- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocol for Measuring Compulsive-like Feeding Behavior in Mice
(*contributed equally to this work) Published: Vol 9, Iss 14, Jul 20, 2019 DOI: 10.21769/BioProtoc.3308 Views: 6021
Reviewed by: Arnau Busquets-GarciaYann HeraultMadeline Keleher

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1770 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1809 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 80 Views
Abstract
Obesity is an important health problem with a strong environmental component that is acquiring pandemic proportion. The high availability of caloric dense foods promotes overeating potentially causing obesity. Animal models are key to validate novel therapeutic strategies, but researchers must carefully select the appropriate model to draw the right conclusions. Obesity is defined by an increased body mass index greater than 30 and characterized by an excess of adipose tissue. However, the regulation of food intake involves a close interrelationship between homeostatic and non-homeostatic factors. Studies in animal models have shown that intermittent access to sweetened or calorie-dense foods induces changes in feeding behavior. However, these studies are focused mainly on the final outcome (obesity) rather than on the primary dysfunction underlying the overeating of palatable foods. We describe a protocol to study overeating in mice using diet-induced obesity (DIO). This method can be applied to free choice between palatable food and a standard rodent chow or to forced intake of calorie-dense and/or palatable diets. Exposure to such diets is sufficient to promote changes in meal pattern that we register and analyze during the period of weight gain allowing the longitudinal characterization of feeding behavior in mice. Abnormal eating behaviors such as binge eating or snacking, behavioral alterations commonly observed in obese humans, can be detected using our protocol. In the free-choice procedure, mice develop a preference for the rewarding palatable food showing the reinforcing effect of this diet. Compulsive components of feeding are reflected by maintenance of feeding despite an adverse bitter taste caused by adulteration with quinine and by the negligence of standard chow when access to palatable food is ceased or temporally limited. Our strategy also enables to identify compulsive overeating in mice under a high-caloric regime by using limited food access and finally, we propose complementary behavioral tests to confirm the non-homeostatic food-taking triggered by these foods. Finally, we describe how to computationally explore large longitudinal behavioral datasets.
Keywords: Compulsive overeatingBackground
Research in rodents and humans has shown that exposure to food rich in sweets and fats increases the risk for obesity and compulsive eating behavior (Avena et al., 2009). Exposure to such diets induces meal-pattern changes on patients defined by large meal size of dense caloric foods, rapid food consumption and loss of self-control during the eating episodes. Nevertheless, the development of such feeding behavior disturbances has not been studied, and intermediate states when changes in behavioral output occur remain obscure. These periods are particularly interesting because during weight gain, individuals may shift from non-pathological food consumption to compulsive overeating. In fact, short-term laboratory-based measures of eating in weight stable obese individuals may not be functionally relevant to understand long-term changes in body weight gain.
To address this, we used longitudinal meal pattern analysis for the study of obesity development (Espinosa-Carrasco et al., 2018a). Here, we describe a diet-induced obesity protocol based on ad libitum access to high-fat and chocolate diets. The protocol combines continuous recording of mouse feeding behavior and the use of specific standalone behavioral tests. This longitudinal analysis allows researchers registering and analyzing the time-dependent appearance and evolution of behavioral and cognitive changes upon body weight increase. Water consumption is also measured since it is strongly linked to food intake and for example declines dramatically in fasted or food-restricted animals (Ellacott et al., 2010; Goltstein et al., 2018). Additional tests measure the preference for rewarding stimuli (e.g., voluntary sucrose intake: Towell et al., 1987) and help quantify the perception of reward (‘liking’), whereas reward seeking (‘wanting’) is reflected by the intervals between feeding bouts. Inflexibility is a key indicator of compulsive feeding and can be assessed by temporally limiting the access to palatable food. A flexible response would result in a change to still available standard chow, whereas inflexibility would be revealed by neglect of the alternative (standard chow again). Other ways to search for compulsive components are to create a conflict by adulterating palatable food with bitter-tasting quinine (Heyne et al., 2009; Di Segni et al., 2014). The selected battery of tests, combined with the longitudinal food intake and motor activity recordings, enables i) a more comprehensive and in-depth characterization of the behavioral changes upon free access to palatable diets, ii) the examination of the etiological and susceptibility genetic factors and iii) the exploration of the basic mechanisms underlying compulsive overeating.
Materials and Reagents
- Mice
Adult (12 weeks of age or older) male mice (C57BL/6J) (Charles River, L’Arbresle, France, Strain Code 027), weighing 22-28 g at the beginning of the experiments. Mice are housed individually, each in their respective cages, in an environment with controlled temperature (around 23 °C) and humidity under a 12-12 h light-dark cycle with free access to food. Young adult mice (> 12 weeks) are used to avoid a bias due to the increase of body weight of the developmental growth curve.
