- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simple Synthesis of Functionalized Paramagnetic Beads for Nucleic Acid Purification and Manipulation
(*contributed equally to this work) Published: Vol 9, Iss 20, Oct 20, 2019 DOI: 10.21769/BioProtoc.3394 Views: 11310
Reviewed by: Imre GáspárManish Kumar PatelAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Optimized RNA Extraction Method From Micro-quantities of Guinea Pig Cartilage and Synovium for Osteoarthritis Research
Nidhi Bhardwaj [...] Jyotdeep Kaur
Jun 20, 2025 1607 Views

A Comparative Protocol for Preserving Deep-Water Marine Invertebrate Tissues: DNA/RNA Shield vs. Liquid Nitrogen for Dual Extraction of High-Quality Nucleic Acids
Ana S. Gomes [...] Olivier Laroche
Nov 20, 2025 1327 Views

Plasmid DNA Purification Using Filterprep With an Optional Endotoxin Removal Step
Yu-Qian Lin [...] Chung-Te Chang
Dec 20, 2025 1068 Views
Abstract
The purification of nucleic acids is one of the most common procedures employed in modern molecular biology laboratories. Typically, commercial column-based protocols are utilized to isolate DNA or RNA from various sources. However, these methods not only require specialized equipment, but are also extremely expensive for high-throughput applications. Although an elegant answer to this issue can be provided by paramagnetic beads, bead-based open-source protocols have been limited in the past. Here, we provide an easy to follow step-by-step manual for the synthesis of paramagnetic beads, as well as their functionalization with either a silica- or a carboxyl-surface that can be used to replace the commercial columns with self-made magnetic beads. Together with a variety of detailed protocols for their use in high-throughput nucleic acids extractions, this bead synthesis method forms the recently published open platform Bio-On-Magnetic-Beads (BOMB), which is available on PLOS Biology (Oberacker et al., 2019). Updated protocols can be found on the associated webpage (https://bomb.bio).
Keywords: Carboxyl-coatingBackground
The ability to achieve solid-phase reversible immobilization (SPRI) (DeAngelis et al., 1995) is one of the most useful characteristics of functionalized paramagnetic nanoparticles (MNPs). Together with their scalability and potential for automation, MNPs are ideal candidates for the development of high-throughput protocols regarding nucleic acid purification (Hawkins et al., 1994; DeAngelis et al., 1995; Rohland and Reich, 2012). The small nano- or microparticles are able to reversibly bind nucleic acids under dehydrating conditions and can be safely immobilized by a strong magnet during consecutive wash or manipulation steps. Bead-based protocols can be adapted easily for multi-well formats, allowing the processing of hundreds of samples simultaneously. Additionally, MNPs are very cost-effective as the beads can be obtained very cheaply, both commercially and self-made. Although their advantages over more established column-based methods are obvious, surprisingly little effort has been put in the development of open-source protocols featuring paramagnetic beads.
Protocols for the synthesis of ferrite nanoparticles have been published before (reviewed in Houshiar et al., 2014; Singh et al., 2014; Wu et al., 2015; Majidi et al., 2016). We adopted the commonly used co-precipitation method due to its efficient and robust performance and the fact that it does not require any specialized equipment (Choi et al., 2007). For this, a solution of FeCl2 and FeCl3 in a 1:2 molar ratio is prepared and slowly dripped into a preheated NaOH solution. This forms a black precipitate consisting of Fe3O4 particles with an approximate diameter of 5 to 20 nm as judged by electron microscopy (Figure 1A). As oxygen is known to interfere with ferrite precipitation reactions, the NaOH solution should be degassed and/or preheated to 80 °C or higher before adding the iron solution. After the synthesis reaction, the nanoparticles are washed multiple times with deionized water. We recommend starting the respective coating reaction immediately after the synthesis as the particles are prone to oxidation. However, it is possible to stabilize them using various chemicals, such as detergents, sodium oleate or polyvinylpyrrolidone (PVP) (reviewed in Singh et al., 2014; Majidi et al., 2016). Upon lyophilization, the core MNPs can be stored in an air-tight container under inert atmosphere.
