- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Imaging the Vasculature of Immunodeficient Mice Using Positron Emission Tomography/Computed Tomography (PET/CT) and 18F-fluorodeoxyglucose Labeled Human Erythrocytes
Published: Vol 9, Iss 19, Oct 5, 2019 DOI: 10.21769/BioProtoc.3391 Views: 5329
Reviewed by: Zinan ZhouAli Asghar KermaniFarah Haque

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Rat Aortic Ring Model to Assay Angiogenesis ex vivo
Isabelle Ernens [...] Daniel R. Wagner
Oct 20, 2015 11713 Views
Abstract
Nuclear blood pool imaging using radiolabeled red blood cells has been used in the clinical setting for the evaluation of a number of medical conditions including gastrointestinal hemorrhage, impaired cardiac contractility, and altered cerebrovascular blood flow. Nuclear blood pool imaging is typically performed using Technetium-99m-labeled (99mTc) human erythrocytes (i.e., the “tagged RBC” scan) and gamma camera-based planar scintigraphic imaging. When compared to typical clinical planar scintigraphy and single-photon emission computed tomographic (SPECT) imaging platforms, positron emission tomography (PET) provides superior image quality and sensitivity. A number of PET-based radionuclide agents have been proposed for blood pool imaging, but none have yet to be used widely in the clinical setting. In this protocol, we described a simple and fast procedure for imaging the vasculature of immunodeficient mice through a combination of a small animal positron emission tomography/computed tomography (PET/CT) scanner and human erythrocytes labeled with the PET tracer 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG). This technique is expected to have significant advantages over traditional 99mTc -labeled erythrocyte scintigraphic nuclear imaging for these reasons.
Keywords: Blood pool imagingBackground
Gamma camera-based imaging of radiolabeled erythrocyte is often used for visualizing the blood pool in nuclear medicine. For example, 99mTc-labeled human erythrocytes and 99mTc-labeled human serum albumin blood pool imaging with planar scintigraphy and single photon emission computed tomography (SPECT) have been used clinically to evaluate cardiac function (Thrall et al., 1978; Hacker et al., 2006; Mohseni et al., 2015), gastrointestinal bleeding (Sadri et al., 2015), vascular diseases (Liu et al., 2017), and orbital cavernous hemangioma (OCH) (Dong et al., 2017). As PET scanners have superior nuclear tracer sensitivity and image resolution compared to clinical gamma-camera scintigraphy and SPECT scanners (Rahmim and Zaidi, 2008), development of a robust PET-based blood pool imaging method is of significant clinical interest. Various PET tracers have been proposed as blood pool imaging agents, but suffer from certain limitations. For example, 15-oxygen-labeled (15O) H215O, 13-nitrogen-labeled (13N) 13NH3, 11-carbon-labeled (11C) 11C-Acetate and 82-rubidium chloride (82Rb) have been used to investigate myocardial blood flow, but are not widely used clinically, as the short radioactive half-life of 15O (122 seconds), 13N (9.97 minutes), 82Rb (76 seconds), and 11C (= 20.334 minutes) require the presence of a cyclotron in close proximity to the PET scanner or to an 82Rb generator. (Herrero et al., 2006; Nakazato et al., 2013; Lee et al., 2017). As the positron emitter 18-fluorine (18F) has a tracer half-life (108 minutes) well-suited for clinical imaging, 18F-based tracers are considered attractive for PET imaging, especially given the wide spread prevalence of FDG-specific PET imaging for oncology patients, as well as other 18F-based agents, such as 18F-fluorothymidine (18F-FLT) used for visualizing tumor proliferation (Cysouw et al., 2017).
In this protocol, we describe the method for labeling human erythrocytes with FDG and washing the FDG-labeled erythrocytes. We also describe the injection of labeled erythrocytes into NSGTM immunodeficient mice and subsequent imaging of the mouse vasculature using a small animal microPET/CT platform. Given that the erythrocyte labeling and washing procedure is straightforward, and that the FDG leakage rate from labeled erythrocytes is relatively slow (~10% in 46 min), we believe this method allows for robust PET imaging of the mouse vasculature. We believe that this imaging technique would be of interest to investigators seeking to visualize the vasculature of immunodeficient mice for other applications. In addition, we speculate that this technique would offer significant advantages over 99mTc-based nuclear blood pool imaging for the evaluation of occult gastrointestinal bleeding in patients, and may be useful for evaluating other clinical pathologies, including those involving the cerebral and myocardial vasculature.
Materials and Reagents
- 1.8 ml cryovial tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 343958)