Note: C57BL/6J mouse is chosen because it is widely used as a model for diet induced obesity since it is prone to develop severe obesity, elevated adiposity, glucose intolerance and moderate insulin resistance. However, other strains and ages of mice may also be used, but taking into account that strains such as SWR/J and A/J mice are more resistant to obesity development. The weight at the beginning of the experiments is just an indication (mean weight of C57BL/6J 12-weeks mouse). Of note, the initial weight of the animals could differ if a different strain is used. - Diet
In the diet-induced obesity protocol, animals can be allocated to receive free access of chocolate-mixture (CM) or exclusive access of high-fat diet (HF) for the study of obesity development as shown in Figure 1A. From now on, CM experiment is referred to as condition 1 and HF experiment as condition 2.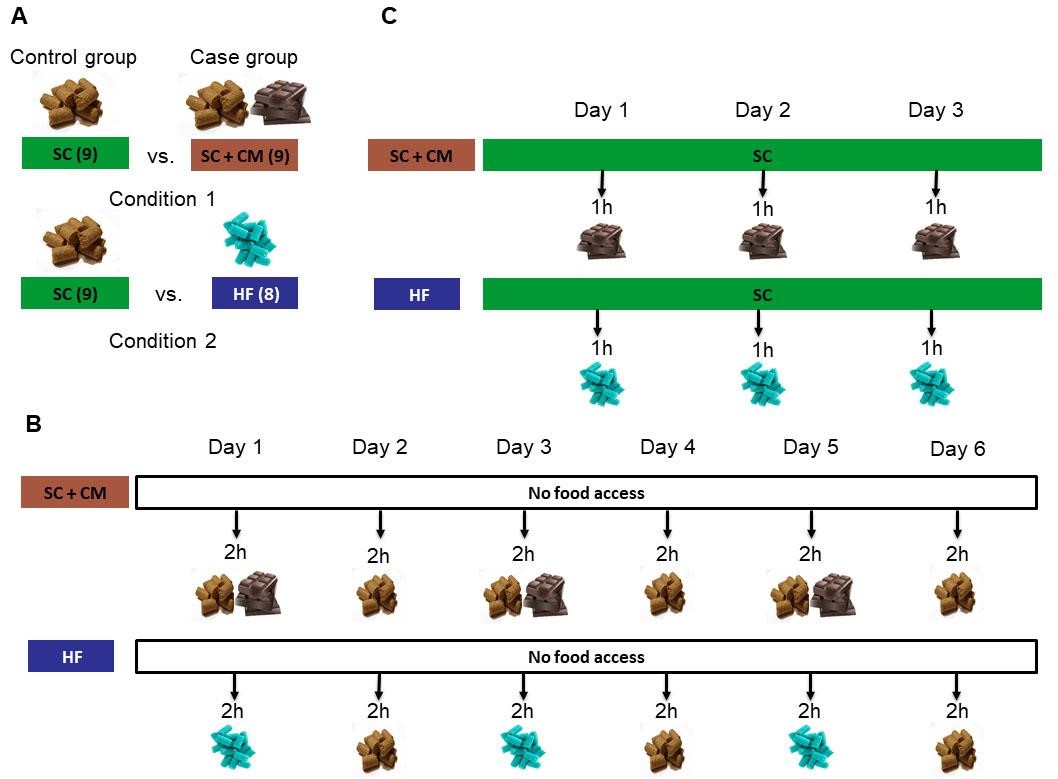
Figure 1. Schematic representation of the main experimental procedures. A. Two different experiments were carried out each of them with a separate control group, namely condition 1 and condition 2. Control group in both conditions 1 and 2 received standard chow (SC) ad libitum. After the habituation period, in condition 1 case group was provided with a free-choice between a standard chow diet and a chocolate-mixture diet (CM), while in condition 2 case group was offered only a high-fat (HF) hypercaloric food. Sample size is shown in parentheses denoting the number of mice in each group. B. Timeline of the limited access to energy-dense foods test in condition 1 (above) and condition 2 (below). Overweight mice were offered CM (condition 1) or HF (condition 2) pellets a single hour during the light (resting) phase of the light-dark cycle for 3 consecutive days to measure feeding inflexibility. C. Representation of the setup used to evaluate compulsive-intake in diet-induced obese mice of condition 1 (above) and obese mice of condition 2 (below). Mice have restricted the access to food to 2 h per day at the beginning of the dark phase during 6 consecutive days. On Days 1, 3 and 5 control mice received standard chow, while case mice in condition 1 are given standard chow/chocolate mixture and case mice in condition 2 are presented with high-fat diet. On Days 2, 4 and 6 all animals received only standard chow.- Standard chow (SDS, Witham, UK, catalog number: 801151)
Standard chow contained 10.76 MJ kg-1 digestible energy (17.5% coming from protein, 75% from carbohydrate and 7.4% from fat; Table 1). Typically, standard chow carbohydrates are contributed to by 45% starch and approximately 4% simple sugars (monosaccharide plus disaccharides) as a proportion of total carbohydrates by weight. - Pellets of a mixture of commercial chocolates (CM)
CM are made from equal amounts of Bounty®, Snickers®, Mars® and Milka® chocolates (local supermarket). CM pellets contained 20.6 MJ kg-1 digestible energy (17% coming from protein, 52% from carbohydrate and 24% from fat; carbohydrate is contributed to by 8% starch and approximately 44% simple sugars (monosaccharide plus disaccharides, Table 1). - High-Fat (HF) diet (Test Diet®, catalog number: 58Y1)
HF consisted of commercial pellets of purified diet with 60% energy derived from fat. HF pellets contained 22 MJ kg-1 digestible energy (24% coming from protein, 30% from carbohydrate and 35% from fat; carbohydrate is contributed to by 5.4% starch and approximately 6.4% simple sugars (monosaccharide plus disaccharides, Table 1).
- Diets listed above describe what has been used successfully using the protocol provided; however, using alternative diets with similar composition could also be successful.
- Food was renewed every 3-4 days to ensure the maintenance of the organoleptic properties. We have consistently observed in several of our experiments that around 15% of mice under a high-energy free choice obesogenic diet do not gain weight (obese-resistant).
