- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Optical Stimulation and Electrophysiological Analysis of Regenerating Peripheral Axons
Published: Vol 9, Iss 12, Jun 20, 2019 DOI: 10.21769/BioProtoc.3281 Views: 5636
Reviewed by: Shaarika SarasijaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optogenetic Approach for Investigating Descending Control of Nociception in Ex Vivo Spinal Cord Preparation
Volodymyr Krotov [...] Pavel Belan
Nov 5, 2025 1977 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Whole-Mount Immunostaining for the Visual Separation of A- and C-Fibers in the Study of the Sciatic Nerve
Valeriia Ustymenko [...] Nana Voitenko
Dec 5, 2025 1421 Views
Abstract
Although axons in the peripheral nervous system can regenerate, functional recovery after nerve injuries is poor. Activity-based therapies, such as exercise and electrical stimulation, enhance the regeneration of cut peripheral axons. Despite their effectiveness, clinical application of these experimental techniques has been limited. At least part of the basis for this translational barrier has been a lack of information as to the precise mechanism of activity-based therapies on peripheral axon regeneration. To evaluate the requirements for neuron-type specific activation to promote regeneration using these therapies, in the current protocol, we employed optogenetics. Utilizing the advantages of transgenic mouse lines we targeted opsin expression to different neuron types. Using fiber optics we activated those neurons with high temporal specificity as a model of activity-based intervention after nerve injury and to measure functional recovery achieved after such a treatment.
Keywords: ChannelrhodopsinBackground
Optogenetics has emerged as a potent tool in experimental neuroscience. The expression of light-gated ion channels in neurons can be used to control neuronal activity with great temporal precision, and by targeting of that expression to different neuronal populations the role of that activity can be studied very specifically. Our lab and others have demonstrated that activity-based experimental therapies, such as exercise and electrical stimulation, if applied after peripheral nerve injury will enhance the regeneration of cut axons of motor and sensory neurons (Al-Majed et al., 2000; Brushart et al., 2002; Wood et al., 2012; Thompson et al., 2014). Exercise in the form of treadmill running is a powerful promoter of axon regeneration but it is difficult to separate the effects of exercise-induced activation of injured neurons from other effects, such as alterations in available hormones, changes in muscle properties, modulation of immune cells or activation of uninjured neurons in networks that generate the locomotion. Furthermore, when modeling the effects of exercise on recovery from peripheral nerve injury, it is difficult to determine whether the effect is due to the production of action potentials in the neurons whose axons have been severed or whether exercise may alter the membrane potential of axotomized neurons without directly causing it to fire an action potential. To determine these parameters would be highly challenging in an awake behaving animal running on a treadmill.
Brief electrical stimulation of a cut peripheral nerve also enhances axon regeneration (Elzinga et al., 2015; Gordon and English, 2016). It offers some technical advantages in probing the mechanism of action of exercise on peripheral axon regeneration. A specific peripheral nerve can be unilaterally targeted by applying stimulation to the nerve, and the frequency and duration of stimuli (action potentials) applied to the nerve can be controlled. However, other cell types within a peripheral nerve also respond to electrical stimulation (Schwann cells and probably immune cells), and the conductive nature of tissues can facilitate current spread. The lack of cell-type specificity of electrical stimulation and exercise also have limited some of the mechanistic questions that can be proposed. Here, we describe a way to use optogenetics to apply a cell-type specific activity-based therapy and also to use optogenetics to provide a functional outcome measure (direct muscle response, i.e., the direct M response) following nerve injury.
We used mouse genetics to target the expression of the light-sensitive cation channel, channelrhodopsin 2, selectively in sensory neurons, motoneurons, or both. In the mice we used, only a subset of the neurons with axons in peripheral nerves expressed this opsin, those that also express yellow fluorescent protein (YFP); other neurons acted as internal controls. We then activated the opsin-expressing neurons by application of light to their axons in peripheral nerves. We found that brief optogenetic activation enhanced peripheral axon regeneration regardless of whether motor or sensory neurons were treated individually or synchronously (Figure 1). We also used optogenetics to measure functional recovery using electrophysiology (Ward et al., 2016).
A major advantage of the use of optogenetics in this model system is that activity can be discretely controlled in genetically targeted neuronal populations of interest. Optogenetics can be used to evaluate the mechanism behind the success of activity-based therapies on peripheral axon regeneration. For example, we have also used an inhibitory opsin, Natromonas halorhodopsin, fused to a light-emitting Renilla luciferase and Venus (a protein for bioluminescent resonance energy transfer that amplifies the emitted light) (Tung et al., 2015; Berglund et al., 2016) to inhibit neuronal activity during treadmill exercise. The luciferase oxidizes a small molecule substrate, h-Coelenterazine, to generate yellow photons and activate the halorhodopsin. Thus, after administration of h-Coelenterazine, neurons expressing this iLMO2 fusion protein become hyperpolarized. In motoneurons whose axons were regenerating, we found that inhibiting motoneuron activity during treadmill exercise blocked the enhancing effect of exercise (Jaiswal et al., 2017). Considered together, the results of these optogenetic experiments lead us to conclude that neuronal activity is both necessary and sufficient to promote the regeneration of injured peripheral axons.