- 3.5 ml cryovial tube (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 343958)
- 15 ml conical tube (Corning, catalog number: 430790)
- 400-600 µl Lithium Heparin tube (BD. Microtainer® catalog number: 365985)
- 1 ml sterile syringe (NIPRO Medical Corporation, catalog number: JD+01D2238)
- 10 cc BD Luer-LokTM disposable syringe (Thermo Fisher Scientific, catalog number: BD 309604)
- 15 ml FalconTM conical centrifuge tube (Thermo Fisher Scientific, catalog number: 14-959-53A)
- 10 ml and 25 ml FisherbrandTM Sterile Polystyrene Disposable Serological Pipets (Thermo Fisher Scientific, catalog numbers: 13-676-10F and 13-676-10M)
- 22 gauge needle (BDTM Needle 1, BD, catalog number: 305155)
- 26 gauge x ¾” mouse and rat tail vein Monoject IV catheter (Patterson Veterinary, catalog number: 07-836-8403)
- ParafilmTM M PM996 all-purpose laboratory film
- 200 µl and 1,000 µl TipOne sterile filter pipet tips (USA Scientific, catalog numbers: 1120-8810 and 1122-1830)
- 0.2 µm Nalgene® bottle top sterile filter unit (Sigma-Aldrich, catalog number: Z370576-12EA)
- 4-8 week old male splenectomized NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSGTM) immunodeficient mice (The Jackson Laboratory, stock number: 005557)
- 10 ml of human whole blood in standard ACD anticoagulant solution shipped next day (Zen-Bio, Inc, SER-WB10ML-SDS)
- 1 ml of 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) with specific activity calibrated at a minimum of 20 milliCuries/ml at the time of cell labeling (United States Pharmacopeia (USP) grade, Cardinal Health nuclear pharmacy)
- 0.9% Sodium Chloride Injection USP, 100 ml Fill in 150 ml PAB® (B. Braun, NDC number: 00264-1800-32)
- 250 ml bottle of IsoThesia (Isoflurane) solution (Henry Schein Animal Health, catalog number: 029405)
- Sterile de-ionized water
- NaCl (Sigma-Aldrich, catalog number: S7653-1KG)
- KCl (Sigma-Aldrich, catalog number: P9333-1KG)
- K2EDTA dihydrate (Sigma-Aldrich, catalog number: 03660-1KG)
- Heparin sodium salt (Sigma-Aldrich, catalog number: 375095-100KU)
- Phosphate Buffered Saline (PBS) solution, pH 7.4 (Thermo Fisher Scientific, catalog number: 10010023)
- Filter sterilized 100 ml 5x EDTA solution (see Recipes)
- 100ml 1x EDTA solution (see Recipes)
- 1 Unit/ml of heparin-PBS solution (see Recipes)
Equipment
- Inveon PET/CT (Siemens Medical Inc., INVEN, catalog number: 138757)
- Centrifuge 5810 R (Eppendorf, catalog number: 108308)
- Centrifuge 5810 R (Eppendorf, catalog number: 119548)
- 200 µl single channel manual pipetter
- 1,000 µl single channel manual pipetter
- AtomLabTM 500 dose calibrator (Biodex Medical Systems, Inc)
- Tissue culture incubator (Sanyo Scientific, catalog number: 133060)
- Biological safety cabinet (The Baker company, SterilGARD, catalog number: 101951)
- Rotator platform (Labnet International, Inc GYROMINI)
- Blood Glucose Test Strips (TrueTrack from @Nitro Diagnostics TM (SN 7688648), Code 4698, Lot RS4698)
- BioVet® (m2m Imaging) physiological monitoring and heating system (m2m imaging Corp. USA. http://www.m2mimaging.com/)
Software
- Microsoft Excel program (Microsoft Excel 2010)
- Inveon PET/CT small animal imaging platform (Siemens Medical Inc., Knoxville, Tennessee)
- Inveon Workstation Software (Siemens Medical Inc., Knoxville, Tennessee)
- BioVet® (m2m Imaging) physiological monitoring and heating system (m2m imaging Corp. USA. www.m2mimaging.com)
Procedure
- Human red blood cell (RBC) preparation
- Gently pipet 10 cc’s of whole human blood in standard anticoagulant citrate dextrose (ACD) solution (overnight shipping from Zen-Bio, Inc) into a sterile 15 ml conical centrifuge tube with a 10 ml sterile disposable pipet. The original blood sample should have a good quality shown in Figure 1. Blood should be slowly pipetted to minimize mechanical trauma/shear injury to erythrocytes.
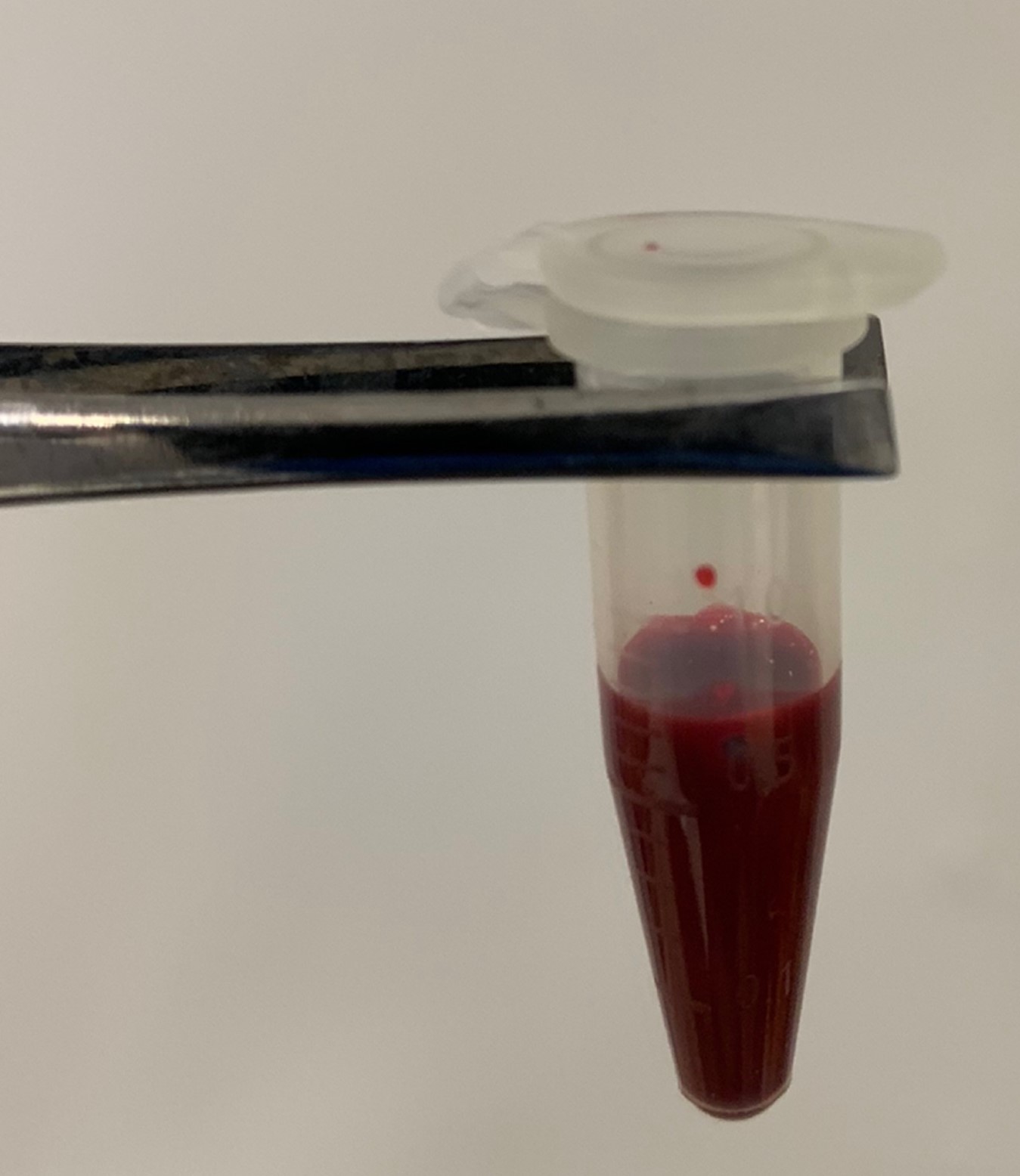
Figure 1. Original anti-coagulated blood. The original anti-coagulated blood sample should have no clot and have good fluidity. - Centrifuge blood at 1,000 x g (RCF) for 10 min in a desktop centrifuge (no brake) at room temperature.
- Slowly remove nearly all of the plasma from the red blood cell (RBC) fraction using a 10 ml sterile pipet. The plasma should be close to straw colored or have a slightly reddish tinge as Figure 2. Plasma that has a frankly turbid or significant reddish coloration implies either improper centrifugation or significant pre-existing hemolysis of the original blood sample that may render the sample unsuitable for radiotracer labeling. Slowly transfer 2 ml of pelleted RBCs into a 15 ml conical centrifuge tube using 10 ml sterile pipet. Residual plasma and buffy coat should be avoided during erythrocyte aspiration. Add 8 ml of 1x EDTA solution to RBCs. Close cap on tube and gently invert tube several times to wash cells.

