- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunohistochemical Staining of CD8α in Diabetic Mouse Kidney
Published: Vol 9, Iss 18, Sep 20, 2019 DOI: 10.21769/BioProtoc.3364 Views: 3633
Reviewed by: Xiaoyi ZhengAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Isolate Germinal Centers by Laser Microdissection
Farbod Bahreini [...] Kathrin Kalies
Jun 5, 2022 2523 Views

Immunohistochemistry of Immune Cells and Cells Bound to in vivo Administered Antibodies in Liver, Lung, Pancreas, and Colon of B6/lpr Mice
Kieran Adam and Adam Mor
Jul 20, 2022 3606 Views

Monitoring Group 2 Innate Lymphoid Cell Biology in Models of Lung Inflammation
Jana H. Badrani [...] Taylor A. Doherty
Jul 20, 2023 2681 Views
Abstract
Immune cell infiltration, particularly cytotoxic CD8α lymphocyte infiltration, plays an important role in development of diabetic nephropathy. Although CD8α infiltration can be evaluated by its production of cytokines, its localization in the kidney is of particular importance. The current protocol describes CD8α immunostaining using a Vectastain ABC kit. This protocol works well with most commercially available antibodies, including CD8α antibodies in kidneys of diabetic mice.
Keywords: Diabetic nephropathyBackground
Diabetic nephropathy (DN), a complication of diabetes, is the most common cause of end-stage renal disease. Pathological changes in DN are closely correlated with the degree of renal immune cell infiltration, particularly toxic CD8α lymphocyte infiltration. Therefore, a sensitive and reliable method to localize CD8α lymphocytes in the kidney will facilitate the research in diabetic nephropathy as well as other fields relating inflammation (Li et al., 2018).
Materials and Reagents
- Syringe
- Filter (Tisch Scientific, Millex, syringe-driven filter unit, 0.22 µm)
- Paraffin slides
- Glass staining jar (IMEB, SKU:SD-09)
- M.O.M Kit for detecting primary antibodies on mouse tissue (Vector Laboratories, Inc., catalog number: PK-2200)
Note: This Kit includes blocking solution and diluent for dilution of both primary and secondary antibodies (stored at 4 °C). - Biotinylated goat anti-mouse IgG antibody (Vector Laboratories, catalog number: BA-9200, stored at 4 °C)
- Vectastain Elite ABC HRP Kit (Vector Laboratories, catalog number: PK-6101, stored at 4 °C)
- Biotinylated goat anti-rat IgG antibody (Vector Laboratories, catalog number: BA-9400, stored at 4 °C)
- Rat anti-mouse CD8α antibody [AbD Serotec (now Bio-Rad Laboratories), catalog number: MCA2694, stored at -20 °C]
- Normal goat serum [Sigma-Aldrich (now Millipore, Sigma), catalog number: G9023-10ML, stored at -20 °C]
- Toluidine blue O (Sigma-Aldrich, catalog number: T3260-5G)
- 0.1 M Phosphate buffer (AlboChemicals, SKU: B0787MV4W2)
- Xylene
- Ethanol
- Methanol
- H2O2
- PBS
- Permount (Fisher Scientific, catalog number: SP15-100)
- Tris-HCl
- Blocking solution (see Recipes)
- Counterstain stock solution (see Recipes)
- Counterstain working solution (see Recipes)
- DAB solution (see Recipes)
Equipment
- Beakers (Nalgene, catalog number: 1542E06)
- Orbit shaker (Labline, catalog number: 3520)
- Regular light microscope (Wetzlar, Leitz Orthoplan)
- Oven
Procedure
Note: In this protocol, the staining does not require antigen retrieval.
- Choosing paraffin slides
- If possible, choose appropriate slides (with intact tissue sections) with date (experiment) and number (block and slide).
- Label slides with staining date, antibody and dilution, host species using pencil.
- Place slides in glass staining jar, being careful not to scrape other slides/tissues.
Note: Be sure to record slide date, number, antibody, dilution information in staining book along with start times on small form.
- Pre-treatment of slides
- Deparaffinate: Soak slide in xylene: 2 x 15 min.
- 100% ethanol (ETOH): 3 x 10 min.
- Freshly prepared 100% Methanol with 0.3% H2O2: 20 min to remove endogenous peroxidases (200 ml 100% methanol + 2 ml 30% H2O2).
- Rehydrate: Soak slide for 1-min intervals with: 95%, 90%, 80%, 70%, 60%, 50%, and 30% ETOH.
- PBS: 3 x 10 min.
- Block non-specific reactions
- Take out slides from the glass container and absorb excess PBS from slides with a tissue wipe, being careful not to disturb tissue.
- Add 500 μl blocking solution (or according to tissue size) per slide, allowing the solution to cover all tissue.
- Place the slides into slide chamber that has ddH2O in the bottom for 1-2 h at room temperature.
- Primary antibody
- Take out slides from chamber, discard blocking solution and absorb residual solution with a tissue wipe, being careful not to let the tissue dry.
- Add 500 μl primary antibody solution (or according to tissue size) per slide, allow solution to cover all tissue.