Future advances in excitatory and inhibitory opsins, bioluminescence, luminopsins and chemogenetics will continue to facilitate the development of neuromodulation and novel gene therapy approaches. Via chemogenetic manipulation using DREADDs (Cre-dependent excitatory designer receptor exclusively activated by designer drugs), we have shown that subthreshold excitation of motoneurons and sensory neurons is sufficient to enhance their regeneration (Jaiswal et al., 2018). The application of these new optogenetic tools will expand our ability to further define the activity requirements of highly specific neuronal populations.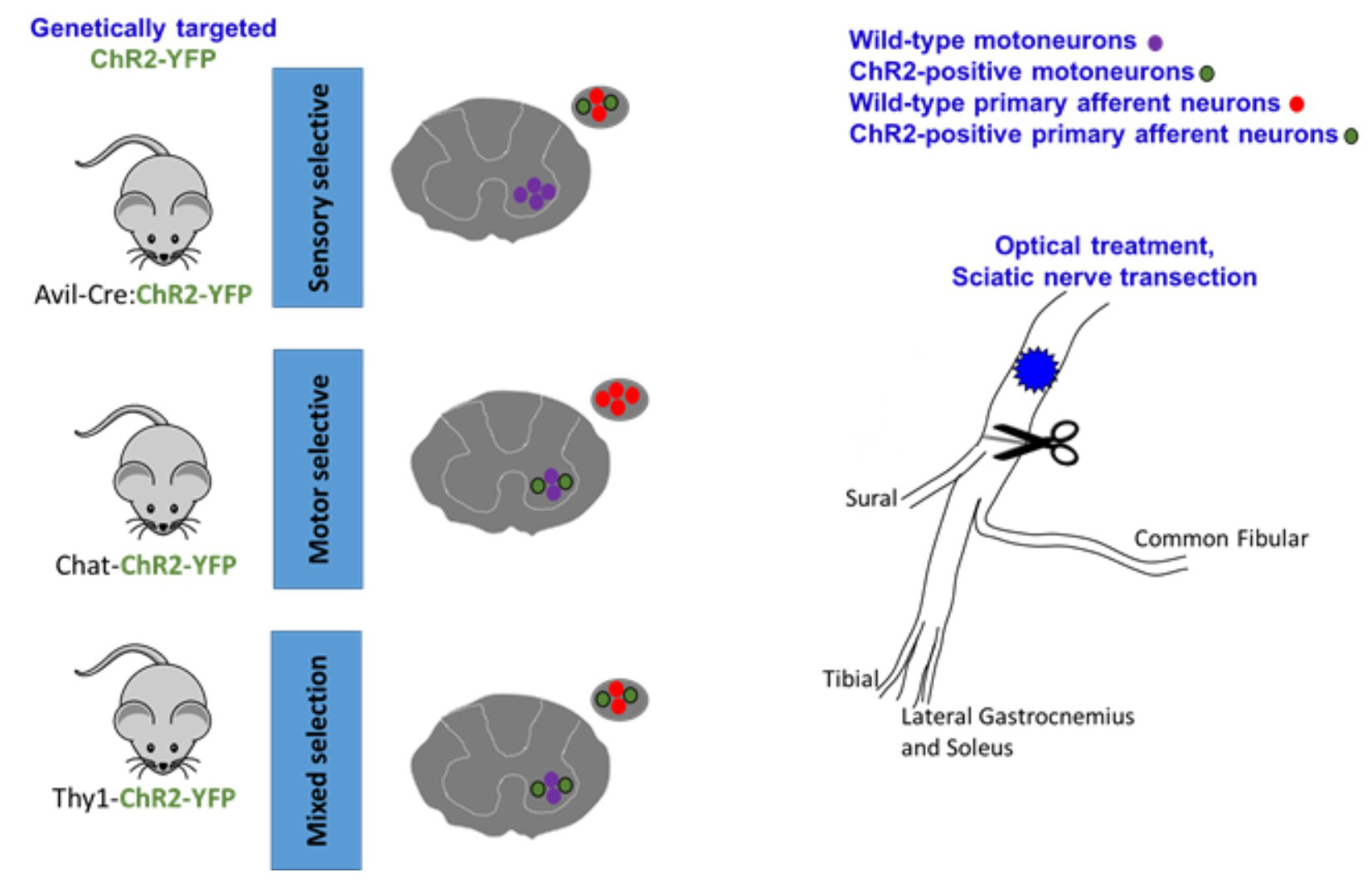
Figure 1. graphical representation of the mouse model and sciatic nerve. Multiple promoters can be used to selectively express ChR2 (or other opsins) in targeted cell types. In the cartoons of spinal cords and dorsal root ganglia cross-sections, different cell types express ChR2 and are responsive to blue light activation in the periphery. The whole sciatic nerve and its terminal branches are depicted in the cartoon. Using different transgenic mouse models, we have shown that excitatory neuronal stimulation and exercise enhance axon regeneration following sciatic nerve injury, and this effect can be blocked with inhibitory luminopsins.
Materials and Reagents
- 0.5 ml tubes (Bio PLAS, Inc.)
- Silastic sheeting (Dow Corning Corporation Medical Products)
- 10 μl pipette tips (BioExpress)
- 1 ml Syringe 25 G (BD, Franklin lakes, NJ)
- Gauze pads 2 x 2 (Fisher brand)
- Betadine wipes (Purdue Products, L.P.)