Figure 2. The original blood after first centrifuge step. Ideally, the plasma should be close to straw colored or have a slightly reddish tinge. - Centrifuge at 1,000 x g for 10 min at room temperature, then slowly aspirate the supernatant which shown in Figure 3 with a 10 ml sterile pipet. Rapid supernatant aspiration will disturb the supernatant/RBC interface and result in aspiration of RBCs.

Figure 3. The RBCs after washing with 4x volume of 1x EDTA solution - Transfer 250 µl of RBCs with 1,000 µl pipet tip into 1.8 ml cryovials (Labeled “1” and “2”). Care should be taken to avoid pipetting any residual supernatant.
- To each RBC cryovial, add 100 µl 5x EDTA solution and 50 µl sterile de-ionized water (total volume = 400 µl) via manual pipette.
- Gently pipet 10 cc’s of whole human blood in standard anticoagulant citrate dextrose (ACD) solution (overnight shipping from Zen-Bio, Inc) into a sterile 15 ml conical centrifuge tube with a 10 ml sterile disposable pipet. The original blood sample should have a good quality shown in Figure 1. Blood should be slowly pipetted to minimize mechanical trauma/shear injury to erythrocytes.
- 18F-FDG labeling of human erythrocytes
- To maximize the radiolabeling efficiency of human RBCs for in vivo PET imaging of the vasculature of NSGTM immunodeficient mice, the specific activity of USP grade 18F-FDG should be calibrated to at least 20 milliCurie (mCi) per ml at the start of cell labeling.
Note: (The Jackson Laboratory, stock number: 005557) has been used, due to its severe immunodeficiency. Other immunodeficient mouse strains have not been tested, but may be feasible as well. - Add 100 µl 18F-FDG solution to each RBC cryovial (F.V. = 500 µl) behind appropriate radioactivity shielding. Methods for handling radioactive materials for this protocol should follow those established by the radiation safety officer at your institution. Directly count radioactivity in each cryovial using the AtomLabTM dose calibrator (mCi’s) and record the time. The amount of activity should be at least 1 mCi of FDG in each vial. Ensure the cap is tightly screwed on the cryovial. Care should be taken when choosing an appropriate vial for FDG RBC labeling, as some cryovials are composed of certain plastics that do not maintain a good seal about the cap when exposed to higher temperatures and can result in local radioactivity leakage/contamination. A thin strip of ParafilmTM can be wrapped around the margins of the cap to ensure no radioactivity leakage from the tube and reduce water loss from the cryovial lumen.
- Gently finger resuspend RBCs. Place vials in an upright position in a tube rack tied/taped to a rotator platform as Figure 4. Incubate rotating samples in an upright position at 37 °C for 30 min at ≤ 60 RPM. Avoid inverting tube during rotation, as this likely increases the amount of dried cells adherent to the tube wall.

Figure 4. RBCs labeled with 100 µl 18F-FDG solution on rotator platform and placed in an upright position in the 37 °C incubator - Centrifuge RBC samples at 1,000 x g for 10 min (no brake) at 4 °C. The present sample is shown in Figure 5.
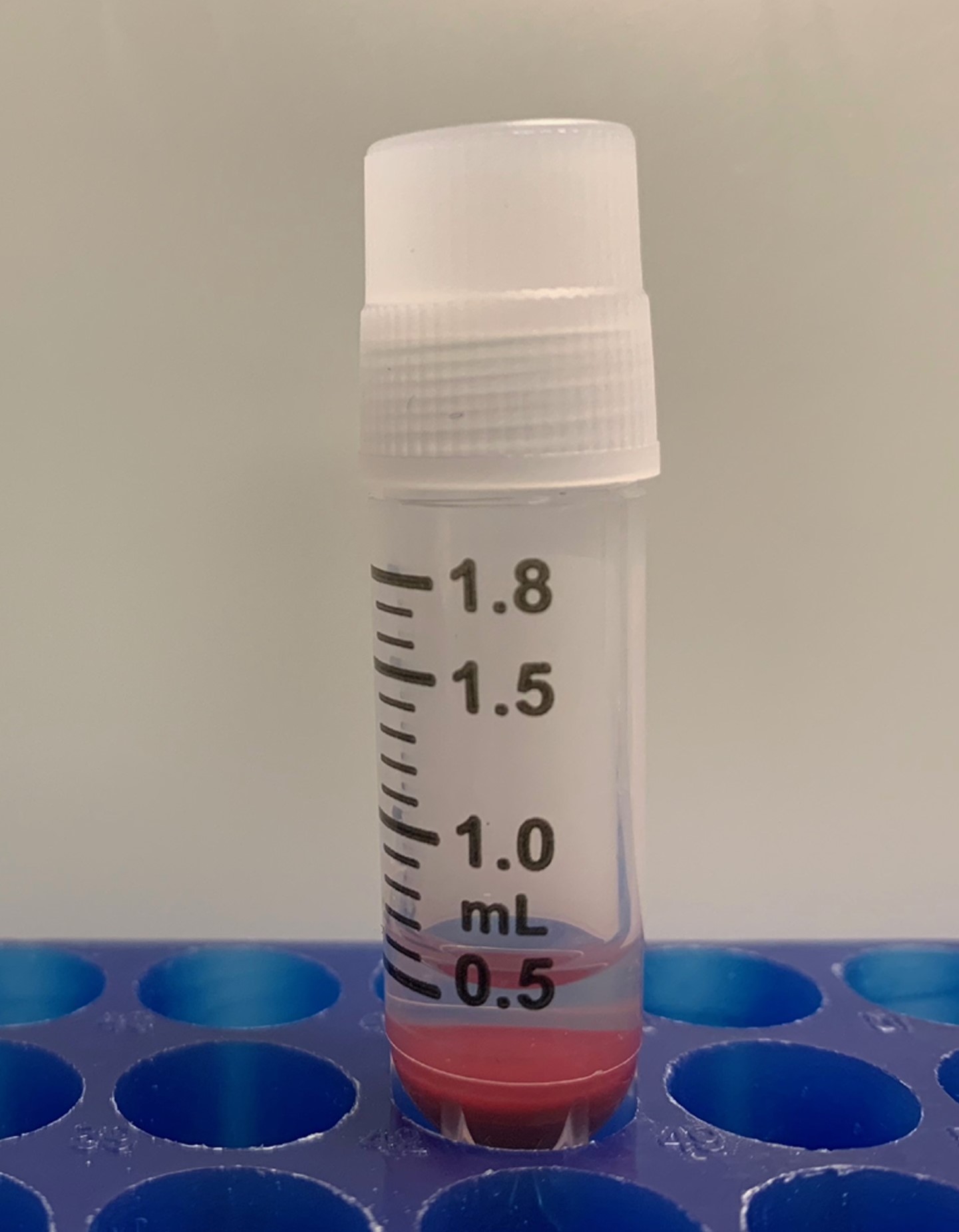
Figure 5. The free 18F-FDG fraction (upper clear half of solution) and the precipitated RBCs fraction (red bottom half of solution) - Place samples in a ventilated laboratory hood behind appropriate radioactivity shielding and gently transfer the supernatant (about 250 µl) to a 3.5 ml cryovial labeled“SUPE 1 and 2”.