Table 1. Nutritional composition and energy content of the experimental diets. The composition of the SC and HF diets was provided by the manufacturers. CM diet was prepared in the lab and its composition was analyzed by Alquimisa laboratories S.L (Salamanca, Spain).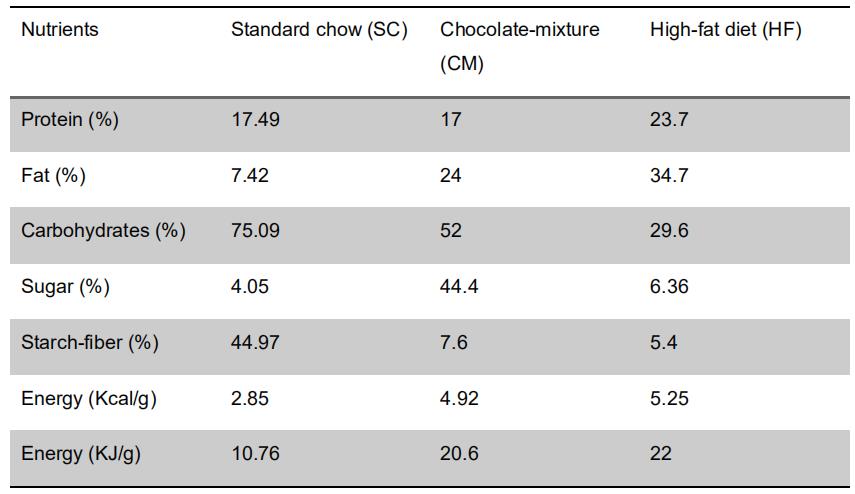
- Standard chow (SDS, Witham, UK, catalog number: 801151)
- Quinine hydrochloride dehydrated (Sigma-Aldrich, Madrid, Spain, catalog number: Q1125) for the adulteration of food in order to test mice feeding inflexibility.
Equipment
- Grinder and spoon for chocolate mixture (Bounty®, Snickers®, Mars® and Milka®) pellet preparation
- Diet storage refrigerator
- Personal computer for data recording using RS232/USB port
- PheCOMP System (Multitake model, Panlab, Barcelona, Spain, catalog number: LE0851B)
The equipment consists of experimental cages (Mouse polycarbonate home cages; Allentown Caging Equipment, ACE, 197 weight x 306 depth x 212 height mm, see Bura et al., 2010) provided with a grid floor and a filter top. The platform supporting each cage contains 2 infrared frames (16 x 16 beams, 16 mm spaced) allowing simultaneous recordings of horizontal activity and rearing (Panlab LE8827 Rearing IR frame).
Notes:- The cages contain 2 feeder units and 2 drinking units and give intake data resolutions of 0.02 grams per second allowing higher precision of the amount of food (g) or liquid consumed (ml) compared to standard cages; see Figure 2. The quantity of water drunk from a nipple, and any possible dripping, are also accounted by using the same system of transducers with 20-μl precision. The modular structure allows multiple dispenser combinations made them suitable for our free-choice experiments.
- One cage can only be used for one mouse.
- Before each experiment, the weight transducer system of the cages are calibrated. To do so, the manufacturer (Panlab, Barcelona, Spain) provides a 20 grams precision weight that is used to calibrate both the drinkers and feeders.
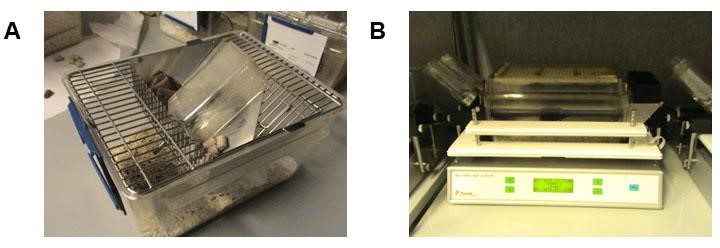
Figure 2. Representative images for housing cages used during the study. A. Standard individualized cage for mouse housing. B. A PheCOMP cage (Panlab, Spain), the equipment used to automatically record the food/water intake and activity of individualized mice.
Software
- Compulse software (Panlab, Barcelona, Spain) for automated data collection
- GraphPad PRISM 6.0 software (GraphPad Software. Inc., San Diego, CA, USA), a software for graphing
- SPSS 17.0 (SPSS Inc, Chicago, IL, USA) statistical analysis software
- “mtb2int.pl” and “int2combo.pl” Perl scripts for processing Phecomp cages output files into intervals comma-separated values (CSV) files. The scripts are available on github at: https://github.com/cbcrg/phecomp/blob/master/lib/perl/
- Pergola (Espinosa-Carrasco et al., 2018b) (https://github.com/cbcrg/pergola): Pergola is a Python library and command line tool for the conversion of longitudinal behavioral data into genomic file formats for its analysis and visualization
- Integrative Genomics Viewer (IGV) (Robinson et al., 2011), (http://software.broadinstitute.org/software/igv/), a desktop application for the visualization of genomic data
- Pergola web-server (Espinosa-Carrasco, 2019) (http://pergola.crg.eu/), a user-friendly web interface to process and visualize longitudinal behavioral data using Pergola
Procedure
- Experimental setup
- The Phecomp cages should be placed on a stable rack to avoid vibrations that can be detected by the food/liquid sensors and lead to inexact registers.