Figure 1. Synthesis of BOMB paramagnetic beads. A. Equipment setup for BOMB MNP synthesis. Place a magnetic stir bar in both the water bath as well as the round bottom flask in order to avoid heat accumulation. B. Synthesized and washed paramagnetic core particles. C. Paramagnetic core particles in transmission electron microscopy (TEM). D. Silica-coated paramagnetic beads in scanning electron microscopy (SEM) and TEM. E. Carboxyl-coated paramagnetic beads in light microscopy (LM), with and without an applied magnet.
Encasing of the ferrite nanoparticles in a silica coat prevents oxidation and leakage of iron ions and provides an inert surface for nucleic acid precipitation without the risk of irreversible binding. The silica deposition reaction outlined here is based on a modified version of the Stöber method (Stöber et al., 1968). It utilizes tetraethyl orthosilicate (TEOS) which is hydrolyzed in a basic environment, leading to the deposition of a SiO2 layer surrounding the paramagnetic core. Conveniently, the thickness of the silica coat, and therefore the size of the beads, can be controlled by the amount of TEOS added to the reaction (Kim et al., 2007), allowing a precise adjustment depending on the respective downstream application. The provided standard coating protocol yields silica-coated beads with an average diameter of approximately 400 nm (Figure 1B), which perform well for a wide range of nucleic acid purification and manipulation experiments from bacteria, mammalian cells, tissues (Figure 2) and many more (Oberacker et al., 2019).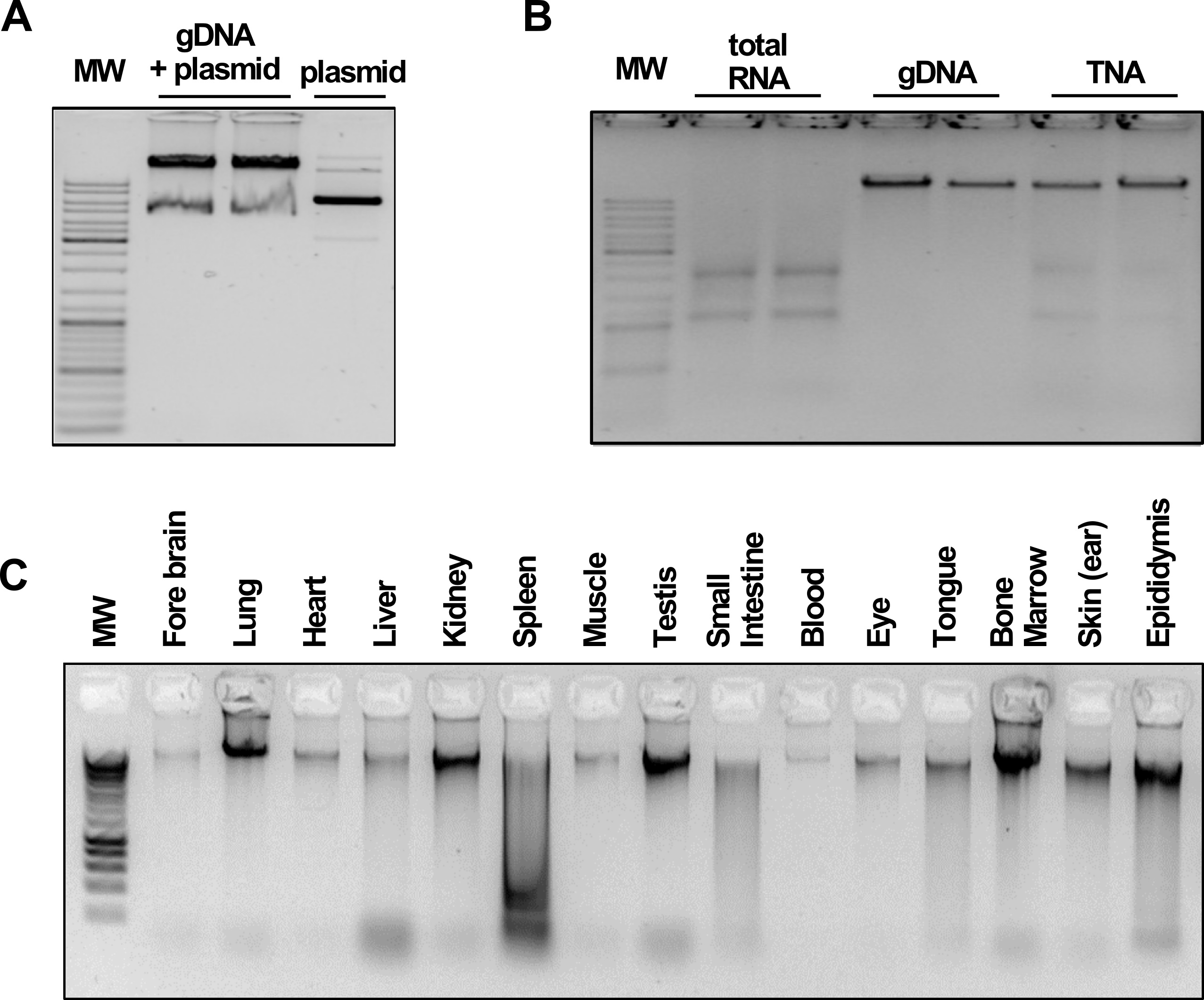
Figure 2. BOMB nucleic acid extractions. Data and figures are taken from Oberacker et al. (2019). A. Total DNA and plasmid extracted from E. coil. MW: GeneRuler DNA Ladder Mix (Thermo). B. TNA, DNA and RNA isolation from HEK293 cells. MW: GeneRuler DNA Ladder Mix (Thermo). C. gDNA isolated from various rabbit (O. cuniculus) tissues. MW: Hyperladder I (Bioline).
