- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
High Resolution Respirometry in Candida albicans
Published: Vol 9, Iss 17, Sep 5, 2019 DOI: 10.21769/BioProtoc.3361 Views: 4979
Reviewed by: Emily CopeEmmanuel Orta-ZavalzaLucy Xie

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Highly Efficient Method for Measuring Oxygen Consumption Rate in Fusarium graminearum
Daniel Gebhard [...] Jörg Bormann
Aug 5, 2016 10896 Views

Isolation of Intact Mitochondria From Drosophila melanogaster and Assessment of Mitochondrial Respiratory Capacity Using Seahorse Analyzer
Christopher M. Groen and Anthony J. Windebank
Feb 5, 2025 2253 Views

Mito Stress Assay of PBMCs With Seahorse XFe96 Flux Analyzer and Comparison of Poly-D-Lysine and Poly-L-Lysine for Cell Affinity
Kumudu Subasinghe [...] Nicole Phillips
Jun 5, 2025 1978 Views
Abstract
Many Candida species, such as the opportunistic human pathogen Candida albicans, are Crabtree-Negative yeasts and are therefore highly dependent on the energy generated through oxidative phosphorylation. Respiration control is linked to a range of aspects of C. albicans cell physiology that appear to be important for virulence, most notably its ability to switch from yeast to hyphal forms and the maintenance of the cell wall. The following protocol allows for the measurement and characterization of respiration in C. albicans using high resolution respirometry. We outline how addition of respiration inhibitors can be used to assay the “mode” of respiration, mitochondrial health and the level of electron transport that is coupled to ATP synthase activity in living cell cultures. These data provide useful insight into the effects of external factors, such as exposure to anti-fungal compounds, or internal changes such as genetic alterations on respiratory performance.
Keywords: CandidaBackground
In the human fungal pathogen Candida albicans, respiration plays a key role in the yeast-to-hypha transition (Grahl et al., 2015), the catabolism of morphogenic amino acids and escape from macrophages (Silao et al., 2019). Respiration inhibitors may be considered for use as antifungals, as they can limit growth, promote cell death and in some cases act in co-operation with current antifungals such as fluconazole (Vincent et al., 2016). Therefore, characterization of C. albicans respiration in response to different environments, nutrients and inhibitors is important to understand pathogenicity and may also prove useful for future drug development.
C. albicans possesses a classical electron transport chain consisting of Complexes I-IV, as well as cyanide-insensitive alternative oxidases (Helmerhorst et al., 2002) and a less well defined parallel pathway (Duvenage et al., 2019). The level of AOX activity increases upon inhibition of classical electron chain components, allowing respiration to continue (Huh and Kang, 2001). C. albicans are still able to respire, albeit at a much reduced level, upon additional inhibition of AOX via the parallel pathway. C. albicans therefore contain a highly robust respiratory network that can resist inhibition.
Analysis of respiration in whole yeast cells can yield physiologically relevant information, such as integration with cellular processes that is not captured when analyzing electron transport within isolated mitochondria. Oxygen consumption in yeast is commonly measured using a Clarke oxygen electrode or chemical extracellular oxygen assays. However, a graphical readout of oxygen flux in real time using high-resolution respirometry can give important information on time-critical events, such as the induction of alternative respiration, and allows for adaptation of the experiment to include more complex titration schedules (Hütter et al., 2006). The following protocol describes the measurement of respiration in yeast-form cells of C. albicans (and other Candida species) in real time, for the characterization of mitochondrial function and the responses to added inhibitors.
