages
The results are expressed as CC50 value which is defined as the concentration of a compound that kills half of the cells in an uninfected cell culture (Smee et al., 2017).- Calculate the average of the OD values acquired from the quadruplicate of macrophages-free complete RPMI-1640 medium that serves as blank.
- Subtract this average value from every other OD value.
- Calculate the average OD values from every triplicate.
- The average OD value acquired from the triplicate of J774A.1 macrophages cultured only in the presence of complete RPMI-1640 medium which serves as negative control, represents the 100% of macrophage growth and therefore a 0% of inhibition.
- Calculate the % of growth and correspondingly the % of inhibition for the various increasing concentrations of TPF, based on the average that refers to 100% of growth.
- Select all the values and insert a scatter plot that depicts the TPF concentrations in the x-axis and the % of inhibition in the y-axis.
Note: The values on the y-axis are depicted as real numbers between 0 and 1. - Set the x-axis to logarithmic scale and final graph must be approximately linear over some range.
Note: The linear regression method of calculating the CC50 value builts on the basis of the fact that all the data points for which the calculated percent of inhibition is more than 0% and less than 100%. This method assumes a linear relationship in the entire dose-response curve, which is rarely the case as it typically has a sigmoidal shape. To employ this method for proper CC50 calculation, it usually has to be combined with the logarithmic transformation of one or both axes to ensure the proper conversion of the dose-response curve into the linear approximation (Nevozhay, 2014). - Select “add trendline” and pick “linear” trendline options.
Note: A trendline is most reliable when its R-squared value is at or near 1. - Using the linear (y = ax + b) equation on this graph for y = 0.5 value, the x point equals to the CC50 value. The CC50 value is expressed in µg/ml.
- Data analysis for the estimation of 50% inhibitory concentration (IC50) for Leishmania spp. amastigotes
- Calculate the average of the OD values acquired from the quadruplicate of parasite- and macrophage-free complete RPMI-1640 medium that serves as blank.
- Subtract this average value from every other OD value.
- Calculate the average of the OD values acquired from the triplicate of promastigotes-free macrophages that serves as infection negative control.
- Subtract this average value from every other OD value.
- Calculate the average OD values from every triplicate.
- The average OD value acquired from the triplicate of Leishmania-infected J774A.1 macrophage cultured only in the presence of complete RPMI-1640 medium without any drug influence which serves as negative control, represents the 100% of parasite survival and growth and therefore a 0% of inhibition.
- Calculate the % of growth and correspondingly the % of inhibition for the various increasing concentrations of TPF, based on the average that refers to 100% of growth.
- Select all the values and insert a scatter plot that depicts the TPF concentrations in the x-axis and the % of inhibition in the y-axis.
Note: The values on the y-axis are depicted as real numbers between 0 and 1. - Set the x-axis to logarithmic scale and final graph must be approximately linear over some range.
Note: The linear regression method of calculating the IC50 value builts on the basis of the fact that all the data points for which the calculated percent of inhibition is more than 0% and less than 100%. This method assumes a linear relationship in the entire dose-response curve, which is rarely the case as it typically has a sigmoidal shape. To employ this method for proper IC50 calculation, it usually has to be combined with the logarithmic transformation of one or both axes to ensure the proper conversion of the dose-response curve into the linear approximation (Nevozhay, 2014). - Select “add trendline” and pick “linear” trendline options.
Note: A trendline is most reliable when its R-squared value is at or near 1. - Using the linear (y = ax + b) equation on this graph for y = 0.5 value, the x point equals to the IC50 value. The IC50 values are expressed in µg/ml.
- Determination of the Selectivity Index (SI)
The Selectivity Index (SI) is defined as the ratio of the 50% cytotoxic concentration of J774A.1 macrophages to the 50% inhibitory concentration of Leishmania spp. amastigotes (CC50/IC50). The in vitro activity of a tested compound against intracellular Leishmania spp. amastigotes in the absence of obvious cytotoxicity on murine macrophage cells (SI > 1) demonstrates its potential in the treatment of leishmaniasis (Makwali et al., 2015).
Notes
It is recommended to optimize experimental parameters, such as incubation time, the number of cells and parasites and the amount of resazurin used, before performing the assay for the first time since these parameters affect the consistency of the metabolic assay (Czekanska, 2011; Kim and Jang, 2018). Moreover, resazurin and resorufin are light-sensitive and need to be protected from light otherwise it results in decreased sensitivity.