- Coated Visorb undyed braided polyglycolic acid suture NSF-2 19 mm 3/8 30” (75 cm)
- Monofilament polyamide suture (Redilon 5-0) (1 metric) 18” (45 cm)
- Mice expressing desired opsins (Jackson Laboratories)
- Thrombin (MP Biomedical)
- Fibrinogen (Sigma)
- Xylazine sterile solution 20 mg/ml Ana Sed Injection (AKORN)
- Ketamine HCl Injection, USP 50 mg/ml (Hospira)
- Ethanol (Fisher)
- CaCl2 (Sigma)
- Fibrin glue (see Recipes)
Equipment
- Surgery
- Dumont forceps #5 (Fine Science Tools, catalog number: 11293-00)
- Spring scissors (Fine Science Tools, catalog number: 15008-08)
- Surgical scissors (Fine Science Tools, catalog number: 14084-09)
- Suture tying forceps (Fine Science Tools, catalog number: 11090-09)
- Needle holders with suture cutters (Fine Science Tools, catalog number: 12502-14)
- Hair clipper (Braintree Scientific)
- Heating pad or warm water-circulator (Braintree Scientific Gaymar TP500)
- Scalpel and handle
- Surgical microscope (Wild Heerbrug Stereozoom)
- Table clamp (to hold the fiber optic cable in place)
- 2.5-10 μl pipette
- Glass-bead sterilizer (Braintree Scientific, catalog numbers: GER 5287-120V and GER 5289)
- -20 °C freezer
- optogenetics and electrophysiology
- Optogenetics patch cable (ThorLabs, catalog number: M82L01)
- Fiber coupled laser source and driver (ThorLabs)
Notes:- Alternatively: We built our own laser diode source and driver and have published the hardware setup in detail with photos at http://web.stanford.edu/group/dlab/optogenetics/hardware.html.
- It is important that the light source must emit light in the wavelength of the opsin being used. For example, we used blue light (473 nM wavelength) to activate channelrhodopsin 2.
- Cuff electrode (or other electrophysiologic recording devices, such as a microchannel nerve interface)
- Silastic tubing (Laboratory Tubing, catalog number: 508-006)
- insulated wire (CoonerWire, catalog number: AS631)
- flexible silicone (Dow Corning, catalog number: Sylgard® 184)
- Recording electrodes
- Fine wire, size 0.002 mm (California Fine Wire Company, MO#M468240)
- Sterile 25 G needle
- Laser safety glasses (ThorLabs, Inc.)
- Silver ground wire (A-M Systems, Inc.)
- Electrical stimulator and stimulus isolation units:
- low-noise 6-channel electrophysiology amplifiers (Figure 2A)
- optically isolated pre-amplifier stage (Figure 2B)
- Linear photovoltaic optocouplers (IXYS Integrated Circuits, catalog number: LOC111)
- Galvanically isolated power supply module (CUI, catalog number: VWRAS2-D5-D12)
- Preamp instrumentation amplifier (Analog Devices, catalog number: AD8421)
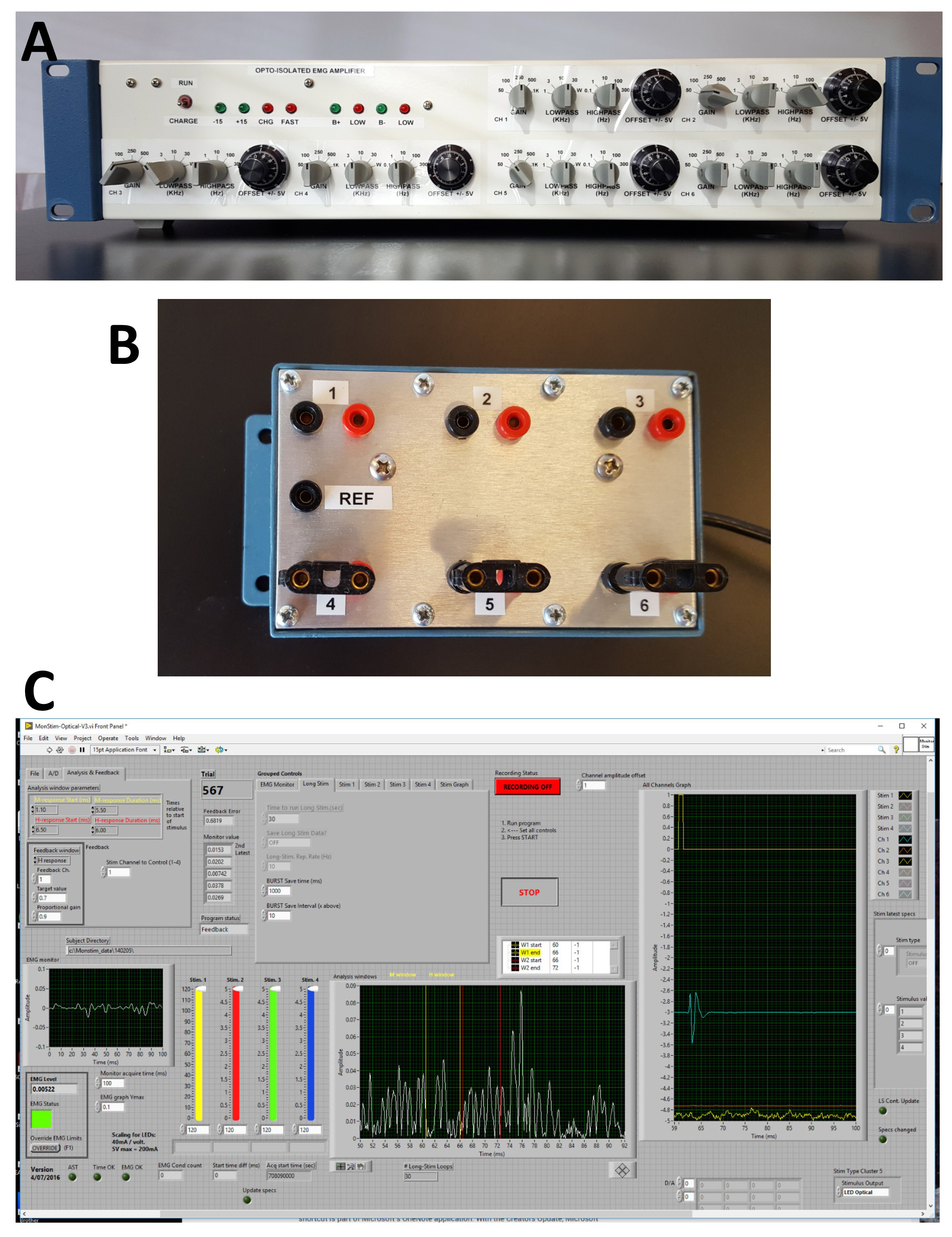
Figure 2. Electrophysiology rig and software. A. Front panel of six channel AC amplifiers used. Separate controls of gain, low pass and high pass filters and offset are present for each channel. B. The optically isolated head stage amplifier unit enables bipolar inputs for each channel and a common reference. C. Front panel of custom Labview® software used for acquisition of electrophysiological data.