As an alternative to a silica-coat around the ferrite particles, a layer of a carboxyl-modified polymer can provide stability as well as a surface with a weak negative charge, thus altering the electrostatic interaction with nucleic acids and consequently the functionality of the beads (Figure 1C). Here, we use methacrylic acid (MAA) monomers, which form a layer of polymethacrylic acid (PMAA) around the ferrite core particles during a free-radical retrograde precipitation polymerization reaction (Aggarwal et al., 1996). Potentially, carboxyl- and silica-surfaces can be further modified to enable a broad range of additional functionalities to the paramagnetic beads.
Materials and Reagents
- 60 ml syringe (HENKE SASS WOLF, catalog number: 8300006680)
- 0.2 µm sterile filter (Sarstedt, catalog number: 83.1826.001)
- Protective clothing (lab coat, eye protection, gloves)
- Stand with boss head sockets and retort clamps (Bochem, catalog numbers: 5000, 5300, 5401)
- 250 ml dripping funnel (Buhler, NS29/32)
- Heat resistant bowl for a water bath
- Heat resistant 2.5 L bottle
- 0.5 or 1 L round bottom flask (Schott Duran, NS 29/32, catalog number: 2172154)
- Sterile plastic 0.5 L bottles (Corning® Costar®, Nunc® T175 uncoated PS flask)
- 50 ml conical centrifuge tube (Sarstedt, catalog number: 62.547.004)
- Ammonia solution (25% NH4OH) (EMD Millipore, catalog number: 1.05432)
- Ethanol (99% C2H6O) (Riedel-de Haёn, catalog number: 34963) or lower grade
- Hydrochloric acid (37% HCl fuming) (Roth Chemicals, catalog number: 4625.1)
- Iron(II) chloride 4-hydrate (≥ 99% FeCl2•4H2O) (Honeywell/Fluka, catalog number: 44939)
- Iron(III) chloride (≥ 98.5% FeCl3) (Roth Chemicals, catalog number: 5192.3)
- Methacrylic acid (≥ 99% C4H6O2) (Merck, catalog number: 155721)
- Potassium persulfate (≥ 99% K2S2O8) (Merck, catalog number: 216224)
- Sodium dodecyl sulfate (NaC12H25SO4) (Merck, catalog number: L3771-100G)
- Sodium hydroxide (≥ 99% NaOH) (Roth Chemicals, catalog number: 6771.1)
- Tetraethyl orthosilicate (≥ 99% Si(OC2H5)4) (Merck, catalog number: 86578)
- 2 M NaOH (see Recipes)
- 0.1 M HCl (see Recipes)
- Fe2/3 solution (see Recipes)
Note: Please consult appropriate MSDS information before working with these chemicals! Use a lab coat, gloves and eye protection at all times! The chemicals are available from other providers as well. No preference is given to the indicated vendors.