- Gently resuspend each 250 µl RBCs cell pellet in 1 ml 1x EDTA solution via manual pipette. Care should be taken to resuspend cells slowly to minimize pipet-induced mechanical damage/shear injury to cells.
- Repeat centrifugation of RBC samples at 1,000 x g for 10 min (no brake) at 4 °C. The centrifuged sample is shown in Figure 6.

Figure 6. Centrifuged 18F-FDG-labeled RBC sample after washing with 1 ml 1x EDTA solution - Transfer 2nd supernatant to an appropriate “SUPE” tube.
- Gently resuspend 250 µl RBCs cell pellets again in 1 ml 1x EDTA solution, as described above.
- Repeat centrifugation of RBC samples at 1,000 x g 10 min (no brake) at 4 °C.
- Transfer supernatant to an appropriate “SUPE” tube. Carefully pipet residual supernatant from the interface with the cell pellet, leaving only a tiny meniscus overlying the pellet.
- Directly count SUPE and RBC pellet cryovials using AtomLab detector via dose calibrator (µCurie) and record time. Typical amounts of intracellular radiotracer activity are limited by the total allowable volume of cell solution (250 µl pelleted RBCs) that can be injected into a mouse per day as per our institutional IACUC guidelines, but usually range from ~150 to 300 µCuries, and can be higher depending upon the chosen 18F-FDG specific activity at the start of cell labeling. Gently resuspend the pellet in 250 µl of 1x EDTA solution. The labeling of two RBC samples allows for titration of a larger volume of labeled RBCs, as needed.
- Transfer the RBC suspension to a 1 ml syringe using a 22 gauge needle. Any residual clumps of cells should not be aspirated, as these likely represent damaged/coagulated cells. Cells should be slowly aspirated into the syringe lumen to minimize mechanical damage/shear injury to cells. The radioactivity in the syringe should be measured before and after animal injection to determine net activity injected in vivo, as there will be residual activity left in the syringe after injection.
- To maximize the radiolabeling efficiency of human RBCs for in vivo PET imaging of the vasculature of NSGTM immunodeficient mice, the specific activity of USP grade 18F-FDG should be calibrated to at least 20 milliCurie (mCi) per ml at the start of cell labeling.
- Small animal PET/CT imaging
- Prepare 4-6 week-old male splenectomized NODSCIDgamma (NSGTM) immunodeficient mice for experiment.
- The mouse can be fasted the night before PET/CT imaging to encourage shifting of mouse metabolic activity from glucose to fatty acids, thus minimizing myocardial uptake of any free FDG in the labeled cell preparation. The mouse can also be placed on a very low carbohydrate diet for this purpose as well, as needed. Draw 500 µl of mouse blood through retro-orbital venous plexus puncture, or other approved venous blood draw at your institution, as seen in the example shown in Figure 7. Blood should be collected into heparinized blood collection tube. If the blood is drawn too slowly, there is a risk of significant coagulation occurring, rendering the blood sample unsuitable for FDG labeling. Measure blood glucose level with a blood glucose monitor for PET calibration.
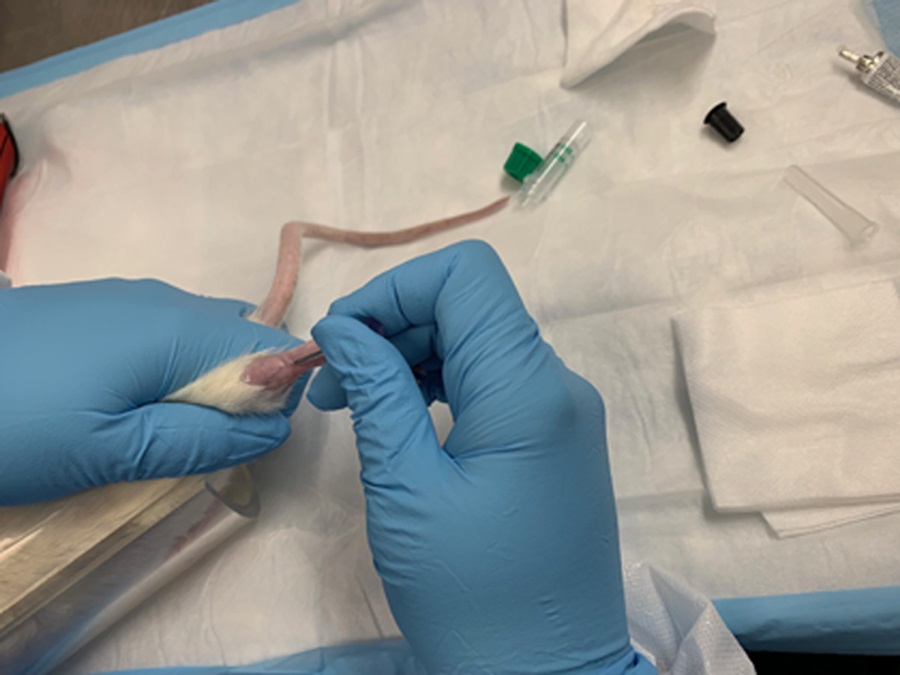
Figure 7. Example of drawing blood through rat leg vein - Anesthetize the mouse via a nosecone manifold under 2-4% inhalational isoflurane. The lowest level of inhalational isoflurane possible is recommended, as isoflurane may induce vasodilation of the mouse vasculature. Record the level of inhalational isoflurane used per mouse.
- Warm the mouse tail with either a warm soaked towel to stimulate dilation of the mouse tail veins. Insert a tail vein micro-catheter into one of the dilated mouse tail veins. Flush tail vein catheter with 1 U/ml heparin-PBS solution. See example shown in Video 1. Video 1. Standard microcatheter insertion into mouse/rat tail (This video was made at University of south Florida, according to guidelines from the University of South Florida on Animal Care and approved by the Animal Research Ethics Board of University of South Florida under protocol IS00004376.)
- Secure the mouse onto the micro-PET/CT scanner bed under 2-4% inhalational isoflurane anesthesia via nose-cone manifold as shown in Figure 8. The animal can be secured to the micro-PET/CT scanner bed by gently wrapping the animal in bubble packaging to minimize animal movement and preserve body warmth. Care must be taken when wrapping the animal, as tight wrapping may impede animal respiratory motion.