Materials and Reagents
- Culture tubes (Falcon 50 ml conical centrifuge cubes, Fisher Scientific, catalog number: 14-432-22)
- Microcentrifuge tubes, 1.5 ml (Sigma-Aldrich, catalog number: Z606340)
- Cuvettes (Fisher Scientific, catalog number: 11547692)
- Microlitre syringes (Hamilton®: 10 µl- and 25 µl microlitre syringes, Sigma-Aldrich, catalog numbers: 28615U, 20735)
- Candida albicans (e.g., SC5314) overnight culture in YPD
- Triethyltin bromide (TET) (Sigma-Aldrich, catalog number: 288047)
- FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone) (Sigma-Aldrich, catalog number: C2920)
- Potassium cyanide (KCN) (Sigma-Aldrich, catalog number: 60178)
- Salicylhydroxamic acid (SHAM) (Sigma-Aldrich, catalog number: S607)
- Bacto peptone (Fisher Scientific, catalog number: S71604)
- Yeast extract (Fisher Scientific, catalog number: B11929)
- Glucose (Sigma-Aldrich, catalog number: G8270)
- NaCl (Sigma-Aldrich, catalog number: AlS7653)
- KCl (Sigma-Aldrich, catalog number: P9333)
- Na2HPO4 (Sigma-Aldrich, catalog number: S7907)
- KH2PO4 (Sigma-Aldrich, catalog number: P9791)
- HCl (Sigma-Aldrich, catalog number: H1758)
- Antimycin A
- Sodium nitroprusside
- Yeast extract-peptone media, 2% glucose (YPD) (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
Note: 50 mM TET, 2 mM FCCP, 2 mM Antimycin A and 200 mM SHAM stocks are prepared in absolute ethanol. 1 M KCN stocks are prepared in water. Aliquots should be prepared and stored at -20 °C. Respiration inhibitors are toxic and appropriate safety measures should be taken during preparation, handling and storage.
Equipment
- Oxygraph-2k respirometer (O2k-core) (Oroboros Instruments Corp, Austria)
- Incubator (e.g., New BrunswickTM Innova® 44, Eppendorf, catalog number: M1282-0002)
- Spectrophotometer (e.g., JenwayTM 6305 UV/Visible Spectrophotometer, Fisher Scientific, catalog number: 11400539)
- Microcentrifuge (e.g., centrifuge 5418, Eppendorf, catalog number: 5418000017)
- Haemocytometer (Sigma-Aldrich, BR718605)
- Optical microscope (e.g., Cole-Parmer, catalog number: UY-48922-70)
- Autoclave (e.g., Astell ScientificTM benchtop autoclave, Fisher Scientific, catalog number: 12735375)
Software
- Datlab 6 (or more recent release) software (Oroboros Instruments Corp)
Procedure
- Candida albicans strains are commonly maintained on YPD agar plates and grown in YPD in a 30 °C shaking incubator (100 rpm), this maintains the yeast in a budding form which can easily be counted. Alternative growth media and/or conditions may be used as appropriate for your experiments.
- Calibrate the Oroborus oxygraph at 30 °C (or at desired temperature) for at least 1 h, or until the oxygen flux reading is stable at zero using 2.5 ml PBS per chamber. An air space should be introduced into each chamber during calibration as per the manufacturer’s instructions.