Equipment
- Fume hood
- Vacuum filtration systems (Corning, catalog number: UD-29530-02)
- Magnetic stirrer with heating (Heidolph, catalog number: 504-10000-00)
- Magnetic stir bars
- Strong neodymium permanent magnet (e.g., Neodymium disc magnet 80 x 10 mm grade N45 adhesive force ~300 kg–be extremely careful when using such strong magnets!)
Procedure
All of the following written protocols are also provided as a flowchart (Figure 3).
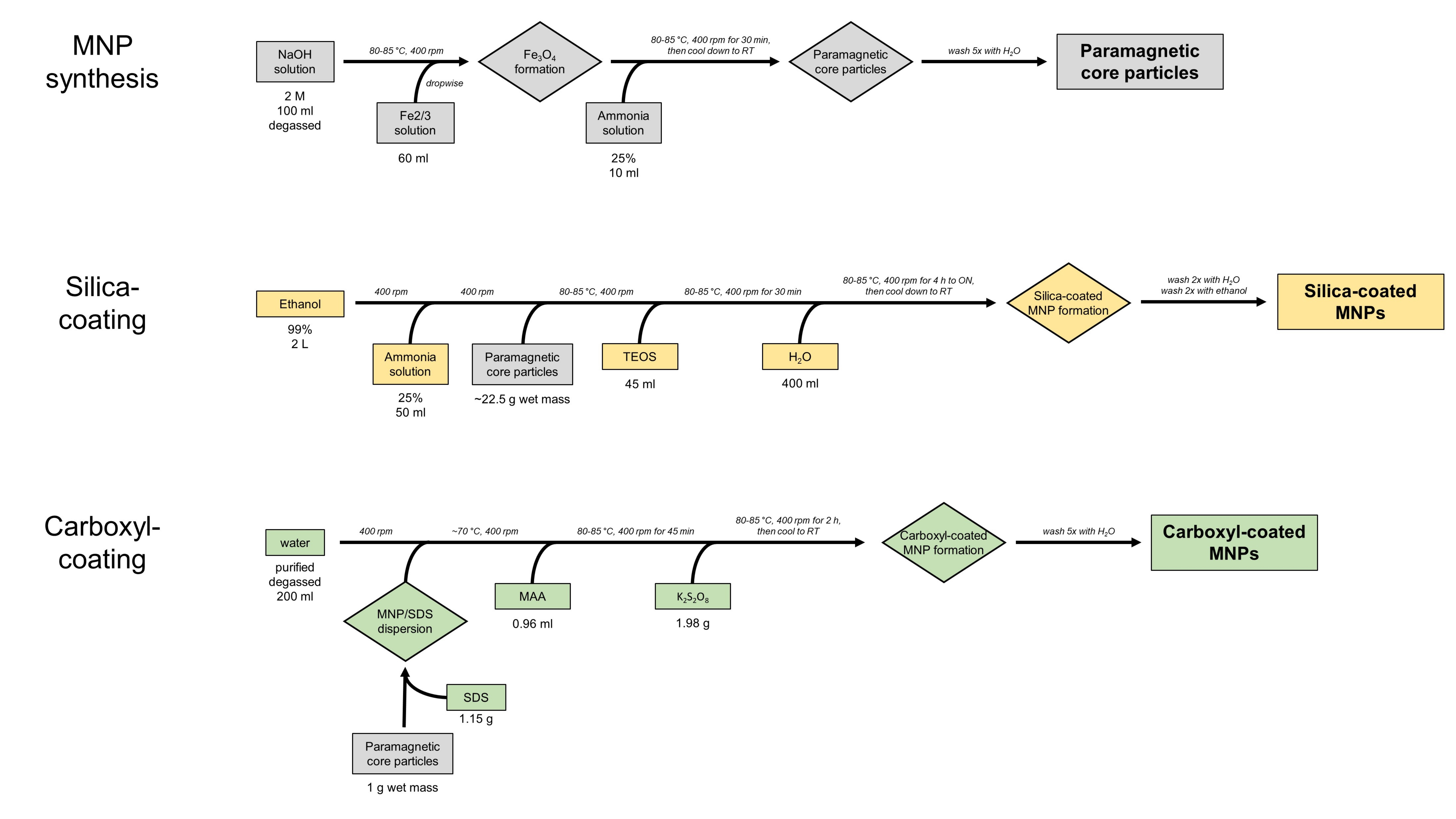
Figure 3. BOMB MNP protocols. Protocol workflow for synthesis of MNP core particles (grey) and their coating with either silica (yellow) or carboxyl (green).