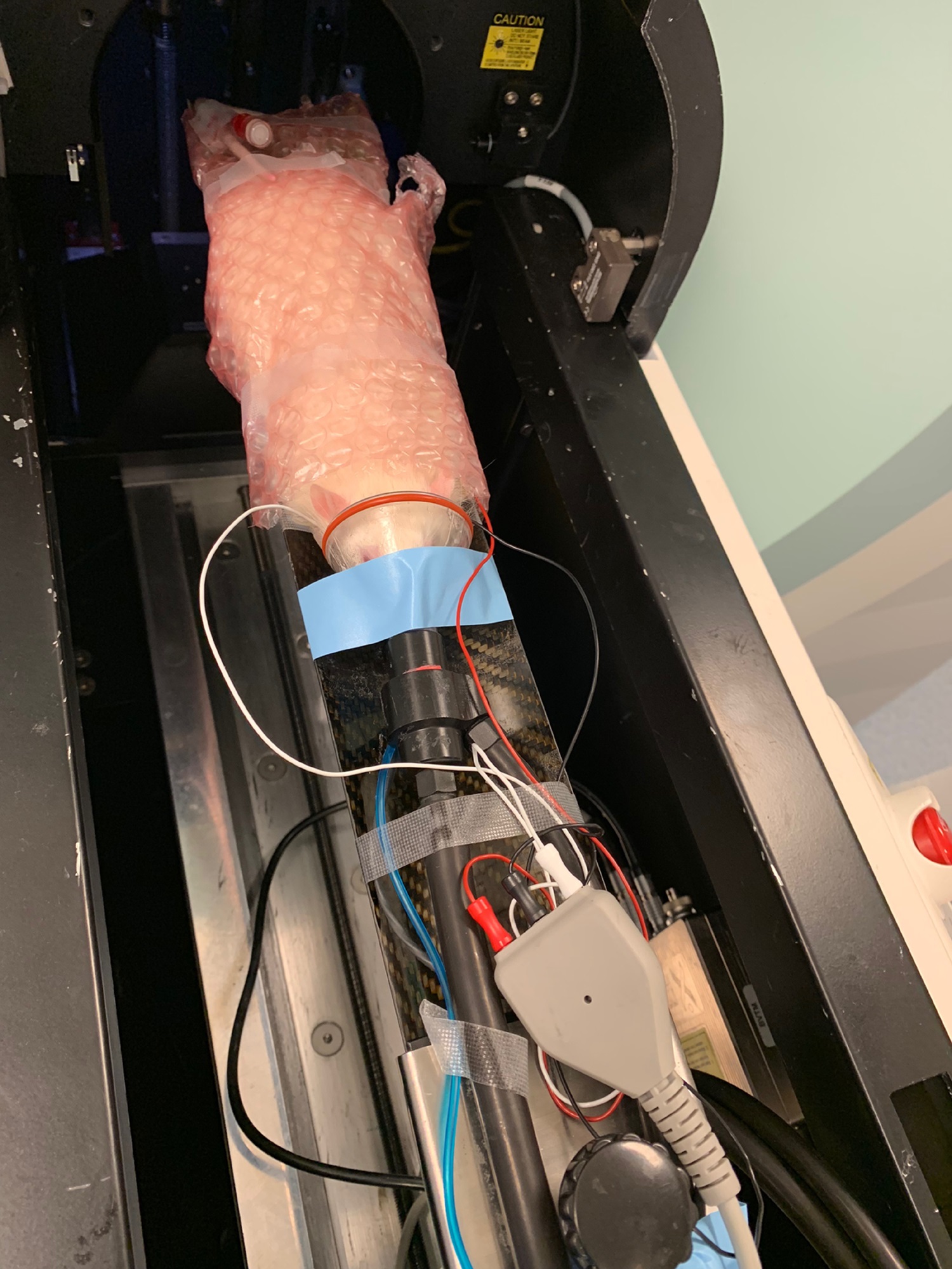
Figure 8. Placement of animal in microPET/CT scanner bed for 18F-FDG RBC imaging of the whole body vasculature - Slowly inject 500 µl of FDG-labeled human erythrocyte suspension into the mouse through the tail vein microcatheter over a course of 1 min to minimize shear injury to cells from passage through the microcatheter lumen. See the example shown in Figure 9.
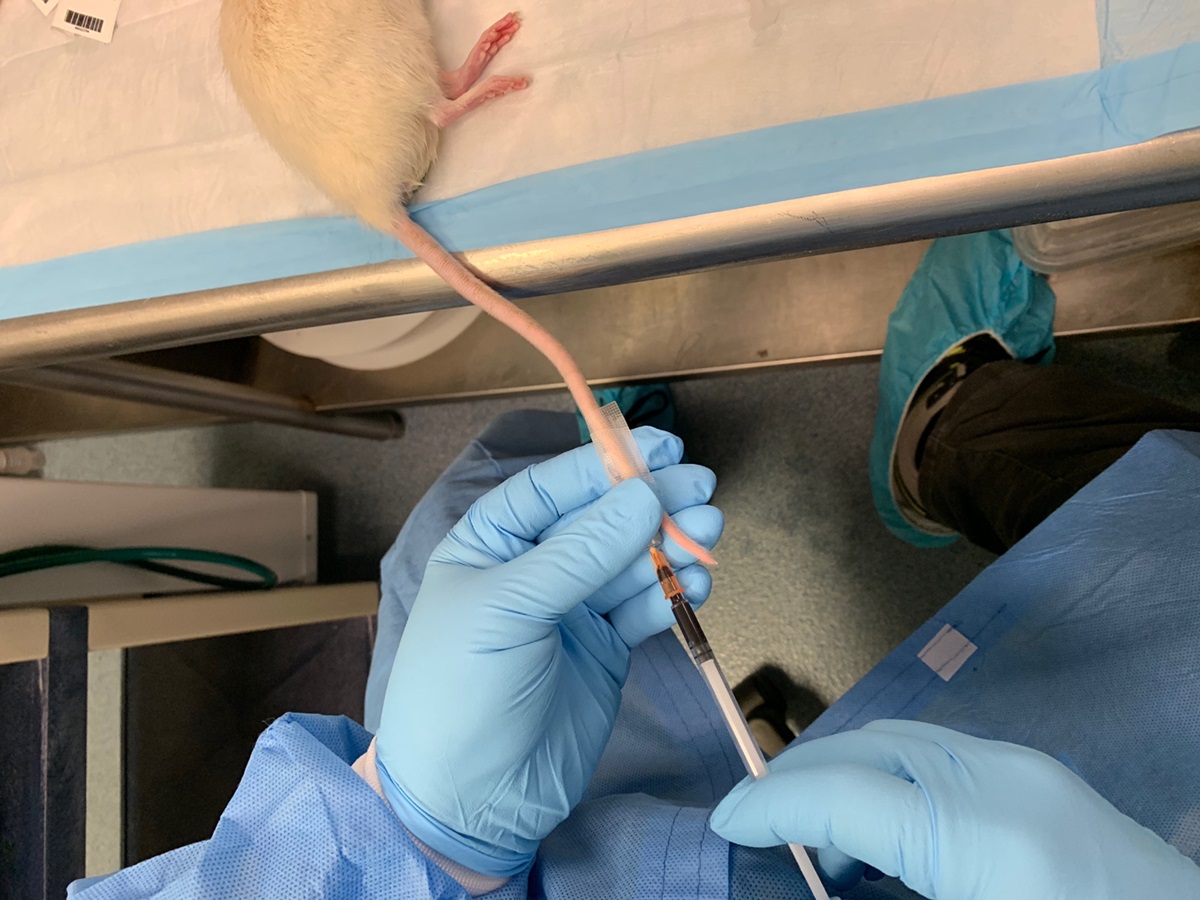
Figure 9. Example of injection of FDG-labeled RBCs through tail vein microcatheter - Acquire ECG-gated whole-body images of the mouse, followed by CT calibration images. PET/CT image acquisition parameters will depend upon the particular experimental indications and unique imaging platform setup at a given institution; as such, the following is a description of the protocol used at our institution.