Software
- Labview® (National Instruments)
Note: Other software is commercially available to run optogenetic hardware (e.g., ThorLabs). - Data acquisition and stimulation program
Our data acquisition and stimulation program were developed in-house using National Instruments’ Labview® programming language. The program monitors ongoing activity for one of the input channels for nerve or electromyography/electroneurogram (EMG/ENG) activity. This is displayed on the left of the front panel (Figure 2C). When the average value of activity falls within a user-defined voltage window for 20 ms, a stimulus is output to wire cuff electrodes or optical devices, either LEDs or laser pulses, and data are recorded for a given period, written to disc and displayed in windows to the right of the front panel (Figure 2C). The data can then be analyzed off-line as described below.
Procedure
- Construction of cuff electrode (Figure 3A) (~2 days)
- On day 1, cut two 12 inch lengths of Cooner wire. Strip off 6 mm of insulation from the tips of the Cooner wires.
- Cut one 0.5 inch length of Silastic tubing. Cut a slit longitudinally through the tubing.
- Thread the Cooner wires through the walls of the tube. Wrap the tip of the wire around itself to form a knot. The two exposed wires should be approximately 2 mm apart on the internal portion of the Silastic tubing.
- For any portion of wire that is exposed (not insulated) on the external portion of the Silastic tube, coat with flexible silicone and allow it to dry overnight.
- On day 2, trim the Silastic tubing so that the entire length of the cuff is ~1 cm.
- Construction of fine wire electrodes (Figure 3B) (~30 min per electrode)
- Cut two 12 inch lengths of 0.002 mm fine wire.
- Thread the two lengths of fine wire through a sterile 25 G needle.
- With a scalpel, gently scrape away the green insulation from the tips of the fine wire so that ~1.5 mm of wire is exposed as the electrode. Fold the de-insulated wires to form “hooks” at the needle tip. Stagger the tips so that the de-insulated portions of the two wires are not in contact with each other.
- On the distal length of the fine wires, gently scrape away ~1 cm of the green insulation. This end will connect to the pre-amplifier.
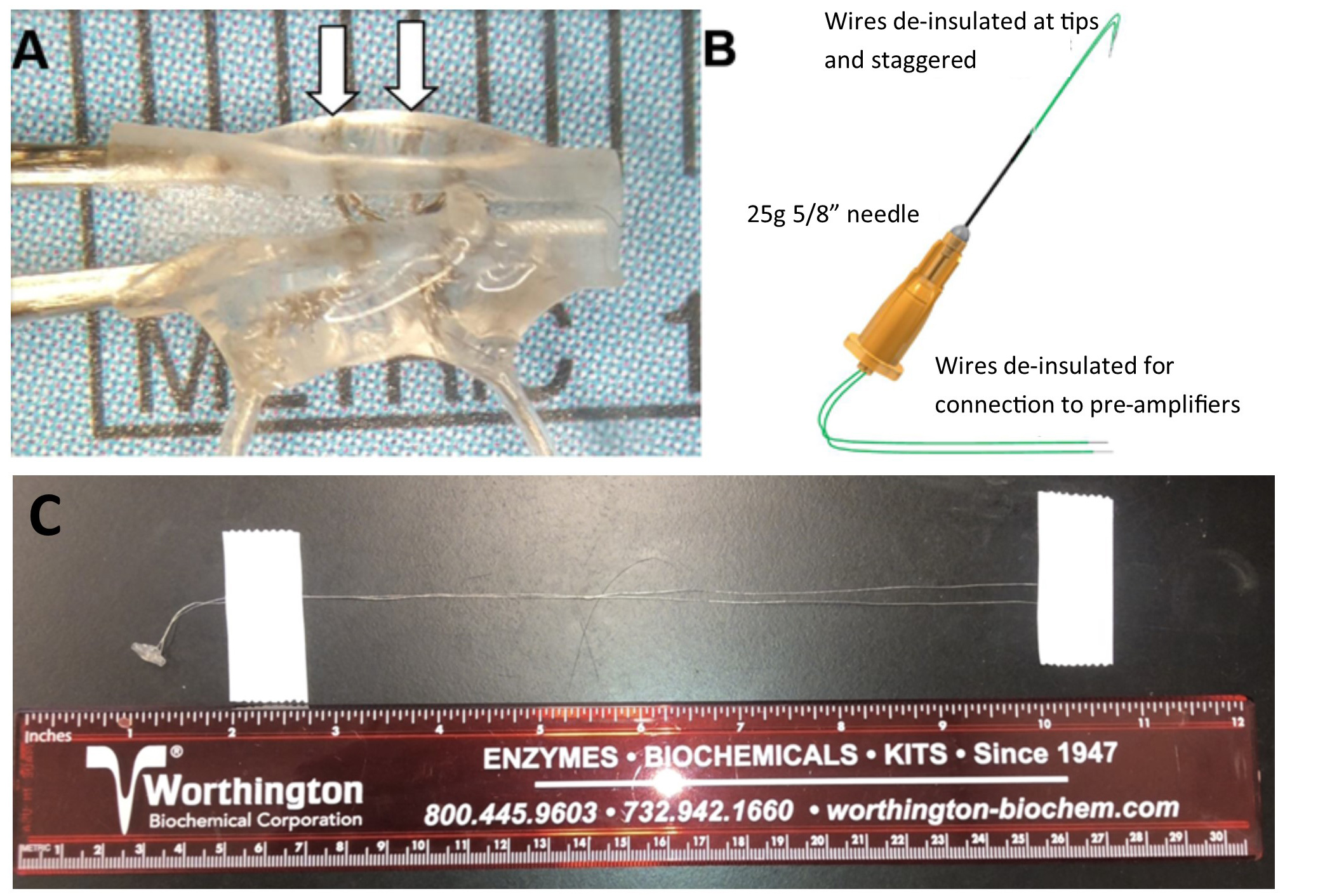
Figure 3. Recording and stimulating electrodes. A. Cuff electrode made with stranded stainless steel microwire. Arrows point to the de-insulated wires coursing around the circumference of the inner surface of the Silastic tubing. B. Fine wire EMG electrode. C. Full view of the cuff electrode.