- For the mice given free access to CM and SC the side of the feeder containing the chocolate mixture, must be alternated to avoid side bias. However, once designated, the food located in each feeder should remain the same along the experiment.
- Chocolate Mixture diet preparation: To prepare the CM, break into small pieces an equal-weighted amount of Bounty®, Mars®, Snickers® and Milka® chocolate and mix in a bowl. Heat the mixture in a microwave oven at 350 W for 2 min to obtain a homogeneous mash. Extend the mixture while it is still melt on a smooth surface and as a result obtain a chocolate table of approximately two centimeters of thickness. Keep the resulting tablet in a refrigerator at least 24 h. Once solidified, cut the resulting tablet in small and equal pellets to obtain pellets that have a similar shape to the commercial standard chow pellet.
- Acclimatization to isolation (habituation phase)
House mice (C57Bl/6J 12 week old males) individually into the Phecomp cages (habituation phase) providing standard chow and water ad libitum for at least 10 days prior to initiating the diet-induced obesity protocol to define the baseline meal pattern and locomotor activity.
Note: The characterization of the time-course and dynamics of obesity-related behavioral changes requires isolation. The protocol can be used in both sexes but females show greater responses to stress than males (Senst et al., 2016). - Obesity development phase
- Weigh the mice and distribute them randomly (using Excel RAND() function) to the different diet conditions (chocolate mixture, or high-fat diet) balanced by body weight (8-12 mice per group). For each condition, use littermates as control pair animals (only given standard chow).
- Feed control group with standard chow and experimental mice with free choice between chocolate and SC or with only high-fat diet for 8 weeks. Animals are recorded uninterruptedly during periods of 3 or 4 days. Between these periods the system is paused during approximately one hour, for refilling the feeders and the cleaning of the cages.
- All diets are offered ad libitum. At the end of the obesity development phase, the increase of body weight must be significant in case groups as compared with controls (approximately 40% higher weight for HF mice and 10% higher weight for CM mice, see Espinosa-Carrasco et al., 2018a). Weigh mice two times a week. Circadian recording of food consumption, water intake and activity are obtained of the overall phase by using the automated collection system (Phecomp cages and Compulse software, see equipment and software sections, respectively).
- Longer recordings (6 days) will be much more informative of the more “natural” animal behavior but shorter recordings are needed for keeping track of all the possible problems arising inside cages (e.g., accumulation of dirtiness on dispensers which could interfere with signal transduction and/or infrared beams disruption by the sawdust). Therefore, we considered 3-4 days long recording is the most suitable option.
- Body weight and feeding behavior analysis
- Register body weight twice a week for the entire experimental period, always at the same time of the day.
- Determine body weight gain by subtracting the body weight on the 1st day of CM or HF exposure from the body weight on subsequent days.
- Testing phase
At the end of the obesity development phase, evaluate the appearance of diet-induced non-adaptive behaviors, such as compulsive behavior or behavioral inflexibility.
Evaluation of compulsive-intake in diet-induced obese mice- Deprive the animals of food during 24 h (water still provided ad libitum) and record feeding behavior upon the return of food (standard chow for control mice, chocolate mixture and standard chow or high-fat diet for case mice of Conditions 1 and 2, respectively). Twenty-four hours of food restriction is a powerful meal inducer having relatively little influence on its overall or long-term well-being (Toth and Gardiner, 2000). The increase in energy intake (KJ) after refeeding compared to free feeding conditions provides information on the influence of hunger on food seeking and food taking.
- Restrict the access to food to 2 h per day at the beginning of the dark phase (20:30-22:30 h) for 6 consecutive days. On Days 1, 3 and 5 the available food is standard chow (for control), standard chow and chocolate mixture (case mice in Condition 1) or high-fat diet (case mice in Condition 2). Days 2, 4 and 6 only standard chow is available to all animals. Water is still provided ad libitum. No food is provided the rest of the day (22 h of starvation), see Figure 1B. This food-restriction schedule allows maintaining the motivation in mice that will be alternately exposed to their non-preferred and their preferred diets. It has been demonstrated to be safe before and mice do recover their body weight (Tucci et al., 2006).
Note: After the 6-day testing phase animals from both conditions are given ad libitum food access to allow the recovery of their normal weight and feeding behavior. For some mice it is common to find pieces of the HF pellets inside the cages. Over-taking is a signature of impulsivity and compulsion, and our main interest is modeling compulsive behavior. For this reason, we recommend using the Phecomp recordings with correction of the spillage (e.g., by subtracting the amount of non-eaten food inside the cage). Otherwise, we cannot assume the entire food intake registered corresponds to the real food eaten.
- Limited access to energy-dense foods
Restrict the access to CM or HF pellets to 1 h per day during the light (resting) phase of the light-dark cycle (from 14:00 to 15:00 h) for 3 consecutive days in overweight mice. Standard chow and water are still provided ad libitum, see Figure 1C.
Note: The rest of the day all mice are fed with SC ad libitum. The test evaluates (i) inflexible behavior, flexible consumers are expected to restore standard chow intake whereas inflexible ones will reject standard chow and keep on waiting for its CM or HF; and (ii) compulsive intake binge-like eating episodes characterized by rapid consumption of high quantities of CM or HF during refeeding. - Food adulteration with quinine hydrochloride
Expose mice in condition 1 or condition 2 to adulterate (CM or HF) diet and standard diet during 24 h food ad libitum access. For food adulteration with Quinine hydrochloride, add 3 grams of quinine hydrochloride per kg of food during the chocolate mixture mash preparation. Stir the mixture to assure the concentration of quinine in the mixture is homogeneous.
