- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Precision Technique for Splenectomy Limits Mouse Stress Responses for Accurate and Realistic Measurements for Investigating Inflammation and Immunity
Published: Vol 9, Iss 15, Aug 5, 2019 DOI: 10.21769/BioProtoc.3317 Views: 6406
Reviewed by: Meenal SinhaLuis Alberto Sánchez VargasAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2542 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Splenectomy in an animal model requires a standardized technique utilizing best practice to avoid variability which can result in adverse impact to the animal resulting in flawed physiologic responses simply due to technique rather than to the studied variables. In the case of the spleen, often investigators are analyzing the animal immune or inflammatory responses. Surgical splenectomy involves many variables from the training and expertise of the surgeon, which directly correlates to surgical technique to the length of operation and ease of the procedure. This operation, in turn, impacts blood loss and insensible fluid losses, sterile technique, unintended trauma to the spleen and surrounding organs, the length of the incision and the duration of the operation with more prolonged exposure to anesthetic agents. All these variables ultimately play a significant role in the experiment since they directly affect the response of the model in terms of inflammation, immune activation, or even suppression. Undesired variables such as these go unnoticed and lead to inaccurate and misleading data.
Keywords: Blunt traumaBackground
The spleen produces protective humoral antibodies via plasma cells, harbors transient and stable populations of B and T cells, filters particulate antigens, and is a reservoir for red and white blood cells. There are specific helper T cells that migrate to and from the spleen serving a role in tumor surveillance and antigen recognition. It has also long been recognized as a domicile for specific B cell populations affecting the individual’s ability to respond to specific gram-negative encapsulated bacteria such as Meningococcus, Streptococcus, and Haemophilus. Splenectomy is sometimes necessary in cases of hemolytic anemias with splenomegaly from hereditary spherocytosis or sickle cell disease or in cases of thrombocytopenia, trauma, and even in some instances of malaria. In many instances, pediatric surgeons try to preserve some splenic function to preserve some immune function and avert long term risks of pulmonary hypertension by performing a partial splenectomy saving about 20-30% of the total splenic mass (Tastaldi et al., 2019). Sometimes due to the inadequate vascular supply of the upper pole of the spleen or in cases of hilar involvement of lesions or massive trauma, total splenectomy may be the only option. Asplenia may result in increased risks of post-splenectomy sepsis, pulmonary hypertension and even some speculation of increased risk of Type II diabetes, childhood obesity (Szendroi et al., 1997) and even inadequate cellular repair responses after cardiac ischemia (Costi et al., 2018).
Recent reports show that therapeutic modality for efficient antigen delivery to spleen shows promising for conquering tumor growth and progression (Shimizu et al., 2018). Current immunotherapy elevated the splenic level of IL-1β, IL-4, IL-10, IL-17, IFN-γ, and TNF-α for immunomodulatory effects of cancer progression (Merheb et al., 2019). Pediatric surgeons have advanced laparoscopic partial splenectomy to preserve hematological functions of the organ while trying to blunt or ameliorate hemolytic dysfunction (Serra et al., 2018). All these studies require the optimal performance of splenectomy to gain immune benefits of spleen function.
Surgery for splenectomy can involve many variables from training and expertise of the surgeon, surgical technique, length of operation, ease of the procedure, blood and fluid loss, sterile technique, trauma to the spleen or other tissues and traumatic manipulation, length of incision and length of exposure to anesthetic agents.
We provide some guidelines for the standardization of the technique for splenectomy and some thoughts in order to create a procedural best practice protocol such that resulting data can have less contamination by factors that can truly impact the right metrics being evaluated such as the degree of inflammation or immunity.