- Optical activation of neurons with axons in the sciatic nerve
- Sterilize instruments in hot beads (~250 °C for 2-3 s).
- Anesthetize mouse with an intraperitoneal injection of ketamine/xylazine cocktail (Ketamine HCl, 80 mg/kg [McKesson, Memphis, TN, USA]; Xylazine, 10 mg/kg [AnaSed Injection, LLOYD Laboratories, Quezon City, Philippines]). The injections volume typically ranges from 0.1cc to 0.25cc based on mouse weight.
- Clip fur from the entire hindlimb.
- Clean the surgical site with betadine and ethanol three times.
- Make a 1-inch skin incision in the skin over the posterior thigh to expose the hamstring muscles overlying the sciatic nerve.
- Gently blunt dissect the hamstring muscles apart in the fascial plane to expose the sciatic nerve.
- Insert the fine wire needle electrode into the gastrocnemius muscle (Figures 4A and 4B). Place the electrode in a common location in each animal (the muscle will atrophy significantly after nerve injury).
- Place the cuff around the sciatic nerve (Figure 4B) and gently hold the tubing closed with a suture.
- Position the fiber optic cable so that the tip is gently contacting the sciatic nerve Figure 4A. Use a clamp to hold the cable in place. Take care that the clamp does not break the fiber optic.
Optional: A specific branch of the sciatic nerve can be selectively stimulated by dissecting the epineurium to separate the tibial and common fibular nerves (Figure 4C). - Stimulate the nerve with a 0.1 ms electrical pulse at ~5 v applied through the cuff electrode. A visible muscle twitch (and M response resulting from direct motor axon stimulation) should accompany the pulse. If a muscle twitch occurs but no evoked EMG response can be recorded, check all connections.
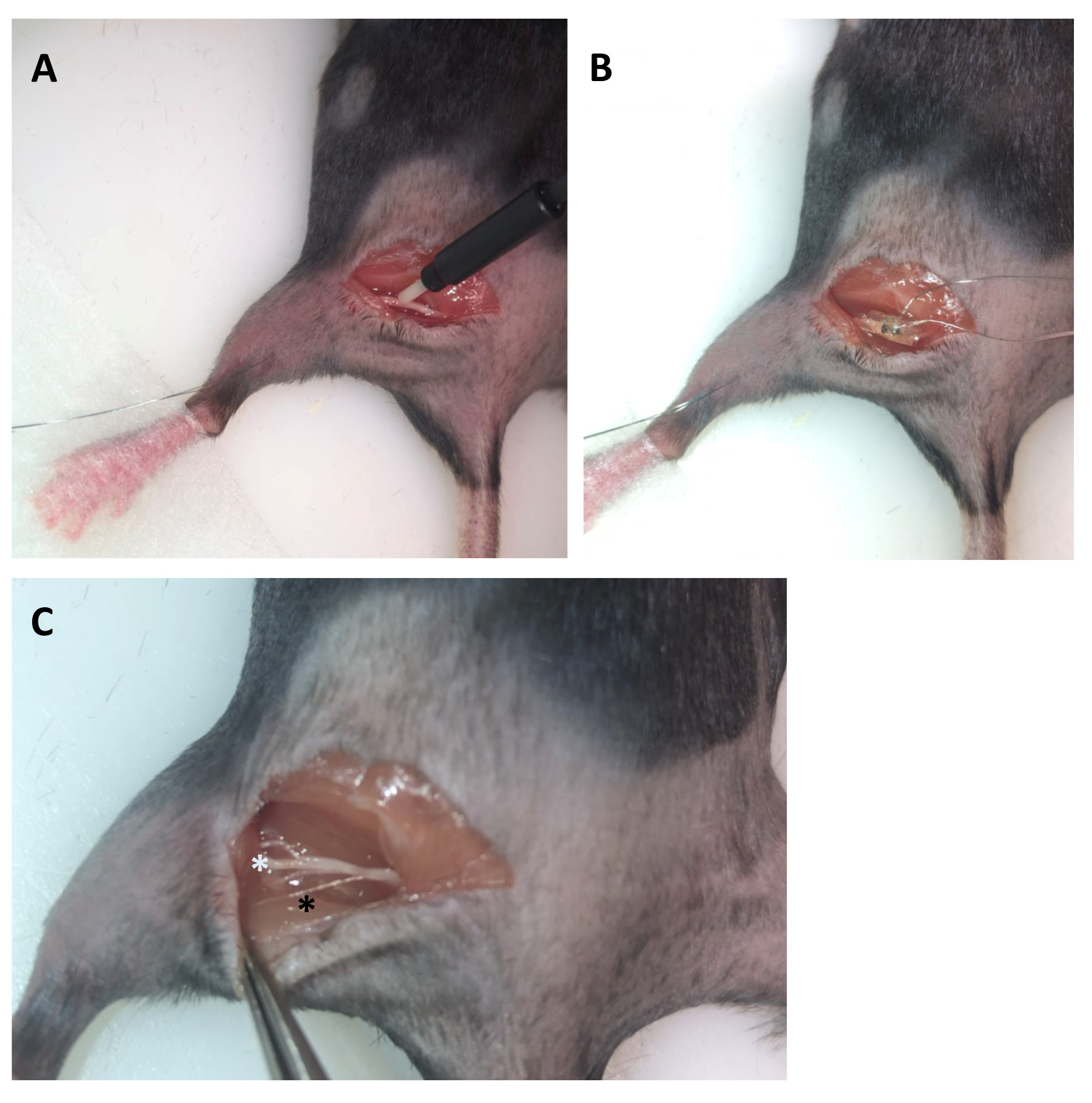
Figure 4. Dissection preparation for stimulation and recording in mouse. A. The tip of the fiber optic cable is in gentle contact with the exposed sciatic nerve. B. The cuff electrode is placed around the sciatic nerve. In A and B, observe that the green fine wire electrode is transcutaneously inserted into the lateral gastrocnemius muscle. C. Branches of the sciatic nerve were gently separated by removing the epineurium with fine forceps. The white asterisk indicates the larger tibial nerve branch, and the smaller common peroneal (fibular) and sural nerves can be seen above and below it, respectively. The black asterisk indicates a small branch of the sciatic nerve that innervates a portion of the hamstring muscle. - Stimulate the nerve with a 5 ms pulse of light delivered through the optical fiber at maximal light intensity (see Note 1 regarding light output). A muscle twitch (and direct M response) should accompany the pulse (if using a mouse that expresses ChR2 in motoneurons, e.g., Thy1ChR2 or Chat:ChR2).
- Record potentials from the muscle and nerve in response to light stimulation. Stimuli should be delivered no more often than once every three seconds to minimize any muscle fatigue.