Note: Because of bitter taste may impair the hedonic characteristics of CM/HF, the rationale of this test is to identify inflexible consumers as an indicator of compulsive intake. Creating this conflict, flexible mice (self-controlled ones) change their consumption to unadulterated non-preferred food whilst inflexible consumers do not respond to this bitter taste and continue to take this food. The concentration of quinine chosen was determined experimentally to be aversive in C57BL/6J mice. Control mice are given choice between standard chow and adulterated standard chow to control for bitter taste detection. Weight, food consumption and water intake of all mice are measured before and after every test.
Data analysis
Meal pattern analysis
Using the Phecomp cage system, we recorded single mouse solid and liquid intake for 9 weeks at 1-second resolution during the experiment. In this section, we summarize all the steps needed to visually explore the effect of the hypercaloric diet on circadian feeding rhythmicity.
Note: The continuous signals from each weight transducer and the infrared frame are amplified, digitized and sent to the computer software for data acquisition and storage.
Along the acquisition process, meal pattern analysis was performed using the COMPULSE software (Panlab, Barcelona, Spain). Meal pattern analysis included recording duration and recurrence intervals of eating or drinking acts, to calculate circadian and ultradian periodicities, and event-related frequency distributions. Feeding/drinking acts were considered as belonging to the
same meal or drinking bout when separated by less than 120 seconds from the next feeding/drinking act and hence, were merged into single food/water bouts (see Heyne et al., 2009).
The feeding parameters computed by the COMPULSE software and their biological significance are listed in Table 2.
For quantification of locomotor activity, we used the ActiTrack software (Panlab, Spain).
For the statistical analysis of meal pattern data, we used a linear mixed model and included the diet as a fixed effect. 'Mouse ID' was included as a random effect since we disposed of repeated measures in the case of the free choice mice. Differences are considered significant at P < 0.05 upon subsequent Bonferroni post-hoc analyses. To quantify the circadian feeding rhythmicity loss, we used three-way repeated measures ANOVA (genotype, diet and phase). For analysis of energy intake during the testing phase we used two-way ANOVA with diet (SC, SC + CM, SC + HF) as factors between subjects. For each test, significance is considered when P < 0.05. The statistical analysis can be performed using the Statistical Package for Social Science program SPSS®.
Table 2. Parameters defining meal pattern and its behavioral significance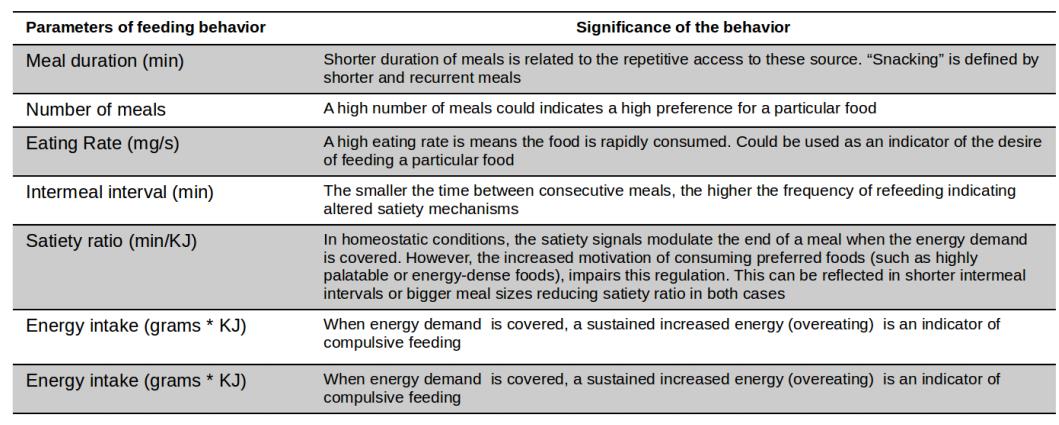
Longitudinal analysis
For the visualization of the longitudinal data, and specifically of food consumption and temporal distribution of feeding events of each mouse, we use a customized open-source software developed in our laboratory (Pergola; http://cbcrg.github.io/pergola) designed for the manipulation, modeling, visualization and integration of (high-throughput) longitudinal behavioral data. On this part of the protocol, we summarized the steps to pre-process the data (steps 1 and 2) and to analyze the data using Pergola (steps 3 to 6). In short, Pergola formats longitudinal behavioral data into files following genomic standards. This formatting enables us to analyze the data using genomic software (see Espinosa-Carrasco et al., 2018b) and simplifies operations such as its visualization.
- Aggregation of Compulse recordings: Compulse, the proprietary software distributed with the Phecomp cages, produces a single text file for each consecutive period of recordings and cage (MTB files) containing the raw behavioral trackings. We created a Perl script to parse these files, mtb2int.pl that aggregates all the data from several MTB files in a single file in the form of intervals.
mtb2int.pl recording_1.mtb recording_2.mtb ... recording_n.mtb -startTime file tac -rename cages Y -out > intervals_feeding.int
Arguments:
recording_1.mtb to recording_n.mtb: MTB files recorded by Phecomp cages.
-startTime file tac: Use the timestamp of the files to set the initial time point.
-rename cages Y: When more than one batch of cages is simultaneously processed, set the IDs of the mice of the additional batches to numeric values starting from last used in the former batch plus 1. n+1 preceding value.