Materials and Reagents
- Animal study for splenectomy
- Silicon tubing (Patterson Scientific,Various Different colors per tubing use, which fit Anesthetic Vaporizer (Patterson Scientific, catalog number: 78703584)
- Balb/C female mice (Jackson Lab, Bar Harbor, ME) (below for specifics)
Balb/C female mice weighing 20-25 g (age of 8 weeks) were acquired for the experiment. The mice were free from pathogens and kept in filter isolation throughout the study. They were housed in an accredited animal care facility at the CHOC Children’s Hospital Research Institute and placed on a regular photoperiod with a normal temperature and given laboratory chow and water, all under standard guidelines. - The filter (Animal Care Systems Inc., Centennial, Colorado, catalog number: C79037B) set which is recommended with Anesthetic Vaporizer (Patterson Scientific, catalog number: 78703584)
- Laboratory chow (Animal Care Systems Inc., Centennial, Colorado, catalog number: T.2919) and water
- Operative Materials
- Absorbable suture (Coated VICRYL*Plus, antibacterial with Irgacare MP* Polyglactin 910 suture, undyed braided, Ethicon Inc.) (Ethicon | J&J Medical Devices, Johnson & Johnson, Somerville, NJ)
- Ketamine/xylazine (dose: 120/20 mg/kg): Ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA), xylazine (Anased, Akorn, Decatur, IL)
- Codeine (1 mg/kg): PubChem SID: 378149847, Purchasable Chemical: C-006_CERILLIAN, (Sigma-Aldrich, Saint Louis, MO, United States, 63103)
- PBS [Phosphate Buffered Saline (1x), 6.7 mM (PO4) without calcium or magnesium] (Lonza, catalog number: 17-516F)
- 5% FCS (Atlanta Biologicals, Flowery Branch, GA)
- Betadine solution, Iodine solution: Betadine® is from Purdue Products, LP (Stamford, CT) (with an isopropyl alcohol application between the steps for Preoperative Skin Preparation)
- ChloraPrepTM, and DuraPrepTM solution (3M Health Care, St. Paul, MN)
- Hanks’ balanced salt solution (HBSS) (Thermo Fisher Scientific, catalog number: 14025092)
- Isoflurane (Piramal Critical Care Inc., Bethlehem, PA)
Note: We got it directly from the Veterinarian, by prescription.
Equipment
- Scissors (Fine Science Tools, catalog number: 11058-11)
- Precisa Bj-2100d Precision Laboratory Balance 2, 100 g x 0.1 g (Digital Analytical Electronic Balance Laboratory Lab Precision Scale) (Precisa Gravimetrics AG, CH-8953 Dietikon, Switzerland)
- Surgical knife, Scalpel (Bard-Parker, catalog number: 372611)
- Twisters: Stainless Steel AISI-420 (Rock-Well-Hardness 48-58)
- Wire Twister, Neurosurgery, Sternal Wire Twister Needle Holder 18 cm hole forceps TC gold surgical suture) (World Precision Instruments, Sarasota, FL)
- Vital WEBSTER Needle Holders (BD V. Mueller, catalog number: RH2561)
- Oxygen tank
- Smooth forceps (Fine Science Tools, catalog number: 11000-13)
- Anesthetic Vaporizer (Patterson Scientific, catalog number: 78703584)
- The nose cone, Anesthesia Machine (Patterson Scientific, catalog number: 78914722)
- Dry cotton-tipped applicator (Q tip): Sterile Cotton Tipped Applicators (Thomas Scientific, Waltham, MA, catalog number: 1213Q15, Manufactory number: 25-806 10WC)
- Cautery pen (Manufactory number: AA03X; Tiger catalog number: TM77563) with high temperature (Bovie® Medical eye cautery) (Symmetry Surgical, Antioch, TN)
- Heat lamp (Fisher Scientific, Thomas Scientific, Waltham, MA, catalog number: NC9631062)
- Induction Chamber (Patterson Scientific, catalog number: 78917833)
- Portable Anesthesia Machine (PAM) (Patterson Scientific, catalog number: 78914722)
Procedure
- Anesthesia for splenectomy
- Preparation for anesthesia and determination of sufficient anesthesia for splenectomy.
- Prepare a sufficient amount of isoflurane. Place into the liquid chamber of the vaporizer (Anaesthetics Vaporizer, Patterson Scientific, as operated by anesthesia technician, see Video 1.)Video 1. Precision technique for mouse splenectomy. This video was made at CHOC Children’s Research Institute according to guidelines from the CHOC Children’s Research Institute on Animal Care and approved by the Animal Research Ethics Board of CHOC Children’s Research Institute under protocol IACUC#_160501.
- Anesthesia procedure
- Attach silicon tubing to induction chamber (red into induction chamber, purple leading out of induction chamber). Attach the long purple line to the vacuum system of the building.
- Turn on the waste pump, attach oxygen manifold to an oxygen tank.
- Turn on an oxygen tank, open oxygen flowmeter. Set the flow rate to 0.9 L/s for oxygen flow rate and 5% isoflurane. Open flow hose to the induction chamber.