Recipes
- Fetal Bovine Serum (FBS)
- Thaw FBS and heat-inactivate it at 56 °C for 30 min in an agitating water-bath under constant agitation
- Store at -20 °C until use
- Complete RPMI-1640 medium
- Add 5 ml of L-glutamine (stock solution 200 mM, stored at -20 °C), 5 ml of Penicillin-Streptomycin (stock solution 10,000 U/ml, stored at -20 °C) and 5 ml of HEPES (stock solution 1 M) to 500 ml of RPMI-1640 medium
- Store at 4 °C
- Take the appropriate volume and add FBS to 10% v/v final concentration
- Complete Schneider’s medium
- Add 5 ml of Penicillin-Streptomycin (stock solution 10,000 U/ml stored at -20 °C)
- Store at 4 °C
- Take the appropriate volume and add FBS to 20% v/v final concentration
- Phosphate Buffer Saline (PBS), 10x, pH = 7.2-7.4
- Place 800 ml of distilled water in a suitable container and dissolve 80 g NaCl, 2 g KCl, 11.5 g of Na2HPO4, and 2 g of KH2PO4
- Agitate until the complete salt dilution and adjust solution to the desired pH (typically 7.2-7.4) with NaOH (1 M) or HCl (1 M) solution, if needed
- Add sterile and distilled water until final volume is 1,000 ml and sterilize through a 0.22 µm pore size syringe filter unit
- Prepare the 1x working solution by diluting 50 ml of 10x stock solution in 450 ml of sterile and distilled water
- Store at 4 °C
- Resazurin solution
- Dissolve the appropriate amount of resazurin sodium salt in the desired final volume of PBS 1x (e.g., 2.46 mg of resazurin in 1 ml of PBS 1x to achieve an intermediate solution and then dilute 5 µl of this per well in order to achieve a final concentration of 60 µg/ml)
- Protect from light during experimental procedure
- Resazurin solution at working concentration must be prepared freshly each time before applying (Czekanska, 2011)
- 0.4% (w/v) Trypan blue exclusion dye
a.Dissolve 0.4 g of Trypan blue in 100 ml of PBS 1x. Agitate well
b.Filter through a pleated filter paper and sterilize through a 0.22 µm pore size syringe filter unit
c.Store at room temperature - 0.01% (w/v) Sodium Dodecyl Sulfate (SDS)
a. Dilute 0.01 g of SDS in 100 ml distilled water. Agitate well
b. Store at room temperature - Lysis solution
Dilute 4.8 µl of HEPES (stock solution 1 M) and 6 ml of 0.01 % SDS solution in RPMI-1640 medium in order to achieve a final volume of 10 ml
Acknowledgments
Part of this research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under the HFRI PhD Fellowship grant (GA. no. 6ΝΔΘ46ΨΖ2Ν-ΣΣΟ). Also this work was supported by KRHPIS II (MIS 5002486) and EATRIS (MIS 5028091).
The protocols were adapted and modified from Koutsoni et al., 2018 and Kyriazis et al., 2013.
Competing interests
Authors declare that they have no competing interests.
References
- Ahmed, S. A., Gogal, R. M., Jr. and Walsh, J. E. (1994). A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 170(2): 211-224.
- Aykul, S. and Martinez-Hackert, E. (2016). Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem 508: 97-103.
- Bates, P. A. and Rogers, M. E. (2004). New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med 4(6): 601-609.
- Bilbao-Ramos, P., Sifontes-Rodriguez, S., Dea-Ayuela, M. A. and Bolas-Fernandez, F. (2012). A fluorometric method for evaluation of pharmacological activity against intracellular Leishmania amastigotes. J Microbiol Methods 89(1): 8-11.
- Burza, S., Croft, S. L. and Boelaert, M. (2018). Leishmaniasis. Lancet 392(10151): 951-970.
- Cheuka, P. M., Mayoka, G., Mutai, P. and Chibale, K. (2016). The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 22(1): e58.
- Corral, M. J., Gonzalez, E., Cuquerella, M. and Alunda, J. M. (2013). Improvement of 96-well microplate assay for estimation of cell growth and inhibition of Leishmania with Alamar Blue. J Microbiol Methods 94(2): 111-116.
- Czekanska, E. M. (2011). Assessment of cell proliferation with resazurin-based fluorescent dye. Methods Mol Biol 740: 27-32.