- Place electrocardiogram (ECG) leads on two front limbs and one hind limb of the mouse (ground lead on a rear leg) for ECG gated PET imaging. The signals detected by these electrodes are recorded during the 10 minute time period by BioVet® (m2m Imaging) physiological monitoring and heating system.
- Set the threshold for TTL cardiac gating signals in a rising mode of R-wave peak.
- Reconstruct the PET list-mode data using 3D-OSEM iterative algorithm with four iterations and eight subsets, with a final image volume of 256 x 256 x 256 voxels. Set effective voxel effective dimensions at 1.4 x 1.4 x 1.4 mm.
- For each animal there are three data sets: standard 3-dimensional (3D) PET reconstruction, resulting in a motion–time average 3D PET image; dynamic 3D PET reconstruction with 30 frames; and the phase-based 4-dimensional (regular 3-dimensional plus time, 4D) PET cardiac reconstruction, with four cardiac gate binning. Please see Figures 10 and 11. In all cases, CT attenuation correction is applied to the PET images (Choi et al., 2019).
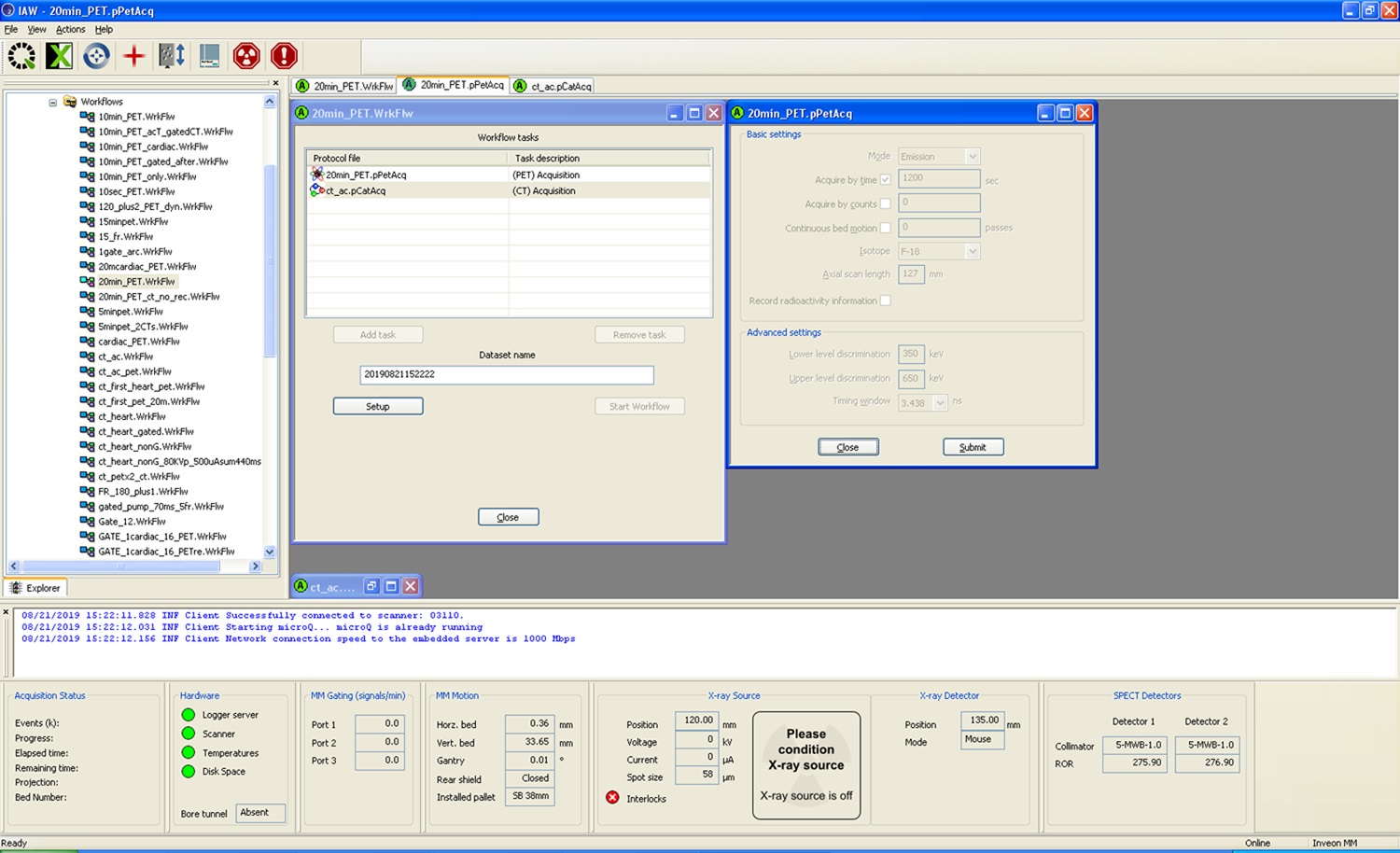
Figure 10. Example for Micro PET acquisition set-up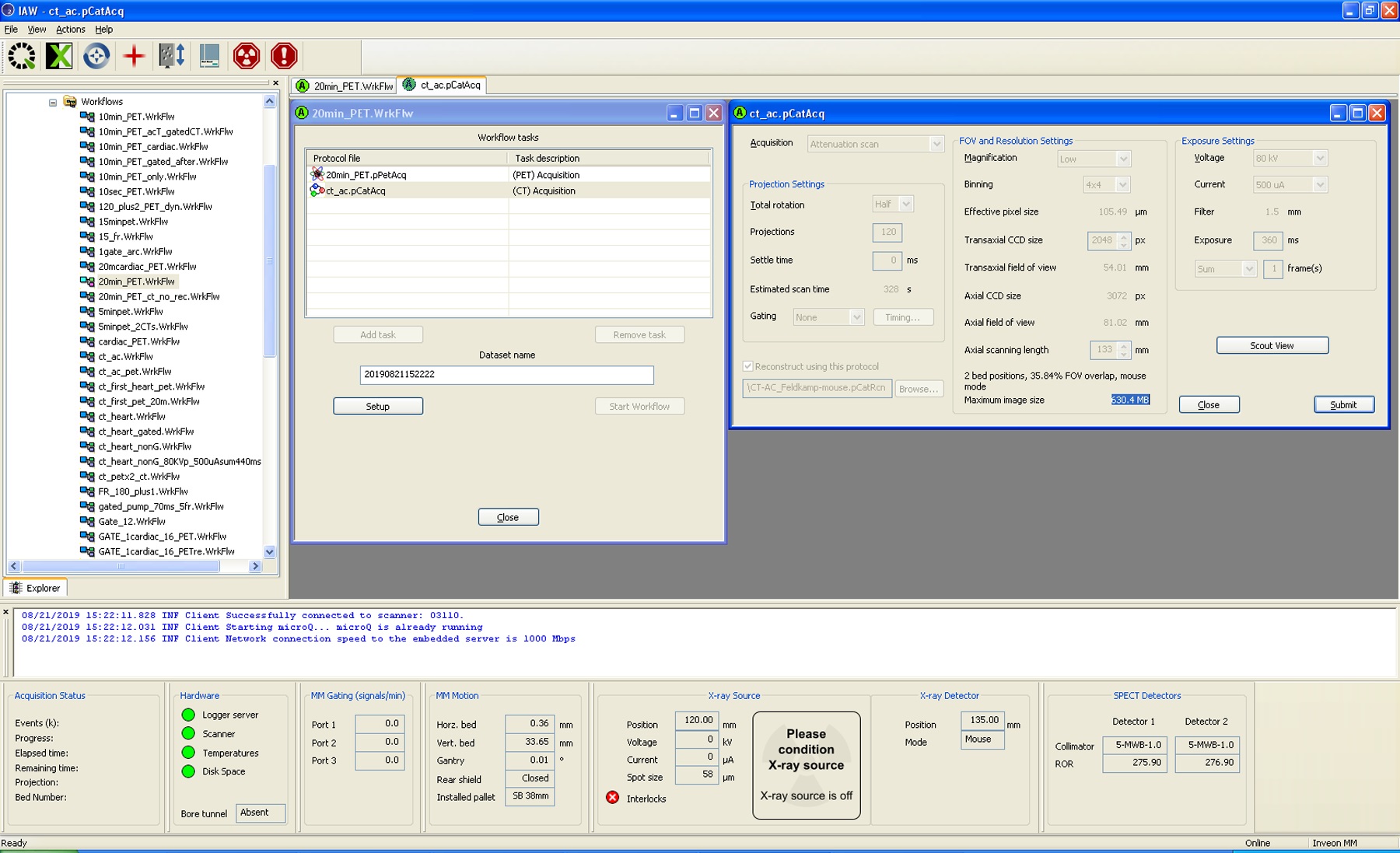
Figure 11. Example for Micro CT acquisition set-up using Siemens Inveon platform
Data analysis
- Micro-PET/CT analysis
PET/CT image processing/analysis parameters will depend upon the particular experimental indications and unique imaging platform setup at a given institution; as such, the following is a description of the protocol used at our institution.- Analyze the whole-body PET images of the mouse using Inveon Workstation Software (Siemens Medical Inc., Knoxville, Tennessee).