- Place mouse into induction chamber. Observe for the mouse to begin to take deep breaths. The mouse is sufficiently anesthetized when breathing rate drops below 60 breaths per minute.
- Splenectomy procedure
- Preparation of surgery
All surgical tools should be sterilized between surgeries appropriate with the nature of tools. Surgical instruments are set up on a sterile surgical table along with sterile supplies such as suture, non-absorbable, suture, forceps, a surgical knife, twisters, scissors. The anesthetized mouse is laid down on the table with nose encased within the nose cone supplying the inhalational gas for anesthesia. The skin is sterilized with iodine solution (ChloraPrepTM, and DuraPrepTM solution). - Incision of skin
A 0.5 cm skin incision is made in vertical midline abdomen just below the xyphoid process with fine scissors. The midline fascia is easily identified just under the skin incision, and scissors can be used to cut a midline incision for the same length.
Note: Be careful to not go into the xyphoid cartilage or any higher so that there is no entry into the thorax, which will result in pneumothorax and death of the mouse. - Mobilization of the spleen
The spleen is brought to the midline incision from the left upper quadrant by bringing out the omentum with the use of smooth forceps and a cotton tip applicator to drag it outward in view. - The spleen is removed to separate from the stomach
The spleen is held up with the smooth forceps, and an eye cautery with a heated tip is utilized to cauterize the attachments and the vessels to the hilum. Often, this portion of handling the spleen and dividing the vessels can be traumatic and result in significant blood loss when performed by untrained or inexperienced individuals. The purpose of some of these simple techniques is to limit trauma and stress to the animal, which are critical factors in avoiding significant systemic responses which can grossly alter the inflammatory and immune response. It is critical to cauterizing attached vessels with a cautery pen using the splenic vessel-preserving technique (Kim et al., 2019). - The midline fascial defect is closed with 4-0 vicryl sutures and skin is closed with interrupted 4-0 Vicryl suture with using Wire Twister for Neurosurgery. Five stitches are used to close the skin opening of 0.5 cm length. (It is the surgeon, who specified the type of knots for patient’s operation, namely, Surgical Tie–Instrument Technique that shows a simple square knot being tied with forceps as shown in the video clip).
- The mouse is anesthetized for a procedure that lasts about 5-10 min in duration, and the mouse is kept under the heat lamp to avoid hypothermia. Also, one cc of saline is given subcutaneously to replace some of the insensible fluid losses during surgery.
- Preparation of surgery
- Recovery of post-splenectomy
- Mice are observed for minutes after surgery to ensure adequate analgesia and again on hourly intervals during the day of surgery for 4 h. Animal appearance, activity, and behavior (e.g., food and water intake) are closely monitored as indications of pain and discomfort. The analgesic drug (Codeine, buprenex, oxycodone, morphine, hydromorphone, i.m. injection) is used if the animals appear in pain as evidenced by visual observation (jerking motions, lack of movement, significant weight loss, vocalizing, and lack of grooming, auditory observation of squealing) for 30 min and again in 4 h post-surgery.
- Codeine is given twice daily for first a few days after the surgery if needed. Post-Procedure Supportive Care: Appropriate food and water are ensured on the cage floor after the procedure. The wound sutures are absorbed through time. The analgesic, tranquilizing or neuromuscular blocking agent(s) and post-anaesthetics recovery: Ketamine/xylazine (dose: 120/20 mg/kg) or Codeine (1 mg/kg), are applied.