-out: Print resulting intervals to stdout.
intervals_feeding.int: Output file containing the parsed intervals. - A second Perl script, int2combo.pl, filters events with an intake less than 0.02 g, merges feeding/drinking acts (inter-meal interval shorter than 120 seconds, see above) and formats the data as a comma-separated values (CSV) file.
int2combo.pl intervals_feeding_act.int -tag field value max 0.02 -filter action rm -bin -compulse -out output R > intervals_feeding_bout.csv
Arguments:
intervals_feeding.int: Input File containing the parsed intervals.
-tag field value max 0.02 -filter action rm -bin: Sets the field to be filtered, the value and the criteria.
intervals_feeding.csv: Output file containing intake intervals.
Note: The data to reproduce steps 3 to 5 and 6 has been archived on Zenodo and can be accessed under the following link https://doi.org/10.5281/zenodo.2668520. - Format raw meals as BED files. Use Pergola to convert the resulting CSV files from the previous step into BED files containing the feeding acts (meals).
pergola -i intervals_feeding.csv -m mapping.txt -f bed -e -dl food_sc food_fat -d all
Arguments:
-i intervals_feeding.csv: Input CSV file containing meal intervals.
-m mapping.txt: A mapping file to describe the content of the input file.
-f bed: Output format.
-e: The first time point on the data becomes time zero.
-dl food_sc food_fat -d all: Data types to keep. - Bin food intake using time windows of 1,800 s and format the data as bedGraph files. Each window value will correspond to the sum of all the intakes of a single mouse.
pergola -i intervals_feeding.csv -m mapping.txt -f bedGraph -w 1800 -e -dl food_sc food_fat -d all
Arguments:
-i intervals_feeding_bout.csv: Input CSV file containing meal intervals.
-m mapping.txt: A mapping file to describe the content of the input file.
-f bedGraph: Output format.
-w 1800: Creates windows of 1800 seconds and sums up the intakes within the corresponding window.