Note: If recording from the nerve, action potentials will propagate in both directions along the nerve from the light source. In mice in which ChR2 expression is restricted to sensory neurons, e.g., the AvilCre:ChR2 mouse, no muscle twitch (or direct M response) should be present. Instead, potentials can only be recorded from the nerve cuff electrode as an electroneurogram (Figure 5A, right). - Stimulate both electrically and optically over a range of stimulus intensities, starting at the lowest intensity (voltage or light intensity) and incrementally increasing to determine a threshold (the voltage or light intensity) at which a response occurs with 50% of the stimulus trials and the maximal response (Vmax). For example, start at 0.1 V and increase stimulus strength by 0.1 V until Vmax is reached.
- Perform optical treatment at a light intensity greater than the intensity required to obtain a maximal M response. The most widely used treatment paradigm is supramaximal stimulation at 20 Hz for one hour.
- Perform nerve transection and repair.
- Nerve transection and repair
- Cut a small (~3 mm x 3 mm) square of Silastic sheeting.
- With forceps, place the Silastic square under the nerve just proximal to the branch point of the sural nerve.
- Mix 2.5 μl of fibrinogen with 5 μl of thrombin and quickly pipette this glue mixture onto the nerve on the Silastic film.
- Allow the fibrin glue to dry.
- Gently probe the film with forceps to ensure that the nerve is secured to the Silastic film.
- With sharp spring scissors, transect the nerve where it is glued to the film and ensure that no fibers remain in continuity at the deepest part of the nerve.
- Apply more fibrin glue to the transected nerve.
- Post-operative recovery and monitoring
Once all wounds have been closed with suture, animals should be placed in a clean recovery cage on an approved water bath-regulated heating pad so that half of the cage is heated and half is at ambient room temperature. Animals should be monitored every 15 min until fully recovered from anesthesia at which time they will be moved into their housing room. The non-absorbable skin sutures should be removed from the skin ~7-10 days. Loss of 25% of body weight from pre-operative body weight is considered evidence that the animal is debilitated, and a veterinary consult should be obtained regarding euthanasia.
Data analysis
In our experiments, recording stimulus-evoked activity starts 20 ms before stimulation (considered background activity) and ends 50 ms after stimulus delivery.
- Raw signals were amplified (1,000x) and band-pass filtered (100-1,000 Hz).
- Data were sampled at 10 kHz.
- Responses to 10-30 stimulus presentations at each stimulus intensity studied were averaged.
- Average background (pre-stimulus) activity was digitally subtracted from recordings.
- Average full-wave rectified voltages of evoked EMG and ENG potentials were measured in user-defined latency windows (Figure 5A, left: dashed lines).