Data analysis
Representative results:
Surgical splenectomy involves many variables from the training and expertise of the surgeon, which directly correlates to surgical technique to the length of operation and ease of the procedure. The actual surgery time varied from the skilled surgeon to two trainees, as shown in Figure 1. The actual surgery time and related complications impacted the recovery time as shown from 5.4 min (running around after the surgery, refer to the end of Video 1), to 2-3 h. Full recovery usually required about a week. Specifically, the skilled surgeon took an average of 7.98 min in the range of 5.32-10.54 min, with ten mice (total 10 mice) to survive up to 42-63 days (sacrificed). The Trainee 1 took the average 11.49 min in the range of 11.49-18.12 min, with six mice (total 6) survived 2-14 days; the trainee 2, 20.43 min, in the range of 18.54-22.36 min, with three mice (total 3) survived 1-5 days (Figure 1).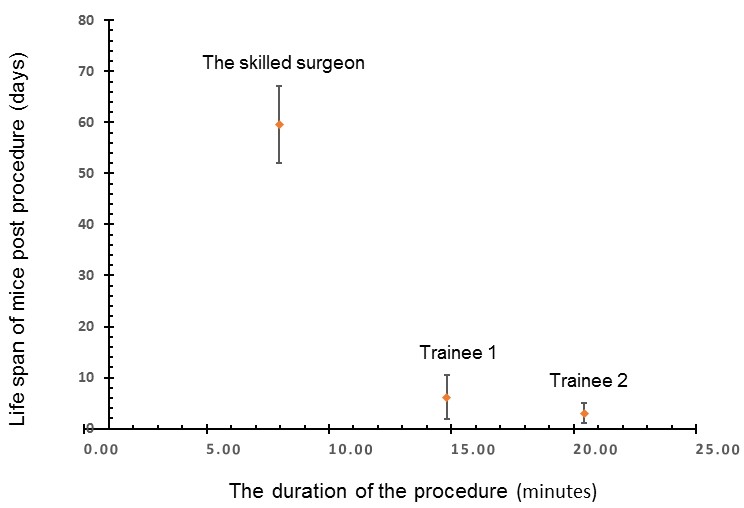
Figure 1. Relationship of survival with the duration of the surgical procedure
Discussion:
We describe a step by step technique for splenectomy with emphasis on performing a quick and easy surgery by limiting the length of the operation and being familiar with mouse anatomy and some techniques to limit the trauma and therefore the risk of blood loss. This technique minimizes fluid and blood loss by limiting the time and focusing on avoiding traumatic manipulation. Also, the length of the surgical incision limits the pain experienced and the shorter duration of surgery means that there is less exposure to anesthetic agents, which can also affect the physiologic responses. Many variations exist in the procedure performed, and therefore, the responses measured can be highly variable and inaccurate. When the procedure is performed swiftly, and without trauma, it limits the adverse effects of surgery and anesthesia such that the stress responses from the body are less significant in their effects on inflammation and infection. All these can explain why the relationship of survival with the duration of the surgical procedure (Figure 1). We have worked on two strains (BalbC and C57BL/6), and both strains worked. We did not work on male mice, and we found ideally, the ages of the mice are eight weeks. The tips and tricks and critical steps include: (1) the incision location is above the spleen, (2) precision cut (minimum incision opening in size sufficient to pull out the spleen); (3) to avoid hitting blood vessels, (4) seal the blood vessel as soon as possible to avoid loss of the blood, (5) seal the incision as soon as possible to avoid body fluidic loss. Cited literature below supports our observation.
The spleen is one of the largest lymphoid organs in the body. It harbors immune cells, including antigen presenting cells, B and T lymphocytes. Many studies have demonstrated the importance of the spleen in mounting a cellular and humoral immune response. Splenectomized Balb/C mice have reduced expression of IL-4 and IL-10, making them more susceptible to infection (Maioli et al., 2007). Humans have been recognized to be susceptible to certain gram-negative organisms when splenectomized. They are at risk of overwhelming post-splenectomy infection (OPSI) since King and Schumacker landmark reported in 1952. Loss of the spleen leads to reduced abilities to mount an adequate humoral response to certain bacteria and antigens due to loss of specific subsets of B lymphocytes (including plasma B cells, memory B cells, and follicular B cells) but also potential reduction in cell-mediated immunity given there are specific Helper T cells (TH1 and TH2) that shuttle around serving in surveillance and possess splenic homing receptors. T cells include cytotoxic T cells, memory T cells, helper T cells, natural killer T cells, suppressor T cells, and gamma delta T cells, natural killer cells, and progenitor lymphopoietic stem cells. Also, the actual physical structure of the spleen allowing large volumes of blood to enter within sinusoids that expose the resident cells to monitor and respond to potential antigens.