- Place the slide into the same slide chamber with ddH2O at the bottom, cover chamber and incubate at 4 °C overnight.
- Secondary antibody
- Fill 3 beakers and the staining jar with PBS.
- Decant primary antibody solution if not recycled. Rinse slide through 3 beakers sequentially, and then place it in staining jar.
- Leave the staining jar at room temperature, changing PBS for at least 5 times, with 10 min intervals. It is optimal to place the staining jar on an orbit shaker that is set at a slow motion (60 rpm).
- Add 500 μl secondary antibody solution (or according to tissue size) per slide to allow solution to cover all tissue.
- Place slides into a slide chamber for 1 h at room temperature.
- Tertiary antibody
- Repeat Steps 5a-5c.
- Add 500 μl tertiary antibody solution (or according to tissue size), allow solution to cover all tissue.
- Place slide into slide chamber for 45 min at room temperature.
- Repeat Steps 5a-5c.
- DAB reaction
- Decant PBS from staining jar.
- Gently pour DAB solution into the staining jar for exactly 10 min.
- Decant DAB solution into designated waster container. Deactivate DAB solution by adding a small amount of bleach to water container.
- Wash slide immediately with H2O twice to stop color development.
- DAB reactive product is brown.
- Counterstain
- Decant H2O from staining jar.
- Pour toluidine blue working solution into the jar. When tissues have a blue color as well as brown, stop counterstain by decanting the counterstain solution into a container. It takes 5-10 min.
- Wash slide with H2O: 3 x 1 min.
- Toluidine blue stains nuclei with blue color.
- Dehydrate-Xylene-Permount
- Dehydrate slides with ETOHs with 30-second interval, 30%, 50%, 70%, 80%, 90%, 95%, and 100%. Repeat 100% ETOH.
- Immerse with xylene: 2 times.
- Permount.
- Place slide on a flat surface and place in a 40 °C oven until the permount dries.
- Inspect slide with microscope and record result.
Software
- ImageJ (https://imagej.net/Downloads)
Data analysis
Immunostaining positive cells can be quantitated using free ImageJ. With this protocol, CD8α-positive cells are primarily found in the interstitium of the kidney. The potential backgrounds and no-specific bindings are minimal. eNOS-/- db/db mice spontaneously develop diabetic nephropathy at 8 weeks of age. These mice were treated with erlotinib (an epidermal growth factor tyrosine kinase inhibitor) or vehicle (water) from 8 to 20 weeks of age. Six photos from each mouse are taken, and the average is used as CD8α-positive cell density form one mouse. The number of CD8a-positive cells in the kidneys is quantitated from 4 mice from vehicle treated eNOS-/- db/db mice and erlotinib treated eNOS-/- db/db mice (Figure 1).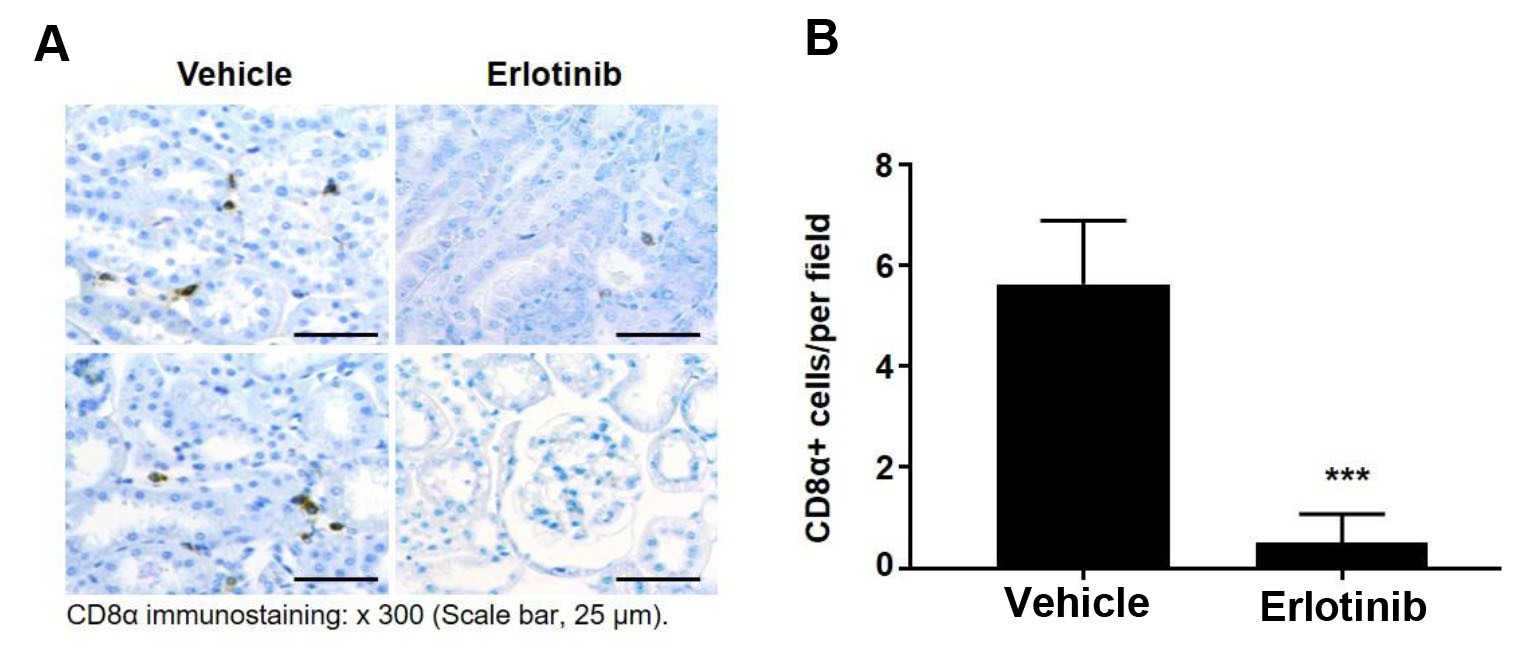
Figure 1. Inhibition of EGFR activity with erlotinib decreased renal CD8α lymphocyte infiltration in eNOS-/- db/db mice. A. The representative pictures of CD8a staining in the mouse kidneys from eNOS-/- db/db mice with vehicle or erlotinib treatment. B. Quantitative data (n = 4, P < 0.001). The value are presented as means ± S.D. The statistical analysis was performed using student’s t-test.