- Add cells from an overnight culture to 3 ml fresh YPD (or other growth media as desired) to an OD600 of 0.1 and grow to an OD600 of 0.5, this represents a dividing population.
Note: Candida albicans cells do not respire appreciably in the stationary phase of growth (Figure 1, red bars).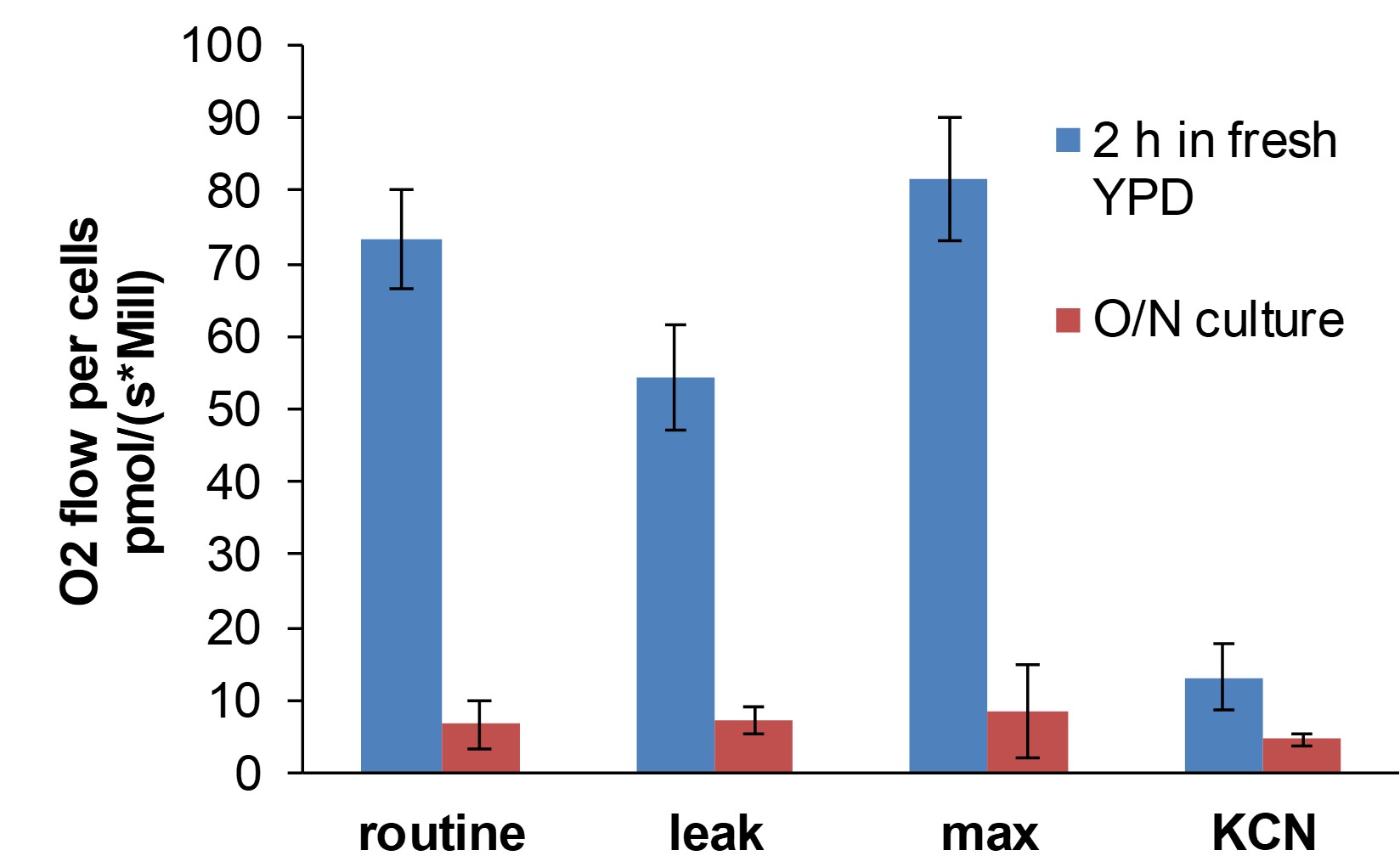
Figure 1. Respiration rate in C. albicans is restored to maximum after 2 h in fresh media at 30 °C. Respiration rate (i.e., oxygen flux per million cells, measured as O2 flow per cells pmol/(s*Mill)) declines in stationary phase cells. We have found that growth for 2 h in fresh media restores respiration levels to maximum. Therefore, it is recommended to transfer cells from overnight cultures (O/N) to fresh media for 2 h prior to respirometry in order to test the efficacy of respiration inhibitors. Leak respiration (respiration state in which proton translocation through the ATP synthase is inhibited) was determined following TET addition (160 µM) and uncoupled (max) respiration following FCCP addition (10 µM). KCN was added at a concentration of 1 mM to inhibit classical electron transport chain (ETC) activity. The data represents the means ± standard deviation for four independent experiments. - Collect 1 ml in a 1.5 ml microcentrifuge tube and centrifuge at 6000 x g for 1 min. Discard media and resuspend cell pellet in 1 ml 1x PBS.
Note: It is important that you do not re-suspend cells in media that will permit further growth as this will prevent the acquisition of stable readings within the respirometer. - Count cells using a hemocytometer and dilute in PBS to give a final cell concentration of 1 x 106 cells/ml in a total volume of 3 ml, per chamber of the respirometer. The respirometer has two chambers to allow for comparative experiments or technical repeats. One or both chambers may be used depending on the needs of the experiment. A sample of the cell suspension should be diluted 1:100 in PBS for counting. Add approximately 10 µl of cells suspension to one chamber of the hemocytometer (with coverslip in place). View the cells using the 10x or 20x objectives as needed. Count all cells in the central gridded square of the hemocytometer. This gives the number of cells x 104/ml. Make further dilutions in PBS as appropriate to give a final cell concentration of 1 x 106 cells/ml.
- Add 2.5 ml of cell suspension, at a concentration of 1 x 106 cells/ml, per chamber of the respirometer and depress the stopper to engage an air-tight seal.
- The respiration rate (O2 flux, pmol/per second/million cells) should be measured when the oxygen consumption rate stabilizes at a constant rate following addition of cells. This can be observed as a stable oxygen flux reading and is referred to as the routine respiration rate.