Splenocytes and specific fractions may have utility for immunotherapy (Li and Kabeer, 2013) in cancer and its responses within the tumor microenvironment (Li and Kabeer, 2018). Autologous splenic lymphocytes may be harvested for reperfusion to preserve specific memory T cell responses against cancer, especially when combined with cancer genome diagnostics (Li et al., 2014). The long-term outcomes of optimal laparoscopic splenectomy for immune thrombocytopenia reached curative in 68% of a cohort study of 109 patients as it is related to relapse-free rates, and predictors of long-term response (Tastaldi et al., 2019). Minimally invasive surgery improves splenic vessel preservation for reducing surgical complications, which was documented in fifteen relevant cohorts with 769 patients (Nakata et al., 2018). Pathologic enlargement (splenomegaly) results in an expansion of cellular proliferation outside of the bone marrow compartment, i.e., extramedullary hematopoiesis (EMH) (Mo et al., 2009), which is not yet fully understood. T- and B-cell recruitment into the restoration of the central (thymus) and peripheral (spleen and lymph node) is required. As T cell compartments are necessary for optimal adaptive immune responses to pathogens and tumor antigens, a comprehensive assessment of immune responses to splenectomy needs to be elucidated in achieving global immunization against cancer (Li et al., 2018) and infection. In particular, a recent study shows that “a novel antigen delivery platform for cancer vaccines that delivers an encapsulated Ag to splenic marginal zone B (MZ-B) cells via the aid of a PEGylated liposome (PL) system shows promise as a therapeutic modality for conquering tumor growth and/or progression” (Shimizu et al., 2018). Such antigen delivery platform for cancer vaccines relies on the quality of control surgery on the spleen. Furthermore, autologous splenic lymphocyte isolation for preserving an individual’s humoral responses to gram-negative bacteria and potentially have a much more robust response to vaccination in patients who have been splenectomized may be of significant benefit to patients.
Taken together, we wanted to emphasize that a precision procedure for splenectomy is essential in spleen related studies. We noted that a previously published procedure (Reeves et al., 2001) differs from our procedure. Specifically, (1) They made skin incision, ∼2.5 cm long, midway between the last rib and the hip joint, while we made only 0.5 cm incision in vertical midline abdomen just below the xyphoid process with fine scissors. (2) They did not seal the blood vessels, which bleeding along with the procedure for up to 60 min of recovery. Note that we used the cautery pen to seal the blood vessel and connective tissue to avoid the ever-lasting wound–natural healing takes much longer time, which needs only 5 min for mice to recovery. (3) Their two stitches for 2.5 cm incision (i.e., subject to infection) were compared with our five stitches for 0.5 cm incision. (4) Their survival rate of 95% following adult splenectomy was in contrast, to our surgeon gained a 100% survival rate.
Conclusions:
This precision technique for splenectomy limits mouse stress responses for accurate and realistic measurements for investigating inflammation and immunity.
Acknowledgments
Author Contributions: SCL and MHK conceived of the study and drafted the manuscript, and all other authors participated in its experimentation of the manuscript. All authors read and approved the final manuscript.
We thank Brent A. Dethlefs and Maria Minon, MD, for their enthusiasm and support.
DISCLOSURES: Funding sources: This work was supported in part by the National Institutes of Health (NCI, R01CA164509; NIEHS, R01ES021801-04S1), by CHOC Children’s Foundation, CHOC-UCI Joint Research Awards (2014, 2015, 2016), and by CHOC Children’s Physician’s Research Pilot grant.
Competing interests
The authors declare that they have no conflicts of competing interest.
Ethics
All protocols were approved by the specific ethics committee (IACUC) that approved the described experiment. IACUC #160501was the approval ID of the animal experiment in this protocol and the validity period was from June 01, 2016 to March 25, 2019.
References
- Costi, R., Castro Ruiz, C., Romboli, A., Wind, P., Violi, V. and Zarzavadjian Le Bian, A. (2018). Partial splenectomy: Who, when and how. A systematic review of the 2130 published cases. J Pediatr Surg.
- Kim, H. S., Park, J. S. and Yoon, D. S. (2019). True learning curve of laparoscopic spleen-preserving distal pancreatectomy with splenic vessel preservation. Surg Endosc 33(1): 88-93.
- Li, S. C. and Kabeer, M. H. (2013) Designer immunotherapy specific for cancer. J Cell Sci Ther 4(1): e116.
- Li, S. C. and Kabeer, M. H. (2018). Spatiotemporal switching signals for cancer stem cell activation in pediatric origins of adulthood cancer: Towards a watch-and-wait lifetime strategy for cancer treatment. World J Stem Cells 10(2): 15-22.
- Li, S. C., Stucky, A., Chen, X., Kabeer, M. H., Loudon, W. G., Plant, A. S., Torno, L., Nangia, C. S., Cai, J., Zhang, G. and Zhong, J. F. (2018). Single-cell transcriptomes reveal the mechanism for a breast cancer prognostic gene panel. Oncotarget 9(70): 33290-33301.