- BOMB synthesis of MNPs
Note: All procedures can be performed under inert argon atmosphere (alternatively purging the system with high purity N2 for 30 min should be sufficient. We generally perform the reaction at atmospheric oxygen levels, however degas and heat up the solutions before use).- Degas the NaOH solution by filtering it with a vacuum filtering system–keep the vacuum running for 1 h while magnetically stirred.
- Assemble the round bottom flask, dripping funnel and water bath according to Figure 1A and place on a magnetic stirrer. Add 100 ml of degassed 2 M NaOH to the collection flask on the bottom and the Fe2/3 solution to the dripping funnel. Heat up the flask containing the 2 M NaOH solution to 80-85 °C.
Note: Using freshly made Fe2/3 solution with the correct iron salts is crucial for the success of the reaction. Otherwise, the forming iron precipitate might lead to lower stability of MNPs as can be observed during washing steps or storing the beads. - Add 60 ml Fe2/3 solution drop wise into 100 ml of preheated (80-85 °C) 2 M NaOH solution while stirring vigorously (> 400 rpm).
Note: A black precipitate of Fe3O4 (FeO•Fe2O3) is formed. - After the complete Fe2/3 solution was added, add 10 ml of 25% ammonia solution to the reaction mixture. Stir for another 30 min and then cool to RT.
- Transfer the solution to a sterile plastic container and magnetically pellet the black precipitate (MNPs), discard the supernatant.
Note: Ammonia containing trash needs to be discarded appropriately. - Resuspend in 200 ml of ddH2O, pellet magnetically and discard supernatant. Repeat for a total of 5 washes (or until the pH of the wash reaches ~7).
- After the last wash, resuspend the MNPs in 30-40 ml of ddH2O and transfer to a fresh 50 ml tube.
Note: To measure the synthesis yield, magnetically pellet the MNPs, remove the water and weigh the product wet-mass. Alternatively, flash freeze the pelleted MNPs in liquid nitrogen and lyophilize and measure the dry mass. Lyophilised MNPs in N2 or argon atmosphere can be stored in a closed container at RT for a longer time. The wet MNPs slowly oxidize over time; therefore, we recommend coating them within a week.
- BOMB Silica-coating
Note: All procedures can be performed under inert N2 atmosphere or atmospheric oxygen conditions. The protocol will work in both cases, however, the formation of brown precipitate (iron oxide) can be reduced or eliminated when working under N2 atmosphere and therefore, the stability of the uncoated beads can be increased.- Mix 2 L of 99% ethanol with 50 ml of 25% ammonia solution and ~22.5 g (wet mass) of the synthesized iron oxide MNPs (A) in a heat resistant 2.5 L bottle using a magnetic stirrer (300-400 rpm). Switch on the heating and allow the solution to heat up to ~80 °C.
Note: It is very important that the core particles are efficiently distributed in the solution. Otherwise, large clusters of MNPs are coated with silica, leading to a decreased performance of the beads in nucleic acid capture applications. - Add 45 ml tetraethyl orthosilicate (TEOS) with constant stirring and incubate for another 30 min.
Note: The size of the particles can be controlled using different ratios of core particles and TEOS. The standard ratio used in the above protocol is: 1 g of paramagnetic core particles to 2 ml TEOS, which results in particles with an average size of ~400 nm. Generally, more TEOS yields larger beads. - Add 400 ml of ddH2O to the solution.
- Allow the reaction to proceed for > 4 h (ideally overnight).
Note: TEOS hydrolyzes spontaneously in water which will result in white silica precipitates which do not contain a paramagnetic core. If large amounts of this precipitate forms, use fresh TEOS for the reaction. - Cool the solution to RT.