-e: The first time point on the data becomes time zero.
-dl food_sc food_fat -d all: Data types to keep. - Visualization of circadian feeding rhythmicity (Figure 3). The resulting BED and bedGraph files can be visualized using a desktop genome browser such as IGV. IGV needs a FASTA file to define a reference sequence genome to which the data are mapped. This file is generated by Pergola when running any of the two above-mentioned commands. Please refer to pergola documentation for a detailed description of how to upload data for its visualization (http://cbcrg.github.io/pergola/visualization.html#igv).
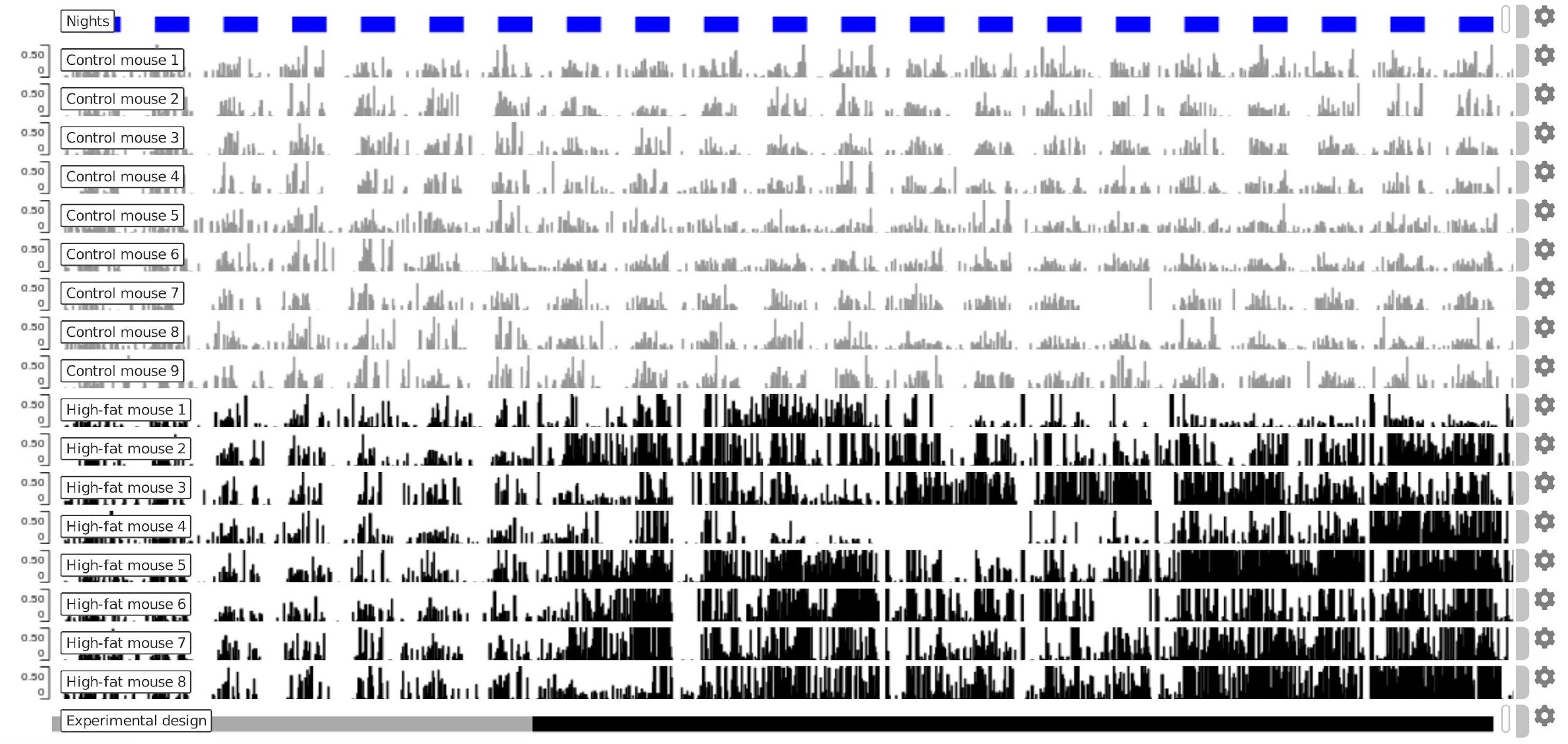
Figure 3. Representative data for disruption of meal pattern during circadian monitoring. Each mouse track displays the accumulated food intakes of mice within 30-minutes time windows as bars. Each row depicts data of a single individual. Top track blue squares depict dark phase of the circadian cycle. Note the circadian microstructure of feeding behavior during the habituation phase (bottom track grey) and how food intake rhythmicity is lost in DIO exposed (DIO initiation indicated by black in bottom track). The displayed period corresponds to the three first weeks of the experiment of condition 2 mice and its controls (barplot tracks in black and gray, respectively). The experimental design track can be found in the Zenodo archived data, see note under Longitudinal analysis step 2. - Alternatively, steps 3 to 5 can be performed using the Pergola web-server (Espinosa-Carrasco et al., 2019). The exact options to process the data as explained in this protocol are shown in Figure 4.
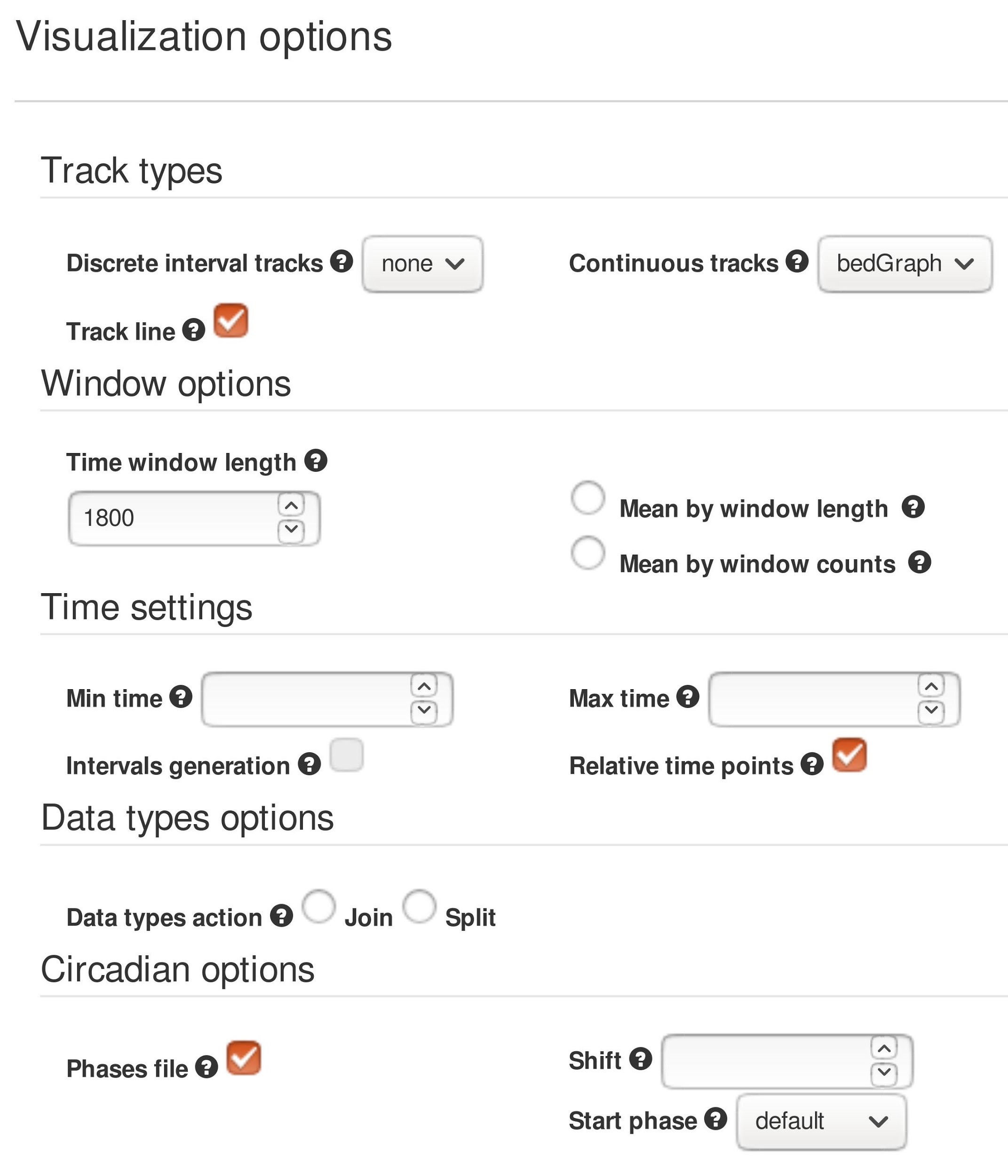
Figure 4. Pergola web server options to obtain the visualization as shown in Figure 3. The set of options shows how to configure the server to obtain the longitudinal visualization of the data.