- Select the vendor software-supplied Patlak compartment plot option for kinetic modeling.
- For 3D PET and 4D PET data sets, manually select multiple volumes of interest (VOI) based on corresponding CT images for the following organs (as needed): heart, leg muscle, liver, kidney and brain. Voxel activities are represented in standardized uptake values (SUV). Plot dynamic activity curves for VOIs using dynamic 3D PET data set for each animal. The 4D PET data are used for defining cardiac function. First, segment the heart on CT images based on anatomical features, then transfer the segmented volume (cardiac PET VOI) for image co-registration, As seen with the example shown in Figure 12. Images are represented as maximum intensity projection (MIP) reconstructions of the source data. ECG guided binning of PET MIP images can be performed to obtain pseudo-dynamic images of mouse cardiac contractility, as shown in Video 2.
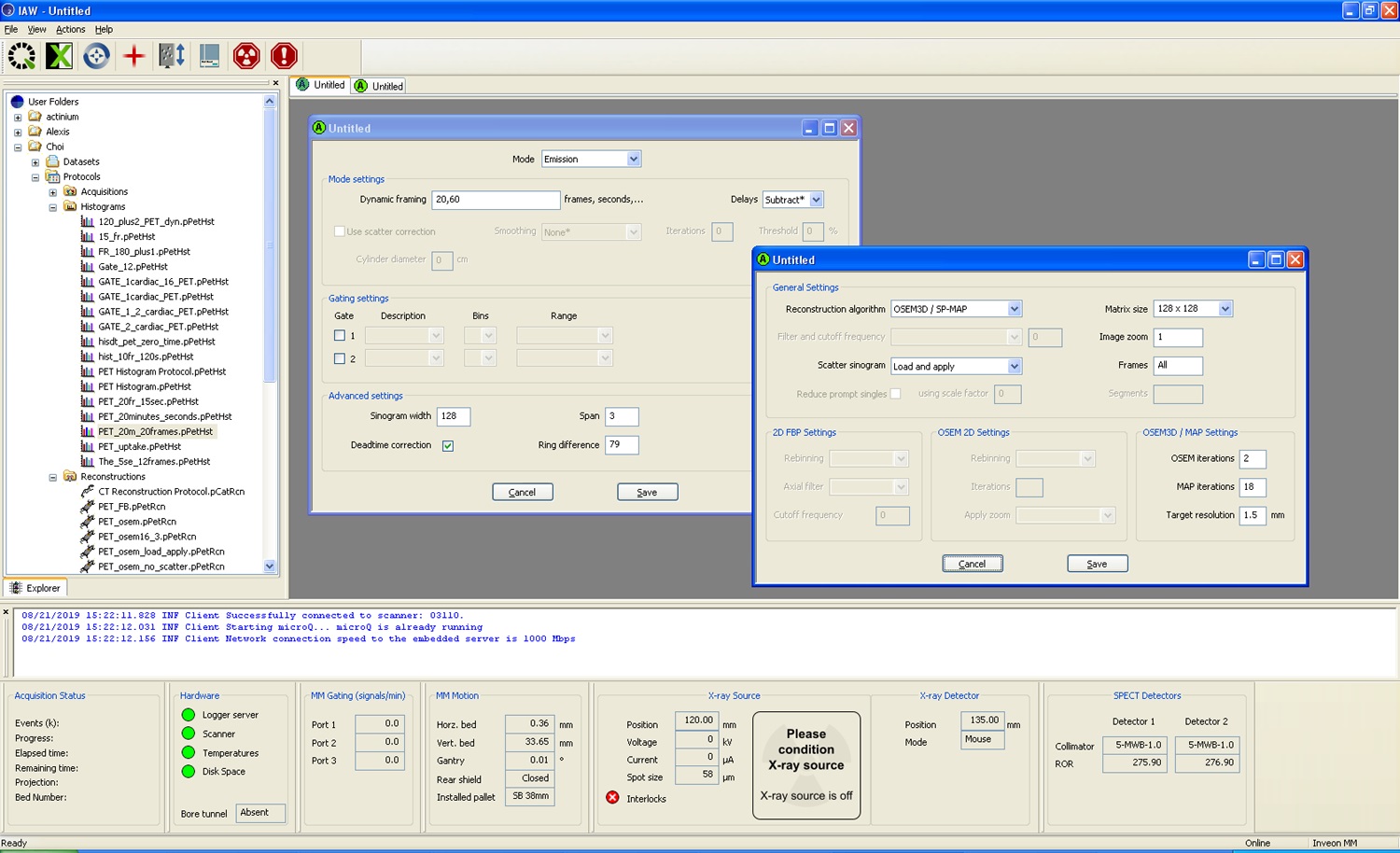
Figure 12. Example of basic parameter setup for microPET/CT reconstruction in Siemens Inveon workstationVideo 2. Rebinned cardiac cine imaging of ECG-gated whole body microPET images of NSG mouse injected with 2.2 MBq (250 μl) of FDG-labeled human RBCs (This video was made at University of south Florida, according to guidelines from the University of South Florida on Animal Care and approved by the Animal Research Ethics Board of University of South Florida under protocol IS00004376.)