The quantitative electrophysiological data gathered using optically evoked potentials can be analyzed in several manners. By comparing EMG response amplitudes in the same latency windows over a range of stimulus intensities (optical or electrical), a full motor recruitment curve can be generated (Figure 5B) and the stimulus threshold for activation and maximal evoked responses can be determined. If a change in neuronal excitability is expected, such as when using an inhibitory opsin (Jaiswal et al., 2018), such an effect can be evaluated as changes in stimulus threshold. Using recording electrodes placed on the nerve, optical nerve stimulation can be used to collect an evoked electroneurogram (Ward et al., 2018) and similar quantitative measurements can be made. When optically stimulating sensory axons in a nerve, the resulting ENG measurements (Figure 5A, right) also can be used to partition the responses of sensory axons of different sizes and conduction velocities by evaluating response amplitudes in different latency windows. Finally, if electrically- and optically-evoked data are collected in the same experiment, the maximal responses can be expressed as an optical/electrical ratio (Ward et al., 2016) and analyzed at different recovery times in comparison to data from intact controls (Figure 5C).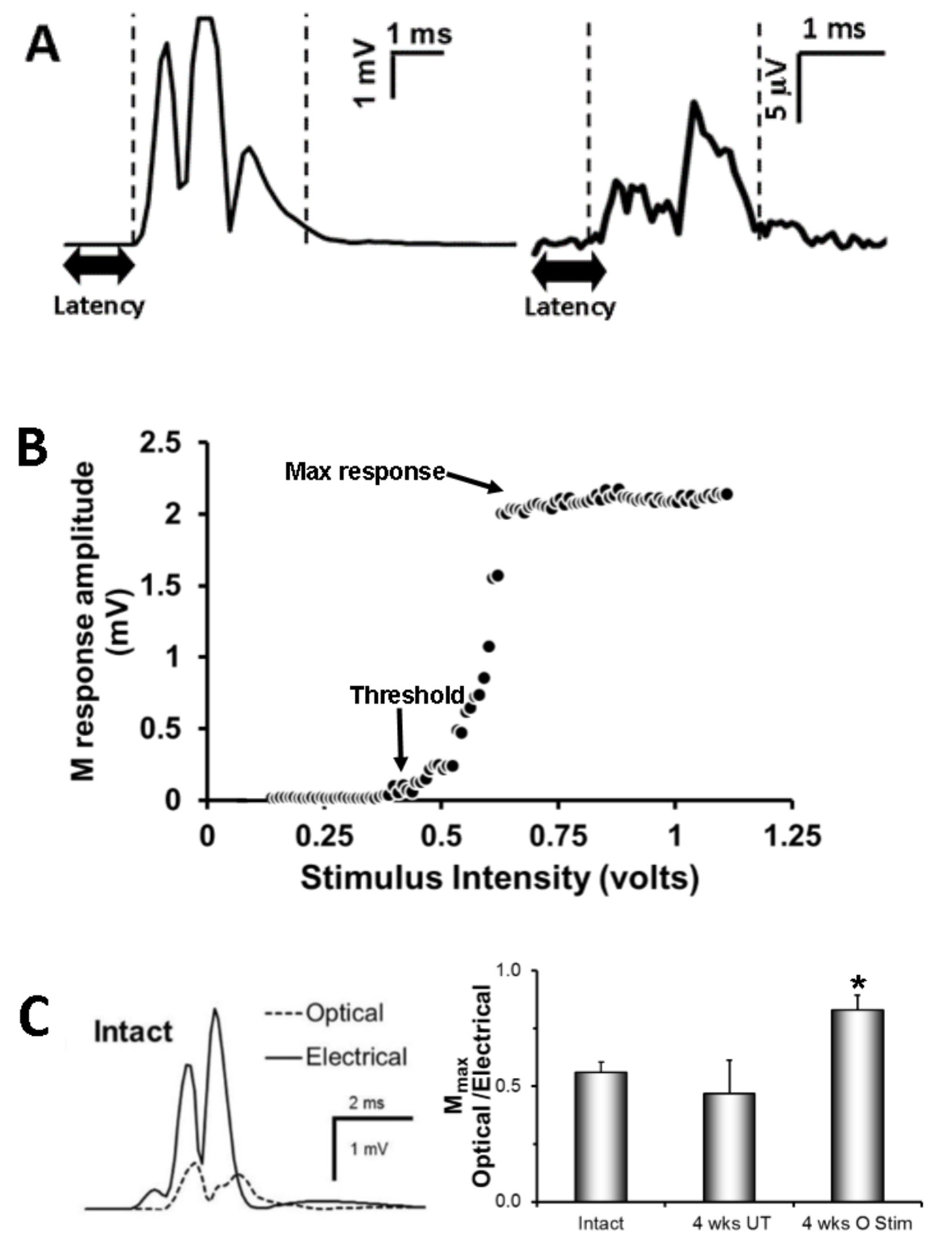
Figure 5. Examples of ways in which quantitative electrophysiological data were analyzed. A. Rectified EMG (left) or ENG (right) traces yield several quantifiable parameters, including latency (time from stimulus onset to response, bold double arrow), duration (time from the detectable response the dashed lines), and response amplitude (mV). After the injury, latencies and amplitudes decrease, while duration increases. B. Ramp of slowly increasing stimulus intensity (voltage if electrical stimulation; luminance [mW/mm2] if optically stimulating) allows the detection of threshold (the stimulus intensity required to evoke a response 50% of the time) and the maximum response. C. Collection of electrical and optical data within the same experiment (left) facilitates the distinction of evoked responses due to electrically excitable (wild type and ChR2 expressing) neurons/axons vs. responses due optically excitable (ChR2 expressing) neurons/axons. Electrically- and optically- evoked M responses can be expressed as a ratio (bar graph-right) to determine the proportion of wild type and ChR2-expressing that have successfully regenerated at different times after injury. In the bar graph, we show that 4 weeks after injury, the maximal M response appears due to the successful regeneration of ChR2 expressing motoneurons that had undergone optical treatment versus the intact and untreated groups.
Notes
- It should be noted that optical fibers can crack if mishandled or bent and could lead to reduced light output due to scattering. The amount of reduction may not be noticeable with one’s eyes. Thus, we recommend that the fiber optic cables be periodically tested for light output by using a photodiode power sensor (available at ThorLabs) suitable for the wavelength of light being utilized.