- Li, S. C., Tachiki, L. M., Kabeer, M. H., Dethlefs, B. A., Anthony, M. J. and Loudon, W. G. (2014). Cancer genomic research at the crossroads: realizing the changing genetic landscape as intratumoral spatial and temporal heterogeneity becomes a confounding factor. Cancer Cell Int 14(1): 115.
- Maioli, T. U., Carneiro, C. M., Assis, F. A. and Faria, A. M. (2007). Splenectomy does not interfere with immune response to Leishmania major infection in mice. Cell Immunol 249(1): 1-7.
- Merheb, R., Abdel-Massih, R. M. and Karam, M. C. (2019). Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int J Biol Macromol 121: 1-5.
- Mo, J. R., Mathur, A., Angagaw, M., Zhao, S., Wang, Y., Gargano, D., DiBacco, A. and Bachman, E. S. (2009). Splenectomy normalizes hematocrit in murine polycythemia vera. PLoS One 4(9): e7286.
- Nakata, K., Shikata, S., Ohtsuka, T., Ukai, T., Miyasaka, Y., Mori, Y., Velasquez, V., Gotoh, Y., Ban, D., Nakamura, Y., Nagakawa, Y., Tanabe, M., Sahara, Y., Takaori, K., Honda, G., Misawa, T., Kawai, M., Yamaue, H., Morikawa, T., Kuroki, T., Mou, Y., Lee, W. J., Shrikhande, S. V., Tang, C. N., Conrad, C., Han, H. S., Chinnusamy, P., Asbun, H. J., Kooby, D. A., Wakabayashi, G., Takada, T., Yamamoto, M. and Nakamura, M. (2018). Minimally invasive preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 25(11): 476-488.
- Reeves, J. P., Reeves, P. A. and Chin, L. T. (2001). Survival surgery: removal of the spleen or thymus. Curr Protoc Immunol Chapter 1: Unit 1.10. doi: 10.1002/0471142735.im0110s02.
- Serra, F., Sorrentino, L., Cabry, F., Biondini, D., Ceccarelli, P. L., Campanelli, M. and Gelmini, R. (2018). First case of laparoscopic partial splenectomy in a child with hamartoma: Case report and review of the literature. Int J Surg Case Rep 53: 140-143.
- Shimizu, T., Abu Lila, A. S., Kawaguchi, Y., Shimazaki, Y., Watanabe, Y., Mima, Y., Hashimoto, Y., Okuhira, K., Storm, G., Ishima, Y. and Ishida, T. (2018). A novel platform for cancer Vaccines: antigen-selective delivery to splenic marginal zone B cells via repeated injections of PEGylated liposomes. J Immunol 201(10): 2969-2976.
- Szendroi, T., Miko, I., Hajdu, Z., Acs, G., Kathy, S., Furka, I. and Szabo, L. (1997). Splenic autotransplantation after abdominal trauma in childhood. Clinical and experimental data. Acta Chir Hung 36(1-4): 349-351.
- Tastaldi, L., Krpata, D. M., Prabhu, A. S., Petro, C. C., Haskins, I. N., Perez, A. J., Alkhatib, H., Colturato, I., Tu, C., Lichtin, A., Rosen, M. J. and Rosenblatt, S. (2019). Laparoscopic splenectomy for immune thrombocytopenia (ITP): long-term outcomes of a modern cohort. Surg Endosc 33(2): 475-485.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, S. C., Rangel, A. D. and Kabeer, M. H. (2019). Precision Technique for Splenectomy Limits Mouse Stress Responses for Accurate and Realistic Measurements for Investigating Inflammation and Immunity. Bio-protocol 9(15): e3317. DOI: 10.21769/BioProtoc.3317.
- Li, S., Couet, J. and Lisanti, M. P. (1996). Src Tyrosine Kinases, Gα Subunits, and H-Ras Share a Common Membrane-anchored Scaffolding Protein, Caveolin: CAVEOLIN BINDING NEGATIVELY REGULATES THE AUTO-ACTIVATION OF Src TYROSINE KINASES. Journal of Biological Chemistry 271(46): 29182-29190.
Category
Immunology > Animal model > Mouse
Microbiology > in vivo model > Bacterium
Cell Biology > Tissue analysis > Injury model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