- Labeling efficiency calculation
Labeling efficiency = (radioactivity dose of RBC pellet)/(radioactivity dose of SUPE + radioactivity dose of RBC pellet).
Recipes
- Filter sterilized 100 ml 5x EDTA solution
700 mM NaCl
20 mM KCl
12.5 mM K2EDTA dihydrate
Add sterile de-ionized water to final volume of 100 ml
Pass final solution through 0.2 micrometer (µm) Nalgene bottle top sterile filter unit - 100 ml 1x EDTA solution
20 ml of filter-sterilized 5x EDTA solution
80 ml of sterile di-ionized water
Pass final solution through 0.2 micrometer (µm) Nalgene bottle top sterile filter unit - 1 Unit/ml of heparin-PBS solution
Heparin sodium salt dissolved in sterile Phosphate Buffered Saline (PBS) solution, pH 7.4
Acknowledgments
We kindly appreciate the suggestions and comments to this work by Dr. Mikalai Budzevich at Moffitt Cancer Center. This protocol was adapted for Choi et al. (2019).
Competing interests
A provisional international patent application was submitted by the H. Lee Moffitt Cancer Center and Research Institute describing the imaging technique outlined in the manuscript (WO Application number WO2017123666A2; PCT/US2017/013063 filed on 2017-01-11). No additional declarations or potential competing interests are made with regards to the patent application by the authors of the manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Ethics
All experiments procedures were approved by University of South Florida (USF) Institutional Animal Care and Use Committee (IACUC). All experiments were performed in accordance to federal regulations and USF IACUC principles (protocol IS00004376) and procedures.
References
- Choi, J. W., Budzevich, M., Wang, S., Gage, K., Estrella, V. and Gillies, R. J. (2019). In vivo positron emission tomographic blood pool imaging in an immunodeficient mouse model using 18F-fluorodeoxyglucose labeled human erythrocytes. PLoS One 14(1): e0211012.
- Cysouw, M. C. F., Kramer, G. M., Frings, V., De Langen, A. J., Wondergem, M. J., Kenny, L. M., Aboagye, E. O., Kobe, C., Wolf, J., Hoekstra, O. S. and Boellaard, R. (2017). Baseline and longitudinal variability of normal tissue uptake values of [18F]-fluorothymidine-PET images. Nucl Med Biol 51: 18-24.
- Dong, H., Zhang, Z., Guo, Y., Zhang, H. and Xu, W. (2017). The application of technetium-99m-red blood cell scintigraphy in the diagnosis of orbital cavernous hemangioma. Nucl Med Commun 38(9): 744-747.
- Hacker, M., Hoyer, X., Kupzyk, S., La Fougere, C., Kois, J., Stempfle, H. U., Tiling, R., Hahn, K. and Stork, S. (2006). Clinical validation of the gated blood pool SPECT QBS processing software in congestive heart failure patients: correlation with MUGA, first-pass RNV and 2D-echocardiography. Int J Cardiovasc Imaging 22(3-4): 407-416.
- Herrero, P., Kim, J., Sharp, T. L., Engelbach, J. A., Lewis, J. S., Gropler, R. J. and Welch, M. J. (2006). Assessment of myocardial blood flow using 15O-water and 1-11C-acetate in rats with small-animal PET. J Nucl Med 47(3): 477-485.
- Lee, B. C., Moody, J. B., Weinberg, R. L., Corbett, J. R., Ficaro, E. P. and Murthy, V. L. (2017). Optimization of temporal sampling for 82rubidium PET myocardial blood flow quantification. J Nucl Cardiol 24(5): 1517-1529.
- Liu, M., Zhao, Z. Q., Fang, W. and Liu, S. (2017). Novel approach for 99mTc-labeling of red blood cells: Evaluation of 99mTc-4SAboroxime as a blood pool imaging agent. Bioconjug Chem 28(12): 2998-3006.
- Mohseni, S., Kamali-Asl, A., Bitarafan-Rajabi, A., Entezarmahdi, S. M., Shahpouri, Z. and Yaghoobi, N. (2015). Effects of filtration on right ventricular function by the gated blood pool SPECT. Ann Nucl Med 29(4): 384-390.
- Nakazato, R., Berman, D. S., Alexanderson, E. and Slomka, P. (2013). Myocardial perfusion imaging with PET. Imaging Med 5(1): 35-46.
- Rahmim, A. and Zaidi, H. (2008). PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 29(3): 193-207.
- Sadri, K., Momenypoor, S., Dabbagh Kakhki, V. R., Sadeghi, R., Aryana, K., Johari Daha, F., Zakavi, S. R. and Jaafari, M. R. (2015). Nano liposomes labeled with 99mTc-HMPAO, a novel agent for blood pool imaging. Iran J Pharm Res 14(4): 981-988.
- Thrall, J. H., Freitas, J. E., Swanson, D., Rogers, W. L., Clare, J. M., Brown, M. L. and Pitt, B. (1978). Clinical comparison of cardiac blood pool visualization with technetium-99m red blood cells labeled in vivo and with technetium-99m human serum albumin. J Nucl Med 19(7): 796-803.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, S. and Choi, J. W. (2019). Imaging the Vasculature of Immunodeficient Mice Using Positron Emission Tomography/Computed Tomography (PET/CT) and 18F-fluorodeoxyglucose Labeled Human Erythrocytes. Bio-protocol 9(19): e3391. DOI: 10.21769/BioProtoc.3391.
Category
Cancer Biology > Angiogenesis > Animal models > Cell migration
Cell Biology > Cell imaging > PET/CT
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