- Latencies are sometimes used in humans to inform clinicians about type of axons (e.g., C fiber versus Aδ) they are recording from. However, in the mouse, the available distances between the recording and stimulating sites are very small and do not lend themselves well to such analyses.
- More optical tools and optogenetic mice will become available. With the three genotypes of mice, we have tested with channelrhodopsin, the duration of the light pulse required to evoke a response varied. Therefore, we strongly suggest that a range of light pulse durations be tested before applying as a treatment or to produce optical data as an outcome measure.
- In the majority of Chat-ChR2-YFP mice, the observed M response produced by optical stimulation dissipated or disappeared completely during optical treatment at 20 Hz. The activation of the channelrhodopsin simply could not follow this rate of light application (Ward et al., 2018). At slower optical stimulation rates (e.g., 10 Hz) M responses could be evoked reliably. In these animals, light stimulation was applied at 10 HZ but for two hours. Others have shown that the absolute number of stimuli, not the rate of stimulation, is the most critical parameter for enhancing peripheral axon regeneration (Park et al., 2015).
- We initially attempted these experiments with light-emitting diodes (LEDs) constructed inside the tubing (similar to Michoud et al., 2018). It is critical to insulate the solder points and frequently test them for electrical leaks. Handling the LED cuff with forceps and the bending that occurs at the solder points can cause an unwanted leak of electrical current (and electrical stimulation of the nerve).
Recipes
- Fibrin glue
- Prepare stock thrombin
- Dilute 5 μl of 25 U/ml thrombin in 45 mM CaCl2
- Pipette the thrombin as 50 μl aliquots
- Freeze (-20 °C) stock solution aliquots until needed on the day of nerve transection surgery
- On the day of nerve transection surgery, fibrinogen must be prepared fresh.
- Weigh out 50 mg of fibrinogen
- Pipette 500 μl of sterile water into a centrifuge tube
- Add the 50 mg of fibrinogen to the 500 μl of sterile water
- Stir gently (do not shake) with the pipette tip until the fibrinogen is dissolved (3-6 min)
- Thaw 1 thrombin aliquot.
- Combine fibrinogen and thrombin at a 2:1 ratio
- Apply immediately to the nerve
- Prepare stock thrombin
Acknowledgments
This research was funded by NIH Grants NS057190, NS087915. Special thanks to Olivia Mistretta and Bill Goolsby for their contributions and continued support.
Competing interests
The authors declare no conflicts of interest.
Ethics
All procedures were approved by the Institutional Animal Care and Use Committee of Emory University (protocol #2003261) and conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience.
References
- Al-Majed, A. A., Neumann, C. M., Brushart, T. M. and Gordon, T. (2000). Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20(7): 2602-2608.
- Berglund, K., Tung, J. K., Higashikubo, B., Gross, R. E., Moore, C. I. and Hochgeschwender, U. (2016). Combined optogenetic and chemogenetic control of neurons. Methods Mol Biol 1408: 207-225.
- Brushart, T. M., Hoffman, P. N., Royall, R. M., Murinson, B. B., Witzel, C. and Gordon, T. (2002). Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci 22(15): 6631-6638.
- Elzinga, K., Tyreman, N., Ladak, A., Savaryn, B., Olson, J. and Gordon, T. (2015). Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol 269: 142-153.
- Gordon, T. and English, A. W. (2016). Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 43(3): 336-350.
- Jaiswal, P. B., Mistretta, O. C., Ward, P. J. and English, A. W. (2018). Chemogenetic enhancement of axon regeneration following peripheral nerve injury in the SLICK-A mouse. Brain Sci 8(5): E93.
- Jaiswal, P. B., Tung, J. K., Gross, R. E. and English, A. W. (2017). Motoneuron activity is required for enhancements in functional recovery after peripheral nerve injury in exercised female mice. J Neurosci Res. doi:10.1002/jnr.24109.
- Michoud, F., Sottas, L., Browne, L. E., Asboth, L., Latremoliere, A., Sakuma, M., Courtine, G., Woolf, C. J. and Lacour, S. P. (2018). Optical cuff for optogenetic control of the peripheral nervous system. J Neural Eng 15(1): 015002.
- Park, S., Koppes, R. A., Froriep, U. P., Jia, X., Achyuta, A. K., McLaughlin, B. L. and Anikeeva, P. (2015). Optogenetic control of nerve growth. Sci Rep 5: 9669.
- Thompson, N. J., Sengelaub, D. R. and English, A. W. (2014). Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev Neurobiol 74(5): 531-540.
- Tung, J. K., Gutekunst, C. A. and Gross, R. E. (2015). Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci Rep 5: 14366.
- Ward, P. J., Clanton, S. L., 2nd and English, A. W. (2018). Optogenetically enhanced axon regeneration: motor versus sensory neuron-specific stimulation. Eur J Neurosci 47(4): 294-304.
- Ward, P. J., Jones, L. N., Mulligan, A., Goolsby, W., Wilhelm, J. C. and English, A. W. (2016). Optically-induced neuronal activity is sufficient to promote functional motor axon regeneration in vivo. PLoS One 11(5): e0154243.
- Wood, K., Wilhelm, J. C., Sabatier, M. J., Liu, K., Gu, J. and English, A. W. (2012). Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol 72(5): 688-698.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ward, P. J. and English, A. (2019). Optical Stimulation and Electrophysiological Analysis of Regenerating Peripheral Axons . Bio-protocol 9(12): e3281. DOI: 10.21769/BioProtoc.3281.
Category
Neuroscience > Peripheral nervous system > Sciatic nerve
Neuroscience > Sensory and motor systems > Spinal cord
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