- When stable routine respiration is achieved, respiration-modulating drugs can be introduced using a 10- or 25 µl Hamilton microlitre syringe inserted into the injection port located in each chamber stopper (Figure 2A). Injection volumes should be between 2- and 25 µl; stock solutions of drugs should be prepared appropriately.
Note: The optimal concentration for a given respiration inhibitor can be determined by a titration experiment, in which increasing concentrations of the inhibitor is applied by additional injections. Further injections of each drug should be made until there is no further change in respiration rate, indicating saturation. We have found that the following concentrations are suitable for use with most Candida species (Table 1).
Table 1. Suggested concentrations for use of inhibitors to electron transport chain complexes in Candida albicans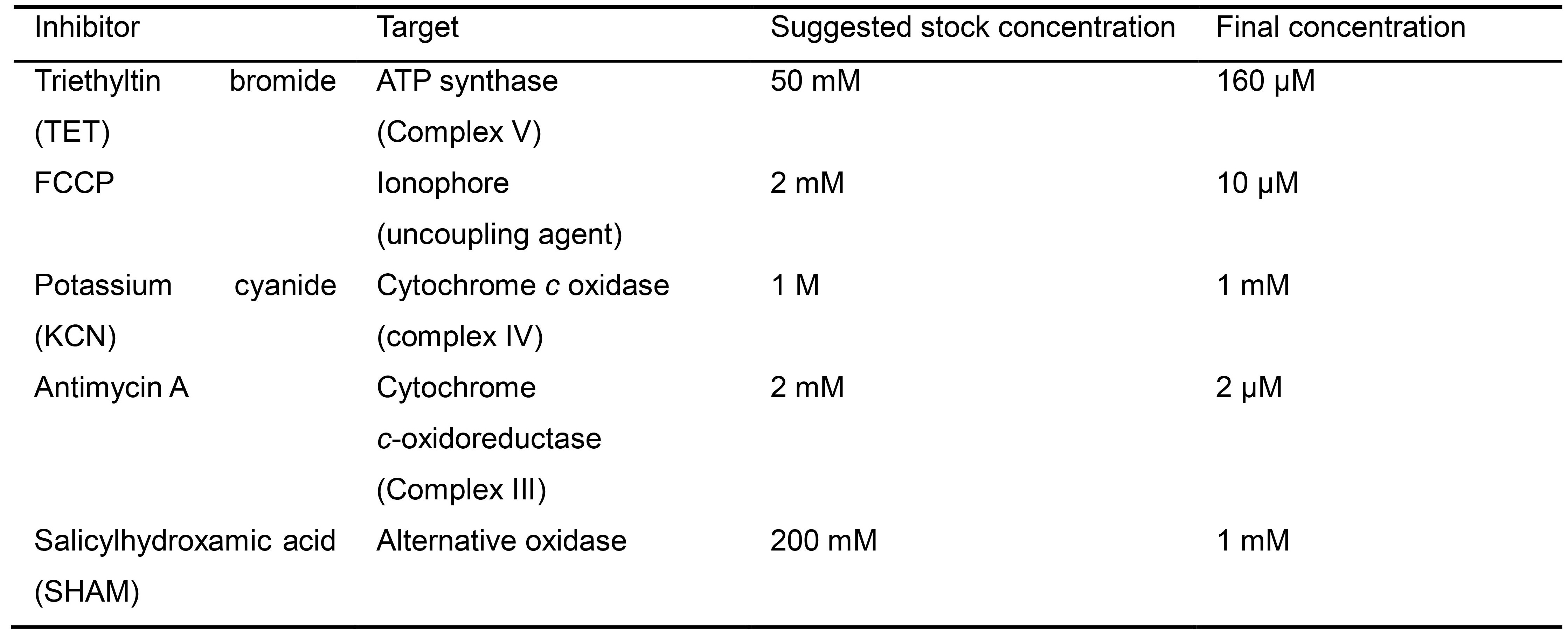
- To characterize “leak” respiration state, in which proton translocation through the ATP synthase is inhibited, TET is added to a final concentration of 160 µM. Respiration rate will decrease and then plateau within 5 min, after which further addition of TET will have no effect. The leak respiration rate should be measured at this point of maximum inhibition following TET addition and represents the level of respiration that is not linked to proton translocation across that ATP synthase. The ratio between routine and leak respiration levels indicates how much electron transport is linked to ATP production, or how “coupled” respiration is. This ratio is also referred to as the respiratory control ratio.