Recipes
- Blocking solution
If the primary antibodies are generated in mouse (mAb), using blocking solution from M.O.MTM Kit (Vector Laboratories, Inc, Burlingame, CA). For primary antibodies generated in non-murine hosts, block the slide with 10% normal serum (in PBS) from host of the secondary antibody. For examples, if the primary antibody is generated in rabbit, and the secondary antibody is generated in goat, then block the slide with 10% normal goat serum diluted in PBS.- Dilute mAb with diluent from M.O.MTM Kit
- Dilute non-mAb primary antibodies in 1% normal serum in PBS from host of the secondary antibody
- Dilute biotinylated goat anti-mouse IgG (H+L) (1:200 dilution) with diluent from M.O.MTM Kit. Dilute all other biotinylated secondary IgG’s with PBS (1:200 dilution, Vector Laboratories)
- Prepare tertiary antibody at least 30 min before use and store at room temperature. Add 16 μl reagent A to 2 ml PBS, mix, and then add 16 μl reagent B and mix. Depending on the number of slides, prepare an appropriate volume of the solution
- DAB solution
- Dissolve 60 mg DAB (aliquoted and stored in freezer) in 200 ml of 50 mM Tris-HCl, pH 7.4
- Then add 80 μl of 30% H2O2
- Protect from light
- Counterstain stock solution
- Add 100 mg toluidine blue to 200 ml 0.1 M phosphate buffer, pH 7.2
- Keep in dark while stirring until completely dissolved. It may take 1-3 days
- Store away from light at room temperature
- Counterstain working solution
- Filter 30 ml of stock solution using a 10 ml syringe with a 0.2 µm filter (Tisch Scientific, Millex, syringe-driven filter unit, 0.22 µm) into a beaker
- Add 170 ml of 0.1 M phosphate buffer, pH 7.2
- Store away from light at room temperature
Note: The working solution can be used twice.
Acknowledgments
The authors would like to thank Dr. James McKanna from Vanderbilt University School of Medicine (retired) for his great contribution to this protocol. This work was supported by NIH grants, DK51265, DK95785 and DK62794 (RCH, MZZ), DK103067 (RCH) and the Vanderbilt O’Brien Center (P30DK114809) (RCH, MZZ), VA Merit Award 00507969 (RCH), and the Vanderbilt Center for Kidney Disease. This protocol is derived from previous research papers published in JHC in1997 (McKanna and Zhang, 1997) and numerous other publications (e.g., Zhang et al., 1997; Zhang et al., 2018).
Competing interests
The corresponding authors declare that there is no conflict of interests.
References
- Li, Z., Li, Y., Overstreet, J. M., Chung, S., Niu, A., Fan, X., Wang, S., Wang, Y., Zhang, M. Z. and Harris, R. C. (2018). Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 67(9): 1847-1857.
- McKanna, J. A. and Zhang, M. Z. (1997). Immunohistochemical localization of lipocortin 1 in rat brain is sensitive to pH, freezing, and dehydration. J Histochem Cytochem 45(4): 527-538.
- Zhang, M. Z., Wang, J. L., Cheng, H. F., Harris, R. C. and McKanna, J. A. (1997). Cyclooxygenase-2 in rat nephron development. Am J Physiol 273(6): F994-1002.
- Zhang, M. Z., Wang, S., Wang, Y., Zhang, Y., Ming Hao, C. and Harris, R. C. (2018). Renal medullary interstitial COX-2 (Cyclooxygenase-2) is essential in preventing salt-sensitive hypertension and maintaining renal inner medulla/papilla structural integrity. Hypertension 72(5): 1172-1179.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, M. and Harris, R. C. (2019). Immunohistochemical Staining of CD8α in Diabetic Mouse Kidney. Bio-protocol 9(18): e3364. DOI: 10.21769/BioProtoc.3364.
Category
Immunology > Immune cell staining > Immunodetection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