- Grekov, I., Svobodova, M., Nohynkova, E. and Lipoldova, M. (2011). Preparation of highly infective Leishmania promastigotes by cultivation on SNB-9 biphasic medium. J Microbiol Methods 87(3): 273-277.
- Griffiths, M. and Sundaram, H. (2011). Drug design and testing: profiling of antiproliferative agents for cancer therapy using a cell-based methyl-[3H]-thymidine incorporation assay. Methods Mol Biol 731: 451-465.
- Kim, H. J. and Jang, S. (2018). Optimization of a resazurin-based microplate assay for large-scale compound screenings against Klebsiella pneumoniae. 3 Biotech 8(1): 3.
- Koutsoni, O. S., Karampetsou, K., Kyriazis, I. D., Stathopoulos, P., Aligiannis, N., Halabalaki, M., Skaltsounis, L. A. and Dotsika, E. (2018). Evaluation of total phenolic fraction derived from extra virgin olive oil for its antileishmanial activity. Phytomedicine 47: 143-150.
- Kyriazis, J. D., Aligiannis, N., Polychronopoulos, P., Skaltsounis, A. L. and Dotsika, E. (2013). Leishmanicidal activity assessment of olive tree extracts. Phytomedicine 20(3-4): 275-281.
- Lage, O. M., Ramos, M. C., Calisto, R., Almeida, E., Vasconcelos, V. and Vicente, F. (2018). Current screening methodologies in drug discovery for selected human diseases. Mar Drugs 16(8): e279.
- Lahlou, M. (2007). Screening of natural products for drug discovery. Expert Opin Drug Discov 2(5): 697-705.
- Makwali, J. A., Wanjala, F. M. E., Ingonga, J. and Anjili, C. O. (2015). In vitro studies on the antileishmanial activity of herbicides and plant extracts against Leishmania major parasites. Res J Med Plant 9(3): 90-104.
- Mikus, J. and Steverding, D. (2000). A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int 48(3): 265-269.
- Mitra, A. K. and Mawson, A. R. (2017). Neglected tropical diseases: epidemiology and global burden. Trop Med Infect Dis 2(3): e36.
- Nasiri, V., Karimi, G., Dalimi, A., Paykari, H. and Ghaffarifar, F. (2013). Effects of sheep and mouse urine on the growth pattern of Leishmania major promastigotes. Biomed Res Int 2013: 748592.
- Nevozhay, D. (2014). Cheburator software for automatically calculating drug inhibitory concentrations from in vitro screening assays. PLoS One 9(9): e106186.
- Nociari, M. M., Shalev, A., Benias, P. and Russo, C. (1998). A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods 213(2): 157-167.
- O'Brien, J., Wilson, I., Orton, T. and Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267(17): 5421-5426.
- Passaging Cells- RAW.swf. Cannon Lab: You Tube.
- Rezende, N., Ceron Jayme, C., Brassesco, M. S., Claudio Tedesco, A. and de Oliveira, H. F. (2019). Standardization of a resazurin-based assay for the evaluation of metabolic activity in oral squamous carcinoma and glioblastoma cells. Photodiagnosis Photodyn Ther 26: 371-374.
- Schneider, A., Hommel, G. and Blettner, M. (2010). Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int 107(44): 776-782.
- Segovia, M., Artero, J. M., Mellado, E. and Chance, M. L. (1992). Effects of long-term in vitro cultivation on the virulence of cloned lines of Leishmania major promastigotes. Ann Trop Med Parasitol 86(4): 347-354.
- Singh, N., Mishra, B. B., Bajpai, S., Singh, R. K. and Tiwari, V. K. (2014). Natural product based leads to fight against leishmaniasis. Bioorg Med Chem 22(1): 18-45.
- Smee, D. F., Hurst, B. L., Evans, W. J., Clyde, N., Wright, S., Peterson, C., Jung, K. H. and Day, C. W. (2017). Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J Virol Methods 246: 51-57.
- TPP Cell Scrapers (www.midsci.com). MIDSCI Biotech: You Tube.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Koutsoni, O. S., Karampetsou, K. and Dotsika, E. (2019). In vitro Screening of Antileishmanial Activity of Natural Product Compounds: Determination of IC50, CC50 and SI Values. Bio-protocol 9(21): e3410. DOI: 10.21769/BioProtoc.3410.
Category
Microbiology > Antimicrobial assay > Antiparasitic assay
Cell Biology > Cell viability > Cell survival
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