- Thereafter, to measure maximal electron transport chain capacity (uncoupled respiration), FCCP is added to a final concentration of 10 µM. Respiration rate will increase and then plateau within 5 min, after which further addition of FCCP will have no effect. At this point, the uncoupled respiration level could be recorded. The ratio of routine respiration to uncoupled respiration can be taken (uncoupling control ratio) and gives an estimation of the reserve capacity of the electron transport system, with a ratio of 1 meaning electron transport is occurring at its maximum.
- Inhibitors of classical respiration may be added to examine the induction of AOX and use of parallel respiratory pathways. Potassium cyanide (KCN), antimycin A or sodium nitroprusside (a nitric oxide donor) can be added to a concentration of 2 mM, 2 µM or 1 mM respectively. Higher concentrations of KCN (10 mM) are needed to inhibit the parallel pathway (Figure 2B).
- AOX can be inhibited by the addition of SHAM at a concentration of 1 mM.
Note: Inhibitors of classical respiration, such as antimycin A, KCN and nitric oxide, cause induction of alternative oxidase (AOX) activity in C. albicans within approximately 20 min (Duvenage et al., 2019). Therefore, SHAM may be applied at the beginning of longer titration experiments to prevent AOX activity and this gives a more accurate reading of the inhibitory effects of subsequent drugs. In our experience, SHAM alone does not appreciably affect routine respiration, as the alternative pathway is not in use during yeast-growth. - Data may be exported from respirometry traces using the manufacturer’s Datlab software as instructed. Respiration rate is expressed as O2 flow per cells pmol/(s*Mill). Experimental parameters such as the concentration of cells or the volume of cells added to each chamber can be modified at any time in Datlab if necessary. Data points can be selected for respiration rate for any given time period (for which mean respiration rate is automatically calculated) using Datlab software supplied with the Oroboros Oxygraph and exported to a spreadsheet program such as Microsoft Excel for further analysis. Three to four independent biological replicates should be performed for each condition for replicating growth conditions, culture times and inoculation OD.
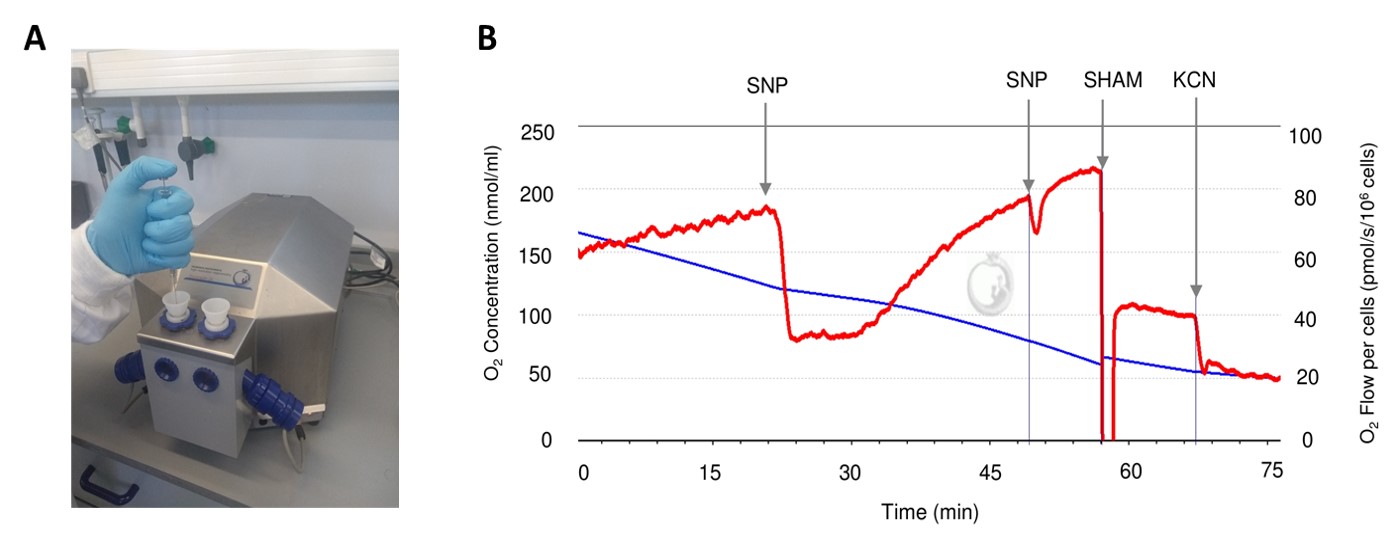
Figure 2. Addition of drugs to the oxygraph chamber and example of respirometry trace data generated by Datlab software. A. Demonstration of drug addition to oxygraph chamber using microlitre syringe. B. Example data of respirometry graph plotted in real time by Datlab software. Nitric oxide donor, sodium nitroprusside (SNP), and alternative oxidase inhibitor, salicylhydroxamic acid (SHAM), were added where indicated resulting in final concentrations of 1- and 2 mM respectively. Potassium cyanide (KCN) was added to a final concentration of 2 mM.