- Separate the coated MNPs using a strong neodymium magnet.
Note: Uncoated MNPs get slowly oxidized over time which is indicated by a brown color of the supernatant after magnetic separation. - Wash twice with pure water.
- Wash twice with pure ethanol.
- Wash with pure water until the pH of the solution becomes neutral (3-4 times).
Note: To measure the synthesis yield, magnetically pellet the silica beads, remove the water and weigh the product wet mass. The coated beads can be stored at RT for at least 1 year.
- Mix 2 L of 99% ethanol with 50 ml of 25% ammonia solution and ~22.5 g (wet mass) of the synthesized iron oxide MNPs (A) in a heat resistant 2.5 L bottle using a magnetic stirrer (300-400 rpm). Switch on the heating and allow the solution to heat up to ~80 °C.
- BOMB carboxyl-coating
Note: All procedures can be performed under inert N2 atmosphere or atmospheric oxygen conditions. The protocol will work in both cases, however, the formation of brown precipitate (iron oxide) can be reduced or eliminated when working under N2 atmosphere and therefore, the stability of the uncoated beads can be increased.- Mix 1 g (wet mass) of the synthesized iron oxide MNPs (A) dispersed in 45 ml of water and 1.15 g sodium dodecyl sulfate with 200 ml of purified, degassed water in a round bottom flask using a magnetic stirrer (300-400 rpm). Switch on the heating and allow the solution to heat up to ~70 °C.
- Add 0.96 ml of methacrylic acid (MAA) into the flask.
Note: The pH drops to about 3. - Equilibrate the reaction mixture for about 45 min while keeping the temperature.
- Add 1.98 g of the initiator potassium persulfate to the solution.
- Let the polymerization reaction progress at ~70 °C for 2 h.
- Cool the solution to RT.
- Separate the coated MNPs from the free MAA and PMAA by concentrating the MNPs with a strong neodymium magnet and discarding the supernatant.
Note: Uncoated MNPs get slowly oxidized over time which is indicated by a brown color of the supernatant after magnetic separation. - Disperse the isolated paramagnetic nanoparticles in deionized water (e.g., in an ultrasonic bath), followed by magnetic extraction as described in Step C7.
- Wash the beads with ddH2O at least 5 times or until the detergent is completely removed and disperse them in 250 ml of ddH2O.
Note: To measure the synthesis yield, magnetically pellet the silica beads, remove the water and weigh the product wet mass. The coated beads can be stored at RT for at least 1 year.
- Benchmarking the BOMB paramagnetic beads
The success of the core particle synthesis, as well as the coating reactions, can be monitored by electron microscopy (TEM, SEM), as well as to a limited extent with light microscopy (LM). The resulting microscopy pictures are displayed in Figures 1C, 1D and 1E. A successful synthesis should yield predominantly single spherical beads which display paramagnetic properties and cluster when exposed to a strong magnetic field. It is also possible to assess further properties of the beads using X-ray Powder Diffraction (XRD), if this information is needed for the respective application. However, for standard applications such as nucleic acid extraction this is not necessary.
We also recommend benchmarking the performance of the beads from each synthesis, and testing them if stored for a longer time. For this, we suggest titring the amount of beads and/or binding buffer in a clean-up of a DNA standard, as described in detail in BOMB protocol #4.1 (https://bomb.bio/protocols). Furthermore, we recommend comparing the results for each application to available commercial setups. A protocol on how to do this can also be found in BOMB protocol #4.1. - Nucleic acid purification with the BOMB paramagnetic beads
The synthesized beads have been tested extensively for a great variety of previously published protocols (Oberacker et al., 2019). Here we display exemplary results for the extraction of nucleic acids from E. coli (Figure 2A), HEK293 cells (Figure 2B) and various mammalian tissues (Figure 2C) using the silica-beads described above. All protocols are also publicly available on the BOMB.bio homepage (https://bomb.bio/protocols).
Data analysis
All data underlying the results shown above can be found in the supporting information of the original paper (Oberacker et al., 2019) in PLOS Biology.