Notes
- During the time between consecutive tests, mice are maintained on their diet conditions depending on the group.
- Since the tests used may alter food intake pattern, it is important to provide the animals with enough time to re-establish their previous food intake pattern during the days between tests. Thus, tests were performed in a set order designed to minimize the effect of testing on following tests and with sufficient inter-test intervals to allow the mice to re-establish their previous feeding behavior.
- Chocolate mixture and high fat must be removed from the cage and replaced with fresh food twice a week to assure the quality, texture and properties of the mixture are optimal.
- PheCOMP cage must be carefully cleaned to avoid any rest of food or sawdust interferes with signal transduction. PheCOMP cages are cleaned every 2 weeks.
Acknowledgments
We acknowledge the help of PRBB Animal facility and especially of Dr. Juan Martín Caballero. The laboratory of M.D. is supported by DIUE de la Generalitat de Catalunya (Grups consolidats SGR 2017/926). This work was supported by Fondation Jérôme Lejeune (Paris, France), MINECO (SAF2016-79956-R), CDTI (‘Smartfoods’), EU (Era Net Neuron PCIN-2013-060) and the Catalan foundation ‘La Marató de TV3’ (#2016/20-30) and the EU Joint Programme–Neurodegenerative Disease Research (JPND) project under grant agreement HEROES AC17/00006. The CRG is a Center of Excellence Severo Ochoa SEV-2012-0208. The CIBER of Rare Diseases is an initiative of the ISCIII.
Competing interests
The authors have no conflicts of interest to declare.
Ethics
All experimental protocols were performed in accordance with recommendations for the proper care and use of laboratory animals [local (law 32/2007); European (EU directive no 86/609, EU decree 2001-486) regulations, and the Standards for Use of Laboratory Animals no A5388-01 (NIH)] and were approved by the local ethical committee (CEEA-PRBB).
References
- Avena, N. M., Rada, P. and Hoebel, B. G. (2009). Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr 139(3): 623-628.
- Bura, S. A., Burokas, A., Martin-Garcia, E. and Maldonado, R. (2010). Effects of chronic nicotine on food intake and anxiety-like behaviour in CB1 knockout mice. Eur Neuropsychopharmacol 20(6): 369-378.
- Di Segni, M., Patrono, E., Patella, L., Puglisi-Allegra, S. and Ventura, R. (2014). Animal models of compulsive eating behavior. Nutrients 6(10): 4591-4609.
- Ellacott, K. L., Morton, G. J., Woods, S. C., Tso, P. and Schwartz, M. W. (2010). Assessment of feeding behavior in laboratory mice. Cell Metab 12(1): 10-17.
- Espinosa-Carrasco, J., Burokas, A., Fructuoso, M., Erb, I., Martin-Garcia, E., Gutierrez-Martos, M., Notredame, C., Maldonado, R. and Dierssen, M. (2018). Time-course and dynamics of obesity-related behavioral changes induced by energy-dense foods in mice. Addict Biol 23(2): 531-543.
- Espinosa-Carrasco, J., Erb, I., Hermoso Pulido, T., Ponomarenko, J., Dierssen, M. and Notredame, C. (2018). Pergola: Boosting visualization and analysis of longitudinal data by unlocking genomic analysis tools. iScience 9: 244-257.
- Espinosa-Carrasco, J., Hermoso Pulido, T., Erb, I., Dierssen, M., Ponomarenko, J. and Notredame, C. (2019). Pergola-web: a web server for the visualization and analysis of longitudinal behavioral data using repurposed genomics tools and standards. Nucleic Acid Research 47(w1): w600-w604.
- Goltstein, P. M., Reinert, S., Glas, A., Bonhoeffer, T. and Hübener, M. (2018). Food and water restriction lead to differential learning behaviors in a head-fixed two-choice visual discrimination task for mice. PLoS One 13(9): e0204066.
- Heyne, A., Kiesselbach, C., Sahun, I., McDonald, J., Gaiffi, M., Dierssen, M. and Wolffgramm, J. (2009). An animal model of compulsive food-taking behaviour. Addict Biol 14(4): 373-383.
- Robinson, J. T., Thorvaldsdóttir, H., Winckler, W., Guttman, M., Lander, E. S., Getz, G. and Mesirov, J. P. (2011). Integrative genomics viewer. Nat Biotechnol 29(1): 24-26.
- Senst, L., Baimoukhametova, D., Sterley, T. L. and Bains, J. S. (2016). Sexually dimorphic neuronal responses to social isolation. Elife 5: e18726.
- Toth, L. A. and Gardiner, T. W. (2000). Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci 39(6): 9-17.
- Towell, A., Muscat, R. and Willner, P. (1987). Effects of pimozide on sucrose consumption and preference. Psychopharmacology (Berl) 92(2): 262-264.
- Tucci, V., Hardy, A. and Nolan, P. (2006). A comparison of physiological and behavioural parameters in C57BL/6J mice undergoing food or water restriction regimes. Behav Brain Res 173(1): 22-29.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fructuoso, M., Espinosa-Carrasco, J., Erb, I., Notredame, C. and Dierssen, M. (2019). Protocol for Measuring Compulsive-like Feeding Behavior in Mice. Bio-protocol 9(14): e3308. DOI: 10.21769/BioProtoc.3308.
Category
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