Recipes
- Yeast-extract peptone broth with 2% glucose (YPD) (Reference 9)
- To an autoclavable flask, add: Bacto peptone 20 g, yeast extract 10 g, distilled water 950 ml
- Autoclave the mixture (121 °C, 20 min)
- Add 50 ml sterile 40% w/v glucose (40% w/v glucose stock is autoclaved separately)
- Phosphate-buffered saline (PBS) 10x stock (Reference 6)
- To an autoclavable flask, add: NaCl 80 g, KCl 2 g, Na2HPO4 14.4 g, KH2PO4 2.4 g, distilled water 800 ml
- Adjust the pH to 7.4 using HCl
- Add distilled water up to 1 L
- Autoclave the mixture (121 °C, 20 min)
Acknowledgments
This work was supported by the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology 097377/Z/11/Z.
Competing interests
The authors declare no conflict of interest.
References
- Duvenage, L., Walker, L. A., Bojarczuk, A., Johnston, S. A., MacCallum, D. M., Munro, C. A. and Gourlay, C. W. (2019). Inhibition of classical and alternative modes of respiration in candida albicans leads to cell wall remodeling and increased macrophage recognition. MBio 10(1).
- Grahl, N., Demers, E. G., Lindsay, A. K., Harty, C. E., Willger, S. D., Piispanen, A. E. and Hogan, D. A. (2015). Mitochondrial activity and Cyr1 are key regulators of Ras1 activation of C. albicans virulence pathways. PLoS Pathog 11(8): e1005133.
- Helmerhorst, E. J., Murphy, M. P., Troxler, R. F. and Oppenheim, F. G. (2002). Characterization of the mitochondrial respiratory pathways in Candida albicans. Biochim Biophys Acta 1556(1): 73-80.
- Huh, W. K. and Kang, S. O. (2001). Characterization of the gene family encoding alternative oxidase from Candida albicans. Biochem J 356(Pt 2): 595-604.
- Hütter, E., Unterluggauer, H., Garedew, A., Jansen-Dürr, P. and Gnaiger, E. (2006). High-resolution respirometry--a modern tool in aging research. Exp Gerontol 41(1): 103-109.
- Phosphate-buffered saline (PBS). (2006). Cold Spring Harb Protoc: doi:10.1101/pdb.rec8247.
- Silao, F. G. S., Ward, M., Ryman, K., Wallstrom, A., Brindefalk, B., Udekwu, K. and Ljungdahl, P. O. (2019). Mitochondrial proline catabolism activates Ras1/cAMP/PKA-induced filamentation in Candida albicans. PLoS Genet 15(2): e1007976.
- Vincent, B. M., Langlois, J. B., Srinivas, R., Lancaster, A. K., Scherz-Shouval, R., Whitesell, L., Tidor, B., Buchwald, S. L. and Lindquist, S. (2016). A fungal-selective cytochrome bc1 inhibitor impairs virulence and prevents the evolution of drug resistance. Cell Chem Biol 23(8): 978-991.
- YPD media. (2010). Cold Spring Harb Protoc: doi:10.1101/pdb.rec12315.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Duvenage, L., Munro, C. A. and Gourlay, C. W. (2019). High Resolution Respirometry in Candida albicans. Bio-protocol 9(17): e3361. DOI: 10.21769/BioProtoc.3361.
Category
Microbiology > Microbial physiology > Respiration
Cell Biology > Cell metabolism > Respirometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