Recipes
- 2 M NaOH
Dissolve in water
Can be stored at RT for at least 12 months - 0.1 M HCl
Dilute in water from stock solution
Can be stored at RT for at least 12 months - Fe2/3 solution (freshly prepared)
3.24 g FeCl3 (0.333 M)
2.00 g FeCl2•4H2O (0.167 M)
Add 60 ml 0.1 M HCl and dissolve by magnetic stirring, sterile filter with a 0.2 µm syringe filter using a 60 ml syringe
Acknowledgments
The authors would like to thank all contributors of the initial publication for their contributions to the BOMB project and the development of protocols employing paramagnetic beads for nucleic acid purifications. We would also like to thank the wider research community for offering unpublished information and resources concerning paramagnetic bead preparation and utility.
Author’s contributions: The idea was conceived by TPJ and TH. Synthesis protocol setup and optimization were done by TPJ. Nucleic acid extraction data as well as light microscopy analysis were provided by PS, DB and PO. The electron microscope analysis was done by KH. The manuscript draft was written by PO, TPJ and TH. All authors contributed to the editing of the manuscript and approved its final version.
Competing interests
The authors declare no conflict of interest.
References
- Aggarwal, A., Saxena, R., Wang, B. and Caneba, G. T. (1996). Studies of the polymerization of methacrylic acid via free-radical retrograde precipitation polymerization process. J Appl Polym Sci 62(12): 2039-2051.
- Choi, J., Kim, J. C., Lee, Y. B., Kim, I. S., Park, Y. K. and Hur, N. H. (2007). Fabrication of silica-coated magnetic nanoparticles with highly photoluminescent lanthanide probes. Chem Commun (Camb)(16): 1644-1646.
- DeAngelis, M. M., Wang, D. G. and Hawkins, T. L. (1995). Solid-phase reversible immobilization for the isolation of PCR products. Nucleic Acids Res 23(22): 4742-4743.
- Hawkins, T. L., O'Connor-Morin, T., Roy, A. and Santillan, C. (1994). DNA purification and isolation using a solid-phase. Nucleic Acids Res 22(21): 4543-4544.
- Houshiar, M., Zebhi, F., Jafari, Z., Alidoust, A. and Askari, Z. (2014). Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods : A comparison study of size, structural, and magnetic properties. J Magn Magn Mater 371: 43-48
- Kim, J. W., Kim, L. U. and Kim, C. K. (2007). Size control of silica nanoparticles and their surface treatment for fabrication of dental nanocomposites. Biomacromolecules 8(1): 215-222.
- Majidi, S., Sehrig, F. Z., Farkhani, S. M., Goloujeh, M. S. and Akbarzadeh, A. (2016). Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed Biotechnol 44(2): 722-734.
- Oberacker, P., Stepper, P., Bond, D. M., Hohn, S., Focken, J., Meyer, V., Schelle, L., Sugrue, V. J., Jeunen, G. J., Moser, T., Hore, S. R., von Meyenn, F., Hipp, K., Hore, T. A. and Jurkowski, T. P. (2019). Bio-On-Magnetic-Beads (BOMB): Open platform for high-throughput nucleic acid extraction and manipulation. PLoS Biol 17(1): e3000107.
- Rohland, N. and Reich, D. (2012). Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res 22(5): 939-946.
- Singh, D., McMillan, J. M., Liu, X. M., Vishwasrao, H. M., Kabanov, A. V., Sokolsky-Papkov, M. and Gendelman, H. E. (2014). Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues. Nanomedicine (Lond) 9(3): 469-485.
- Stöber, W., Fink, A. and Bohn, E. (1968). Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interf Sci 26(1): 62-69.
- Wu, W., Wu, Z., Yu, T., Jiang, C. and Kim, W. S. (2015). Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16(2): 023501.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Oberacker, P., Stepper, P., Bond, D., Hipp, K., Hore, T. A. and Jurkowski, T. P. (2019). Simple Synthesis of Functionalized Paramagnetic Beads for Nucleic Acid Purification and Manipulation. Bio-protocol 9(20): e3394. DOI: 10.21769/BioProtoc.3394.
Category
Molecular Biology > DNA > DNA extraction
Molecular Biology > RNA > RNA extraction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link